Abstract
Preeclampsia (PE) is a multisystemic disorder of pregnancy that not only causes perinatal mortality and morbidity but also has a long-term toll on the maternal and fetal cardiovascular system. Women diagnosed with PE are at greater risk for the subsequent development of hypertension, ischemic heart disease, cardiomyopathy, cerebral edema, seizures, and end-stage renal disease. Although PE is considered heterogeneous, inefficient extravillous trophoblast (EVT) migration leading to deficient spiral artery remodeling and increased uteroplacental vascular resistance is the likely initiation of the disease. The principal pathophysiology is placental hypoxia, causing subsequent oxidative stress, leading to mitochondrial dysfunction, mitophagy, and immunological imbalance. The damage imposed on the placenta in turn results in the “stress response” categorized by the dysfunctional release of vasoactive components including oxidative stressors, proinflammatory factors, and cytokines into the maternal circulation. These bioactive factors have deleterious effects on systemic endothelial cells and coagulation leading to generalized vascular dysfunction and hypercoagulability. A better understanding of these metabolic factors may lead to novel therapeutic approaches to prevent and treat this multisystemic disorder. In this review, we connect the hypoxic-oxidative stress and inflammation involved in the pathophysiology of PE to the resulting persistent cardiovascular complications in patients with preeclampsia.
Keywords: cardiovascular disease, endothelial dysfunction, hypoxia, metabolic disturbances, preeclampsia
INTRODUCTION
Preeclampsia (PE) affects nearly 7% of pregnancies worldwide and remains the second leading cause of maternal mortality, contributing to nearly 70,000 maternal deaths worldwide each year (1). The World Health Organization estimates a higher incidence of PE in developing countries, with a significant burden in middle- and low-income settings (2). Recent evidence highlights disruptions in specific metabolic pathways as key contributors to the complex etiology of PE (3–5). A prior history of PE and factors associated with metabolic syndrome, including chronic hypertension, diabetes mellitus, and obesity, are considered to be the primary risk factors (6). This is the primary reason for the rise in PE cases in developed countries like the United States (7).
PE is a hypertensive disorder of pregnancy, characterized by the new onset of hypertension after 20 wk of gestation, where systolic blood pressure reaches or exceeds 140 mmHg and diastolic blood pressure is at or above 90 mmHg (8). This is accompanied by proteinuria and damage to vital organs such as the liver, heart, kidneys, and brain (e.g., hepatic impairment, heart dysfunction, renal dysfunction, and neurological complications have been observed in preeclamptic mothers) (8–10). Depending on how the symptoms manifest, PE can be broadly classified into early- or late-onset forms (11). Early-onset PE manifests before 34 wk of gestation, primarily attributed to irregular placentation and reduced placental perfusion. This subtype is also commonly termed “placental preeclampsia” (12). Such cases not only give rise to long-lasting maternal cardiovascular complications but also heighten the risk of intrauterine growth restriction and premature birth in the fetus (13). Late-onset PE develops after 34 wk of gestation, accounting for ∼80% of cases (2). It is mainly associated with maternal pregestational chronic inflammation, linked to factors like obesity, autoimmune disorders, diabetes mellitus, and hypertension (2). These underlying conditions heighten the vulnerability of maternal endothelial cells to inflammatory triggers (14). This form is also denoted as “maternal preeclampsia” because of the mismatch between normal maternal hemodynamics and the rising metabolic demands of the placenta and fetus (15).
In both subtypes of PE, the central pathophysiological event is proposed to originate from placental hypoperfusion, leading to significant hypoxia/ischemia (13). The impaired remodeling of the uterine spiral arteries results in inadequate energy supply to the uteroplacental interface, contributing to abnormal implantation and disrupting placental and fetal metabolic processes (16, 17). This sets off a “stress” response within the placenta, marked by metabolic dysregulation, oxidative stress, inflammation, and the release of harmful factors into the maternal circulation (13, 18). These factors, including antiangiogenic biomolecules (soluble fms-like tyrosine kinase-1 or sFLT-1 and soluble endoglin or sEng) and proinflammatory cytokines, promote endothelial vascular dysfunction, primarily by reducing the bioavailability of the vasodilator nitric oxide (NO), contributing to systemic maternal symptoms (9, 16). Consequently, PE emerges as a multisystemic disorder affecting both the mother and fetus (19). This intricate interplay suggests that specific metabolic abnormalities triggered by placental ischemia act as causative factors for PE, leading to widespread alterations in the maternal and fetal metabolic milieu and potentially causing cardiovascular consequences later in life. Recent studies have unveiled concerning statistics regarding the cardiovascular complications of PE, revealing a nearly fourfold increase in the incidence of heart failure, a fourfold increase in subsequent chronic hypertension, and a 2.5- to 4-fold rise in other cardiovascular outcomes, such as stroke, for individuals with a history of PE when compared with those without such a history (20–22). Although prior reviews have primarily focused on specific mechanisms driving the development of the disease, including hypoxia, angiogenic imbalance, and altered mitochondrial metabolism (23–25), the objective of this review is to establish a connection between oxidative stress, a major contributing factor of PE, and the development of cardiovascular comorbidities.
In this narrative review, we conducted a PUBMED search during the period from July 2023 to March 2024 using numerous primary topic headings combined with appropriate terms for each section of the article (e.g., pregnancy, preeclampsia, oxidative stress, mitochondrial dysfunction, hypoxia, inflammation, and cardiovascular disease). Relevant full text articles published in English language were included in this article.
UNDERSTANDING THE BASICS: A CONCISE OVERVIEW OF PREECLAMPSIA ETIOLOGY
Despite significant advancements in our understanding of the pathophysiology of PE, there is limited consensus regarding its true etiology. Multiple hypotheses have been proposed to explain the origin of the disease without cohesive evidence; hence, it has been termed the “disease of theories” (19, 26). The development of PE hinges on a complex interplay of genetic, immunological, and environmental factors (9, 16, 27, 28). The principal pathological contributors to the development of PE are deficient placentation (29), immune system dysregulation (30), systemic inflammatory response (31), endothelial dysfunction (32), and genetic predisposition (28). Importantly, all of these etiological factors are linked with metabolic disturbances (33, 34).
METABOLIC DYSREGULATION IN PREECLAMPSIA
Metabolic pathways direct both normal and aberrant placentation (35–39). The placenta, being a metabolically dynamic organ, responds to various interwoven nutritional and endocrine signals (40), contributing to dysregulations in systemic metabolism that may be associated with organ dysfunction and clinical symptoms observed in PE. Targeting interventions toward placental metabolism, which is significantly influenced by the maternal metabolic status and placental/fetal adaptation (41), could be an innovative therapeutic approach to address PE and its associated complications. Accordingly, the following sections of this review will describe the interconnected metabolic disruptions in the preeclamptic placenta, correlating them with end-organ damage and clinical symptoms in the mother, with a specific focus on the cardiovascular system.
Metabolic Shift during Placental Development
Alterations in the bioenergetics of an organism, known as the “metabolic theory of diseases,” may contribute to the development of various clinical disorders (34, 42–44). Several lines of evidence indicate metabolic disturbances as a central mechanism in cancer and cardiometabolic diseases such as hypertension, type 2 diabetes, obesity, heart failure, and PE (34, 44, 45). In the context of cancer, it is proposed that various biomolecular abnormalities observed in the disease may originate from a common metabolic shift: the transition from mitochondrial oxidative energy production to glycolysis, a phenomenon known as the Warburg effect (46). This metabolic alteration is believed to contribute to cancer development by promoting a hyperproliferative and apoptosis-resistant phenotype in cells with suppressed mitochondrial function (47–49). The persistence of dysfunctional mitochondria, leading to the use of alternative energy sources, further exacerbates this phenotype (50). Thus, the metabolic shift and its consequences on mitochondrial activity may underlie several features of cancer.
Although the Warburg effect is a pathophysiological phenomenon in the context of cancer, during pregnancy, it plays an essential role in providing the necessary energy for the growth of placental cells (51, 52). Placental cells exhibit a hyperproliferative phenotype throughout pregnancy, requiring high energy, which resembles that of cancer cells (53, 54). During the early stages of pregnancy, the development of the placenta occurs rapidly, demanding a significant amount of energy that is primarily provided through anaerobic metabolism (55, 56). The process of aerobic glycolysis is ∼15 times more efficient than anaerobic metabolism (51, 57, 58). However, a circulatory system, which provides a constant supply of oxygen (O2) for aerobic glycolysis, is not established during the early stages of pregnancy when the placenta is still developing (56, 59). Decidualization is the process by which endometrial stromal cells undergo morphological and functional changes to become specialized decidual cells. This transformation is crucial for the successful implantation of the embryo and the formation of the placenta (60). This process occurs in a hypoxic environment at the maternal-fetal interface and relies on the Warburg effect (anaerobic glycolysis as the main energy source) for energy (52). The hypoxic environment induces many factors, such as hypoxia-inducible factors (HIFs), specifically HIF-1α in the context of placental physiology. HIF-1α is a key regulator of glucose metabolism, inducing the transcription of various glycolytic genes and promoting the Warburg effect (52, 61). This phenomenon enables the efficient generation of ATP to sustain the rapid cellular proliferation necessary for placental growth similar to that seen in tumor microenvironment (52). The preference for glycolysis over oxidative phosphorylation is advantageous due to its faster and energetically favorable pathway for ATP production (62). This metabolic reprogramming enables cells within the developing placenta to meet the increasing demands for energy and biosynthetic precursors during rapid cellular expansion and tissue growth, ultimately supporting fetal development (62). The Warburg effect also induces a suppression of mitochondrial activity within the placental cells, linked to their hyperproliferative phenotype, similar to cancer cells (63). Figure 1 illustrates the shared features of placental cells and cancer cells. The mitochondrial suppression during placental development supports the high glycolytic flux. The glycolytic cascade intermediates enter various biosynthetic pathways, generating nucleic acids, fatty acids, and amino acids required for sustained cellular proliferation. This confers resistance to apoptosis and encourages the survival and differentiation of placental cells, even under the hypoxic conditions observed during placental development (52, 64).
Figure 1.

The Warburg effect in pregnancy and cancer. Similarities between the uteroplacental interface and cancer microenvironment. The Warburg effect observed in malignant and placental cells delineates a metabolic phenomenon wherein proliferating cells exhibit heightened glucose uptake. Glucose undergoes glycolysis, a series of enzymatic reactions that convert it into pyruvate. Unlike in most cells where pyruvate is primarily directed to the mitochondria for oxidative phosphorylation to produce adenosine triphosphate (ATP), in cells undergoing the Warburg Effect, pyruvate is preferentially converted into lactate, even in the presence of sufficient oxygen. This metabolic shift is more efficient and favors rapid ATP generation, a characteristic of proliferating cells in cancer and the developing placenta. The tumor microenvironment and the uteroplacental interface present contrasting characteristics in terms of the pathological impacts they exert (on tumors) and the physiological effects they support (during pregnancy). ROS, reactive oxygen species. Created with a licensed version of BioRender.com.
As the supply of O2 to the placenta increases in the subsequent trimester, the transition from glycolysis to oxidative phosphorylation becomes dominant for placental development. Mitochondrial activity and oxidative phosphorylation then become the primary mechanisms for energy production (65, 66). Failure in this transition can affect decidualization and contribute to the development of PE (51, 52, 61, 67). This metabolic reprogramming is necessary to fulfill the escalating energy requirements for placental growth and fetal development in the later stages of pregnancy. Alterations in cellular energy metabolism impact signal transduction, mitochondrial function, and transcriptional regulations (68, 69). Any disruptions in these processes can lead to placental dysfunction and, if not compensated for by physiological mechanisms, contribute to the development of PE. The inability of the placenta to alter its metabolism to the changing environment may underlie abnormal placental development and dysfunction.
In PE, the establishment of the uteroplacental circulation at the beginning of the second trimester is deficient due to defective remodeling of the maternal spiral arteries and shallow placentation. This causes a hypoxic state, leading to the overexpression of HIF-1α (67). HIF-1α plays a major role in altering mitochondrial bioenergetics by regulating cellular energy metabolism, increasing glycolysis, and suppressing oxidative metabolism to continue producing ATP under hypoxic conditions (23, 70). Demonstrating the reliance on the Warburg effect, a study by Vangrieken et al. (4) observed preeclamptic placentas just after delivery and discovered a significant reduction in mitochondrial content alongside an increase in key glycolytic enzymes.
Studies have shown that during stages of moderate hypoxia, defined as intervillous O2 levels below 5% (normal intervillous O2 levels are 6–8%) in midpregnancy, the increase in HIF-1α leads to additional production of substrates for glycolytic metabolism due to an excessive Warburg effect, increasing glucose consumption (4, 67, 71, 72). This adaptation primarily aims to spare O2 for vital fetal organs, such as the heart and brain (73). This excess placental glucose consumption maintains syncytial ATP levels and energy-intensive processes such as protein synthesis and placental growth but leads to decreased transfer of glucose to the fetus, causing a drop in fetal blood glucose levels and impaired fetal development (61, 67, 73). In cases of significant hypoxia, there are no rescue methods, and there is a significant decrease in the supply of nutrients and O2 to the fetus, leading to decreased syncytial ATP levels and inhibition of protein synthesis, eventually resulting in fetal growth restriction (67). This phenomenon is particularly relevant in the context of PE, where the establishment of the uteroplacental circulation is deficient due to a hypoxic state (12, 74).
HYPOXIA IN PHYSIOLOGICAL PLACENTATION AND PREECLAMPSIA
The term “hypoxia” refers to insufficient O2 supply to tissues and cells that do not maintain adequate homeostasis (75). Placental hypoxia is often linked to inadequate vascular remodeling of this organ during pregnancy (76). However, hypoxia plays a substantial physiological role in the normal development of the fetus, contributing to various embryonic processes such as placentation, angiogenesis, and hematopoiesis (77, 78). Around 11–14 wk of gestation, the extravillous trophoblasts (EVTs) invade the myometrium and obstruct spiral arteries forming so-called “trophoblastic arterial plugs” (79, 80). This creates a hypoxic environment that is crucial for the development of uteroplacental and protects the fetus from excessive O2 supply (78, 81). As pregnancy progresses, maternal spiral arteries dilate to meet the increasing blood demands of the fetoplacental structure (15, 82, 83). This structural alteration is influenced by EVTs, which contribute to disrupting the muscular layer of uterine spiral arteries and assume an endothelial cell phenotype, effectively replacing the endothelial layer of these vessels (79, 84). However, when EVTs fail to transition from a proliferative to an invasive phenotype through an epithelial-mesenchymal transition, this failure becomes the primary cause of incomplete remodeling of spiral arteries (79). Consequently, it leads to the narrowing of uterine vessels, compromising placental blood flow (85). In addition, spiral arteries affected by the failure of physiological transformation are more likely to develop atherosis, a process equivalent to the formation of an atherosclerotic lesion (85). Atherosis narrows the vessel lumen by subendothelial foam cells, fibrinoid necrosis, and perivascular lymphocytic infiltration, further compromising placental perfusion (17, 86). Thus, abnormalities in EVTs lead to shallow placentation and inadequate remodeling of spiral arteries, triggering subsequent hypoxia/ischemia of this organ in the early or first stage of PE (Fig. 2) (87–89).
Figure 2.
Diagram illustrating the contrasting spiral artery remodeling during placental implantation in physiological pregnancy vs. preeclamptic pregnancy. During pregnancy, the demand for placental development is addressed through the transformation of maternal myometrial spiral arteries into vessels with high capacitance and low resistance. This process starts in early first trimester and continues until 20–22 wk. However, in cases of preeclampsia, inadequate remodeling occurs because of impaired migration of extravillous trophoblasts (EVTs) and reduced replacement of maternal endothelial cells. This leads to compromised placental blood flow, resulting in hypoxia. Created with a licensed version of BioRender.com.
At the end of the first trimester, the trophoblast arterial plug dissolves, allowing maternal arteries to fully enter the intervillous space (90). It is at this point that O2 levels rise to a considered physiological state (90). HIFs govern responses to hypoxia and are integral to both physiological and pathophysiological processes (91). HIFs, comprising HIF-α subunit (HIF-1α or HIF-2α) and HIF-1β, have their α-subunit regulated by O2 levels (91). HIF-1α levels are rapidly reduced under normal O2 conditions but remain elevated in hypoxic environments (92, 93). In the context of pregnancy, HIF-1α is translocated to the nucleus, activating the expression of target genes that modulate cellular effects such as angiogenesis, cell migration/invasion, and immune cell function in response to low O2 tension (94). In pregnancies complicated by PE, there is typically abnormally high expression of HIF-1α, and the development of EVTs is interrupted at an immature stage. This interruption leads to impaired placentation and placental hypoxia/ischemia, which are key triggers in the pathophysiology of PE (93, 95, 96). Syncytiotrophoblasts from hypoxic and structurally damaged placenta undergo stress, releasing injurious molecules that damage the maternal systemic vasculature (97, 98). This process triggers an inflammatory response, resulting in clinical symptoms. This phase is commonly referred to as the late or second stage of PE (99, 100).
CONTRIBUTIONS OF OXIDATIVE STRESS TO PREECLAMPSIA
A normal pregnancy is inherently in a state of oxidative stress due to increased maternal metabolism and the subsequent metabolic activity of the placenta (101). Oxidative stress also plays a critical role in the pathophysiology of PE, as the ischemic placenta is known to induce the production of reactive oxygen species (ROS) (102). ROS are highly unstable reactive molecules crucial for intracellular signaling, host defense, tissue adaptation, and activation of mitogenic pathways (15). The main sources of ROS in PE are attributed to the mitochondrial electron transport chain (ETC), NO synthases, NADPH oxidases, and xanthine oxidase (103). Some of the most common ROS are superoxide (O2•−), hydroxyl radical (OH•), hydrogen peroxide (H2O2), dichlorofluorescein (DCF), and nitric oxide metabolites (103, 104). As addressed earlier in this review, the placenta develops in varied O2 environments. In the embryonic stage of the first trimester, the intervillous space is maintained in a low O2 state to protect the embryo from radical-induced injury (105). Then, from ∼10 gestational wk, there is a sudden rise in O2 tension in the maternal-fetal circulation, thought to facilitate the transition of EVTs from a proliferative epithelial to an invasive phenotype (106). This transition from low O2 to high O2 levels at the beginning of the second trimester marks the onset of a gradual shift from the Warburg pathway toward a more oxidative metabolism, resulting in the generation of ROS and exposing the placenta to oxidative stress (107, 108). Ischemia-reperfusion (I/R) is characterized by the counterintuitive aggravation of cellular dysfunction and death upon the reintroduction of blood flow to tissues previously subjected to ischemia (109, 110). The restoration of blood flow rescues ischemic tissues, yet paradoxically, reperfusion initiates additional damage, posing a threat to the function and viability of the organ (109, 110). In this regard, impaired placental perfusion leads to repeated events of I/R, which in turn triggers oxidative stress (111). The overproduction of ROS can cause oxidative damage to macromolecules such as lipids, proteins, and nucleic acids and even elicit apoptotic cell death (106). In normal pregnancy, placental mitochondria maintain cellular redox homeostasis with the help of antioxidant enzymes produced in response to a changing O2 landscape (112, 113). However, in PE, these compensatory redox mechanisms become overwhelmed, leading to placental cells experiencing chronic stress and undergoing senescence, which contributes to placental insufficiency (17, 112).
Mitochondrial-Mediated Oxidative Stress in Preeclampsia
Mitochondria serve as the cell’s powerhouse, generating ∼95% of the cell’s required ATP molecules through oxidative phosphorylation (OXPHOS)/ETC. (114). Studies have shown that in PE, the activity of complex IV (cytochrome-c oxidase) and the expression of essential proteins involved in the ETC are reduced, leading to lower oxidative phosphorylation and mitochondrial respiration (18, 115). Instead, there is an enhanced formation of mitochondrial reactive oxygen species (mtROS) at various sites in the respiratory chain, especially in complex III, leading to oxidative stress (Fig. 3) (116). HIFs reduce mitochondrial activity by modifying the enzymes of the tricarboxylic acid cycle and the ETC (106). Simultaneously, intracellular stress, such as oxidative damage, can activate the mitochondria-mediated caspase-9 apoptotic pathway (106). This activation induces the proapoptotic proteins Bax and Bak to inactivate antiapoptotic Bcl-2 and Bcl-xL proteins, resulting in the opening of mitochondrial membrane permeability pores and the release of cytochrome-c (106). This, in turn, activates caspases, leading to programmed cell death (Fig. 2) (106). In addition, these factors collectively contribute to mitochondrial dysfunction, characterized by the loss and reduction of mitochondrial content, cellular respiration, and efficiency of the oxidative phosphorylation (116). This results in a state where mitochondria cannot meet the increasing placental demands for ATP, ultimately leading to cell death (116).
Figure 3.

Schematic representation of mitochondrial dysfunction in preeclampsia and its impact on vital organs. Defective remodeling of the spiral arteries reduces oxygen and nutrient delivery to the placenta, resulting in hypoxia and ischemia in placental cells. Mitochondrial function, particularly the electron transport chain, is highly sensitive to hypoxia, leading to dysfunction and reactive oxygen species (ROS) generation rather than ATP synthesis. ROS exacerbates cell apoptosis by increasing the release of proapoptotic factors and causes placental damage, prompting the release of damaged placental components and inflammatory mediator cytokines into the maternal circulation. In addition, ROS stabilizes hypoxia-inducible factor 1α (HIF1-α), triggering the secretion of various antiangiogenic factors such as soluble fms-like tyrosine kinase-1 (sFLT-1) and soluble endoglin (sENG), which target the maternal endothelium, ultimately affecting vital systemic organs. In the renal system, endothelial damage and podocyte loss result in proteinuria. Increased vascular resistance places strain on the heart, leading to cardiomyopathy. ROS-induced lipid oxidation compromises blood-brain barrier integrity and increases coagulation, contributing to severe outcomes such as stroke, seizures, and cerebral edema. ADP, adenosine diphosphate; ATP, adenosine triphosphate; AT1-AA, angiotensin II type 1 receptor agonistic autoantibody; BBB, blood-brain barrier; CoQ, coenzyme Q; Cyt-c, cytochrome-c; DAMPs, damage-associated molecular patterns; FAD, oxidized flavin adenine dinucleotide; FADH2, flavin adenine dinucleotide; LDL, low-density lipoproteins; miRNA, microRNA; mtDNA, mitochondrial DNA; NADH, nicotinamide adenine dinucleotide; NAD+, oxidized nicotinamide adenine dinucleotide. Created with a licensed version of BioRender.com.
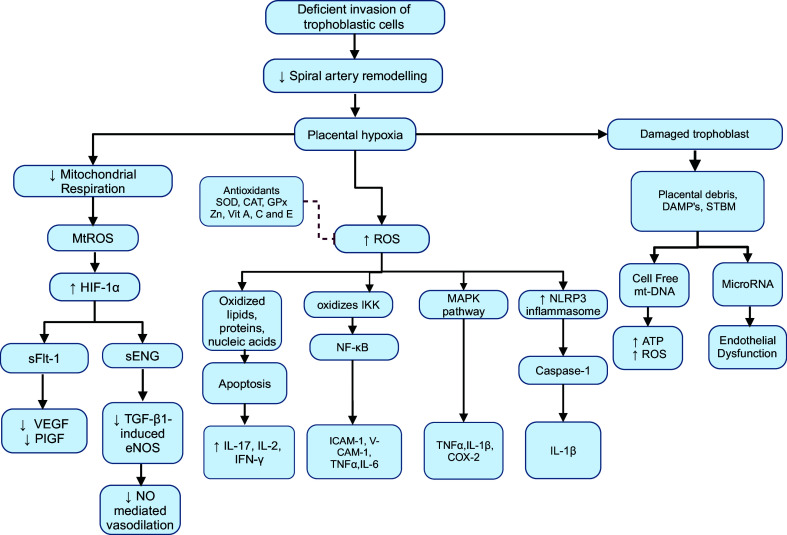
In the context of PE, the syncytiotrophoblasts are damaged because of the dysfunctional mitochondrial state. They are proposed to respond to structural damage and oxidative stress by upregulating and releasing placental damage-associated molecular patterns (DAMPs), apoptotic debris, syncytiotrophoblast microvesicles (STBM), circulating cell-free mitochondrial DNA (mtDNA), proinflammatory cytokines, microRNAs (miRNAs), and antiangiogenic factors into the maternal circulation (Fig. 3) (117–120). Cell-free mtDNA and fetal DNA are metabolically active by themselves and can induce malfunction of ETC resulting in decreased ATP release and overproduction of ROS, imposing direct injury to the maternal systemic endothelium (121). STBM, on the other hand, transfer messenger RNA (mRNA) and miRNA to the maternal endothelium (25). miRNAs are involved in multiple functions, including the regulation of the mitochondrial function (e.g., miRNA-210), responses to ischemia/hypoxia, and promotion of adipogenesis by targeting adipogenic regulators (122). This leads to triglyceride accumulation and insulin resistance, ultimately resulting in endothelial dysfunction when miRNA regulation is disrupted (25, 122). Moreover, HIF-1α directly regulates hypoxia-responsive miRNA-210 and switches mitochondrial metabolism to glycolysis, thereby increasing the release of ROS (120). Concurrently, ROS triggers increased stabilization of trophoblast-specific HIF-1α and HIF-2α (106). HIF-1α subsequently binds to hypoxia-responsive elements, activating the transcription of multiple genes related to angiogenesis and metastasis (120). This regulation of transcription factors leads to the release of various antiangiogenic factors, including sFLT-1 and sEng along with proinflammatory cytokines such as interleukin-17 (IL-17), interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), inhibin A, and oxidants (Fig. 4) (18, 123). These circulating factors target the vascular endothelium, smooth muscle cells, and extracellular matrix (124). This cascade results in maternal vascular endothelial dysfunction, excessive vasoconstriction, and inflammation of end-organs and is suggested to be key pathogenic mechanisms responsible for the maternal clinical symptoms (Fig. 3) (18, 103, 106, 124). Table 1 presents key cardiovascular signaling molecules that are altered during the development of PE.
Figure 4.
Diagram illustrating the progression from inadequate placentation to the release of biomarkers and inflammatory mediators in preeclampsia. Insufficient trophoblastic invasion in preeclampsia leads to defective spiral artery remodeling, resulting in hypoxia. Consequently, mitochondrial respiration is depressed, leading to excessive reactive oxygen species (ROS) production. These ROS activate various proinflammatory signaling pathways, including nuclear factor-κB (NF-κB), mitogen-activated protein kinase (MAPK), and NOD-like receptor protein 3 (NLRP3) pathways, culminating in the release of multiple proinflammatory cytokines. ROS oxidize IκB kinase (IKK), leading to the release of NF-κB, which results in the transcription of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), interleukin (IL)-6, and tumor necrosis factor-α (TNF-α). Similarly, activation of the MAPK pathway results in TNF-α, IL-6, and cyclooxygenase-2 (COX-2), whereas activation of the NLRP3 inflammasome activates caspase-1 and releases IL-1β. Concurrently, soluble fms-like tyrosine kinase-1 (sFLT-1) binds to vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) receptors, impeding their angiogenic response. Soluble endoglin (sEng) diminishes transforming growth factor-β1 (TGF-β1) binding to its angiogenic receptor, inhibiting endothelial nitric oxide (NO) synthase (eNOS)-mediated NO synthesis and subsequently reducing vasodilation. Placental hypoxia also damages trophoblastic tissue, leading to the release of placental debris, including cell-free mt-DNA and microRNA, further exacerbating oxidative stress and causing endothelial dysfunction. HIF-α, hypoxia-inducible factor-α; IFN-γ, interferon-γ. Created with a licensed version of BioRender.com.
Table 1.
Variable biomarkers in PE
| Biomarker | Levels in PE | Measurement Source | Biological Functions | References | |
|---|---|---|---|---|---|
| 1 | sFlt-1 | Increased | Serum; toward end of pregnancy | sFLT-1 binds to free VEGF and PlGF in the maternal circulation, thus reducing their bioavailability for their membrane receptor. | (125, 126) |
| 2 | HIF-1α | Increased | Serum—1st and 2nd trimester | Transcription factor that controls responses to hypoxia, mediating angiogenesis, cell migration, and immune function; also, stimulates sFLT-1 production in trophoblast. | (127, 128) |
| 3 | O2•−, OH• and H2O2 | Increased | Serum—early pregnancy | Causes oxidative damage. | (104) |
| 5 | DCF, reactive nitric oxide metabolites ( and ) | Increased | Serum—early pregnancy | Found in placental mitochondria; this suggests that the primary source of ROS contributing to oxidative stress is mitochondria. | (129, 130) |
| 7 | AT1-AA | Increased | Serum—early pregnancy until nearly 18 mo following postpartum | Bind and activate AT1 receptors to increase angiotensin II sensitivity and increase concentration of endothelin-1, sFLT-1, and ROS, which affect renal function. | (131–135) |
| 8 | sEng | Increased | Serum—from 20–23 wk to 27–30 wk of gestation, and then rapidly increases at 36–38 wk | An antiangiogenic protein that binds to TGF-β1, preventing its interaction with its natural angiogenic receptor, thereby inhibiting TGF-β1-induced eNOS activation and vasodilation (NO-mediated vasodilation). | (3, 136–138) |
| 9 | Inhibin A | Increased | Serum—from 4th wk of gestation | Associated with hypoxia and shows significant elevation by 10 wk of pregnancy. It downregulates activin-A signaling, which enhances extravillous trophoblast migration. This characteristic could potentially aid in the early diagnosis of preeclampsia. | (139–141) |
| 11 | Activin A | Increased | Serum—late 2nd and 3rd trimester | Glycoprotein growth factor involved in cellular differentiation and proliferation. Its levels correlate with cardiac global longitudinal strain. | (139) |
| 11 | VEGF, PlGF | Decreased | Serum—1st trimester | Regulates endothelial cell proliferation, angiogenesis, and vascular permeability. Low-circulating PlGF as a biomarker for poor placental function and a mediator of the angiogenic switch. | (142, 143) |
| 12 | Homocysteine | Increased | Serum—16th wk of gestation to delivery | Elevated levels of homocysteine are observed because of the loss of NO-mediated vasodilation, which is characteristic of oxidative inactivation of the NO synthase cofactor. | (105, 144, 145) |
| 14 | TNF-α, IL-6, IL-17, IL-2, IFN-γ, ICAM-1, VCAM-1, TLR-9 | Increased | Serum—early to midpregnancy | Proinflammatory cytokines. | (146, 147) |
| 15 | SOD, CAT, GPx, selenium, Co-Q10 | Decreased | Serum | Enzymatic antioxidants that promote the conversion of ROS into harmless products. | (23, 113, 148–150) |
| 16 | Thiols in plasma, α-tocopherol, Zinc and Lycopene, Vitamin A, C and E, fatty acids Docosahexaenoic acid | Decreased | Serum | Nonenzymatic antioxidants. | (113, 149, 151) |
| 17 | BNP, ANP, cTnI | Increased | Serum—after 20 wk till 3–6 wk postpartum | Indicators of myocardial strain that are released with the stretching of ventricles. | (13, 152–158) |
AT1-AA, angiotensin II type 1 receptor agonistic autoantibody; ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; CAT, catalase; CLPP, caseinolytic peptidase P; Co-Q10, coenzyme Q10; cTnI, cardiac troponin I; DCF, dichlorofluorescein; DHA, docosahexaenoic acid; eNOS, endothelial nitric oxide synthase; GPx, glutathione peroxidase; HIF-α, hypoxia-inducible factor α; H2O2, hydrogen peroxide; ICAM-1, intercellular adhesion molecule-1; IFN-γ, interferon-γ; IL-2, interleukin-2; IL-6, interleukin-6; IL-17, interleukin-17; LDL, low-density lipoproteins; LDL-C, low-density lipoprotein-cholesterol; mtROS, mitochondrial ROS; NK, natural killer cells; NO, nitric oxide; O2•−, superoxide; OH•, hydroxyl radicals; PAPP-A, pregnancy-associated plasma protein-A; PE, preeclampsia; PlGF, placental growth factor; ROS, reactive oxygen species; sEng, soluble endoglin; sFLT-1, soluble fms-like tyrosine kinase-1; SOD, superoxide dismutase; TC, total cholesterol; TGs, triglycerides; TGF-β1, transforming growth factor β1; TLR-9, Toll-like receptors; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor.
IMPACT OF OXIDATIVE STRESS ON IMMUNOLOGICAL FUNCTION
Emerging evidence suggests that oxidative stress-induced immunological imbalance plays a role in the development and progression of preeclampsia (159). In particular, increased oxidative stress disrupts the equilibrium of proinflammatory and anti-inflammatory mediators, leading to aberrant immune responses and endothelial dysfunction characteristic of PE (111). Accordingly, mitochondrial-mediated oxidative stress not only contributes to the overall oxidative burden but also acts as a trigger for an immunological imbalance (159). The disrupted balance in immune responses creates a cascade of events that further amplifies the complexities of PE (160, 161).
In PE, ROS further stimulates the transcription of various proinflammatory signaling pathways mainly nuclear factor-κB (NF-κB) (162) and results in the synthesis of proinflammatory cytokines causing cellular damage (Table 1) (163, 164). The proinflammatory state can disrupt the equilibrium necessary for a healthy pregnancy, potentially culminating in complications like impaired placental development. Furthermore, the immunological imbalance manifests in functional repercussions such as endothelial dysfunction, altered vascular tone, and heightened susceptibility to cardiovascular events.
ENDOTHELIAL DYSFUNCTION IN PREECLAMPSIA: IMPLICATIONS FOR MATERNAL CLINICAL OUTCOMES
The likelihood of developing coronary heart disease and other cardiovascular events is elevated in individuals with PE (86, 165). Many cardiac and vascular deleterious factors like agonistic autoantibodies to the angiotensin II type 1 receptor (AT1-AA), sFLT-1, and inflammatory cytokines are increased in PE in response to placental hypoxia (166). AT1-AA mainly acts by increasing intracellular calcium levels, causing a chronotropic response in the heart, and also increasing mean arterial pressure (131). In addition, they induce hypertension by binding to and activating the angiotensin II type 1 receptor, which in turn causes the upregulation of vasoactive factors such as endothelin-1, as well as ROS, sEng, and sFLT-1 (Table 1) (166). These factors impair endothelial cell function, leading to reduced production of endothelial-derived relaxing factors such as nitric oxide (NO) and prostacyclin. This results in vasoconstriction and subsequent hypertension (167). This also leads to circulatory dysfunction in the kidneys, decreasing renal blood flow and glomerular filtration rate, which ultimately contributes to hypertension and proteinuria (132, 167). The cardiovascular modifications induced by sFLT-1 in pregnancies complicated by PE are permanent and can potentially establish the basis for future cardiovascular risk (22, 168, 169). Circulating levels of sFLT-1 in plasma correlate with the severity of PE (22). Excess of sFLT-1 binds to placental growth factor (PlGF) and vascular endothelial growth factor (VEGF), preventing their interaction with receptors located on vascular endothelial cells (15). This interference leads to maternal endothelial dysfunction, contributing to abnormal vascular tone and hypertension (Fig. 3) (170). Activin A and inhibin A, members of the TGF-β superfamily, are expressed by the syncytiotrophoblast, cytotrophoblast, and decidual cells. They stimulate trophoblast invasiveness (171, 172). In PE, their levels are dysregulated in the second and third trimesters, and this is considered to be a possible pathogenic mechanism for PE by inducing vascular endothelial oxidative stress (139). There are studies showing the direct relationship between increased activin A and abnormal left ventricular longitudinal strain and the future risk of postpartum cardiac dysfunction in PE cases (173, 174). Also, inhibin A is elevated in all three trimesters of PE pregnancy and disrupts the adaptation of maternal blood vessels and the cardiovascular system in pregnancy (139). Another factor that can disrupt the cardiovascular system is homocysteine (Hcy), which is formed by the demethylation of methionine (175). Hyperhomocysteinemia has been observed in PE (144). Hcy causes oxidative damage to the endothelium through ROS-mediated vascular injury and the oxidation of low-density lipoprotein (LDL) by downregulating the antioxidant enzyme glutathione peroxidase (175, 176).
Among the two subsets of PE, the risk of developing cardiovascular disease later in life is higher in early-onset PE, with an almost seven- to eightfold increase, whereas in late-onset PE, there is only a twofold increase in risk (177). In the clinical setting, when studying arterial stiffness in cardiovascular disease, diagnostic tools such as the augmentation index (AIx) and pulse wave velocity (PWV) are used (178). Researchers have observed that during the postpartum period the AIx and PWV are significantly higher among the early onset preeclamptic group, indicating changes that may lead to cerebrovascular disease (179). Ospina-Tascón et al. (180) demonstrated a significant decrease in the intermittent flow of the small vessels and a reduction in capillary density. These findings highlight the role of microcirculatory alteration in the development of organ dysfunction in preeclamptic subjects. Also, the severe inflammatory state due to cytokine actions generates endothelial derangements that result in damage to microcirculatory blood flow (124). Microcirculatory dysfunction is further evidenced by a decrease in arteriolar and venous calibers in the retinal vessels of patients with preeclamsia (180, 181). Under the influence of oxidative stress and inflammatory stimuli, the endothelium loses its protective glycocalyx that regulates leukocyte and platelet adherence, control of vascular permeability, and protection against the shear effect of blood flow (105, 182). Simultaneously, the endothelial synthesis of NO and prostaglandins, which are vasodilators, and antiplatelet factor is impaired (183). This alteration also leads to the adhesion of platelets, leukocytes, and fibrin to the endothelial surface, promoting thrombosis and vascular inflammation (105). The endothelium acts in a paracrine fashion, stimulating the proliferation and migration of underlying smooth muscle cells that are responsible for the contractile property of the vessels (120). Furthermore, there are noted changes in matrix metalloproteinases and the extracellular matrix, contributing to pathological systemic vascular remodeling and increased arterial stiffening (22, 124). As a result, there is an increase in vascular resistance, which results in hypertension (184). Excessive vascular remodeling is a predisposing feature of atherosclerosis (185). In fact, some studies have reported that PE is an independent risk factor for the development of early coronary atherosclerosis (21, 184, 186). The vasoconstrictive, coagulating, prooxidant, and proinflammatory features of PE are significantly correlated with the progression of coronary artery calcification and atherosclerosis, increasing the risk of ischemic heart disease (186, 187). Consequently, women with preeclampsia have an increased risk of developing ischemic heart disease, mainly after menopause (186). Endothelial damage in PE also triggers the activation of the coagulation cascade and thrombophilia, increasing the susceptibility of the development of venous thromboembolism (188). This includes conditions such as deep vein thrombosis and pulmonary embolism, particularly during pregnancy and the postpartum period (189).
Women with PE may have adverse left ventricular (LV) remodeling, resulting in cardiac dysfunction (190). They face an elevated risk of developing cardiomyopathy not only during the peripartum period but also in the years following a preeclamptic pregnancy (191). An echocardiographic study has identified LV and right ventricle dilation, mitral and/or tricuspid regurgitation, and atrial enlargement in women with preeclampsia (192). Another study showed thicker septal and posterior LV walls (187). Genomic investigations revealed upregulation of genes involved in cardiac fibrosis, inflammation, and myocardial remodeling, leading to heart hypertrophy and increased mass in women with preeclampsia (193–195). Overall, these alterations result from increased vascular resistance, inducing myocardial strain on the ventricular walls and leading to concentric heart hypertrophy and diastolic dysfunction (Fig. 3). This is followed by the release of markers of cardiac stress such as brain natriuretic peptide (BNP) (13, 152), atrial natriuretic peptide (ANP) (153, 173), and cardiac troponin-I (cTnI) (Table 1) (153). Circulating sFLT-1 levels significantly correlate with the severity of PE, including increased thickness of the carotid intima layer, myocardial performance index, global longitudinal strain, and serum levels of ANP, BNP, and cTnI in women diagnosed with PE (13, 22, 196–198). Persistent structural alterations in the heart may lead to serious clinical emergencies such as myocardial ischemia, heart failure, and cardiogenic shock (199, 200). Furthermore, cardiac functional alterations, such as impaired myocardial contractility resulting from structural changes lead to both LV systolic and diastolic heart failure (192).
Neurological complications associated with PE include seizures (eclampsia), ischemic stroke, cerebral venous sinus thrombosis, subarachnoid hemorrhage (SAH), intracerebral hemorrhage (ICH), reversible cerebral vasoconstriction syndrome (RCVS), and posterior reversible encephalopathy syndrome (PRES) (201). Research indicates that PE is associated with an increased risk of stroke due to factors such as poor collateral blood flow, elevated peripheral vascular resistance, hypercoagulation, cerebral vasomotor reactivity, and heightened vascular stiffness (202, 203). Furthermore, in PE, there is an association with heightened levels of circulating oxidized LDL and cerebrovascular complications (204). This phenomenon results in an increased uptake of oxidized LDL by macrophages, triggering their transformation into foam cells, and consequently leading to atherogenesis (204, 205). Oxidized LDL also enhances the permeability of the blood-brain barrier (BBB) and can potentially exacerbate I/R injury, resulting in conditions such as edema and hemorrhage (202). Moreover, the combination of hypertension and endothelial activation, often associated with inflammation, oxidative stress, and vascular dysfunction, can lead to reversible ischemic encephalopathy in the posterior hemispheres resulting in PRES (206, 207). PRES is a syndrome of vasogenic edema and BBB damage (201). It manifests as headache, scotomata, and scintillation and contributes to the occurrence of seizures observed in eclampsia (Fig. 3) (14, 206, 207). RCVS is associated with PE and eclampsia and presents as an acute severe headache due to subarachnoid hemorrhage and also as ischemic stroke due to sudden vasospasm (201, 208). Furthermore, RCVS is believed to be associated with endothelial dysfunction and BBB breakdown (208, 209).
Renal impairment in PE is primarily manifested as proteinuria, resulting from endothelial damage in renal glomeruli (Fig. 3). This damage presents in the form of glomerular endotheliosis, increased glomerular permeability, and the associated loss of podocytes (14, 124). Circulating sFLT-1 levels bind to VEGF produced by podocytes and hinder the fenestration of glomerular endothelial cells (210). In addition, sFLT-1 interferes with the endothelial signaling of VEGF in the kidneys (210). This can even give rise to severe complications like acute kidney injury, acute tubular necrosis, and nephrotic range proteinuria in patients with preeclampsia (14, 140, 211). Damage caused by the loss of urinary podocytes, as well as damage to the endothelium, can persist in the postpartum period, potentially indicating a future link to kidney disease (211). Pregnancies complicated by PE are at a higher risk of developing a reduced estimated glomerular filtration rate (211), albuminuria (211), chronic kidney disease (200, 201), and end-stage kidney disease (201, 202), compared with those with normotensive pregnancies (212).
FUTURE RESEARCH PRIORITIES AND OPPORTUNITIES
Future research should prioritize both the prevention and treatment of PE, as there is currently no universally accepted treatment for this condition. Despite ample evidence that preeclamptic pregnancy increases the risk of adverse vascular remodeling response to injury later in life, there remains a lack of biomarkers for early definitive diagnosis. Identifying women with PE as high risk for cardiovascular disease allows for clinical studies to intervene in the disease progression at an earlier stage. Implementing routine postpartum screening to detect early cardiac and vascular changes may have a positive impact on preventing the development of cardiovascular issues.
There is a significant need for drugs to prevent PE in high-risk groups. These drugs can be modeled to affect early spiral artery remodeling directed toward the vascular endothelium. Further understanding mitochondrial dysfunction and oxidative stress, which are significant contributors to the pathogenesis of PE, and designing drugs to modulate these factors is a promising strategy.
In addition, investigating how angiogenic factors continue to cause endothelial dysfunction even after the delivery of the placenta could advance the prevention of cardiovascular diseases. Such research could lead to novel therapeutic approaches that improve long-term cardiovascular outcomes for women who have experienced PE.
CONCLUSIONS
This review explores how metabolic alterations contribute to oxidative stress and inflammation, ultimately impacting maternal vascular endothelium and leading to multisystemic complications associated with PE. Understanding metabolic alterations can provide insights into the distinct maternal cardiovascular impacts of PE. Changes in placental development during the first trimester trigger oxidative stress, inflammation, and endothelial dysfunction, leading to the onset of cardiovascular complications associated with PE. Cardiovascular disease associated with PE manifests earlier and more severely. As discussed in this review, impaired placental perfusion is a key factor in the development of an ischemic placenta, and likely promotes progression of the cardiovascular disease. New therapeutic strategies, such as effective antioxidant, anti-inflammatory, and novel vasodilator treatments, could have impact on mitigating the long-term cardiovascular consequences of PE. To effectively treat the metabolic disorder, new diagnostic tools are also necessary to detect the disease earlier in gestation. Placenta-targeted therapies for patients with PE may have great impact in reducing adverse events in mothers, while minimizing toxic effects on the fetuses. In summary, understanding PE as a systemic disease of metabolic function, driven by the placenta, could lead to future improvements in treatment of the disease.
GRANTS
This work was partially supported by National Institute of Child Health and Human Development Grant R01HD097466 (to C.L.B.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.M.M. and A.K.N.A. prepared figures; M.M.M., G.C.M., M.A., G.P., and A.K.N.A. drafted manuscript; M.M.M., G.C.M., M.A., K.F.S., G.P., A.K.N.A., and C.L.B. edited and revised manuscript; M.M.M., G.C.M., M.A., K.F.S., G.P., A.K.N.A., and C.L.B. approved final version of manuscript.
REFERENCES
- 1. Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res 124: 1094–1112, 2019. [Erratum in Circ Res 126: e8, 2020]. doi: 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- 2. Staff AC. The two-stage placental model of preeclampsia: an update. J Reprod Immunol 134–135: 1–10, 2019. doi: 10.1016/j.jri.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 3. Zhang L, Li X, Zhou C, You Z, Zhang J, Cao G. The diagnosis values of serum STAT4 and sEng in preeclampsia. J Clin Lab Anal 34: e23073, 2020. doi: 10.1002/jcla.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vangrieken P, Al-Nasiry S, Bast A, Leermakers PA, Tulen CBM, Schiffers PMH, van Schooten FJ, Remels AHV. Placental mitochondrial abnormalities in preeclampsia. Reprod Sci 28: 2186–2199, 2021. doi: 10.1007/s43032-021-00464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma Y, Ye Y, Zhang J, Ruan CC, Gao PJ. Immune imbalance is associated with the development of preeclampsia. Medicine (Baltimore) 98: e15080, 2019. doi: 10.1097/MD.0000000000015080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bartsch E, Medcalf KE, Park AL, Ray JG; High Risk of Pre-eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 353: i1753, 2016. doi: 10.1136/bmj.i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shih T, Peneva D, Xu X, Sutton A, Triche E, Ehrenkranz R, Paidas M, Stevens W. The Rising burden of preeclampsia in the United States impacts both maternal and child health. Am J Perinatol 33: 329–338, 2015. doi: 10.1055/s-0035-1564881. [DOI] [PubMed] [Google Scholar]
- 8. Beckers KF, Sones JL. Maternal microbiome and the hypertensive disorder of pregnancy, preeclampsia. Am J Physiol Heart Circ Physiol 318: H1–H10, 2020. doi: 10.1152/ajpheart.00469.2019. [DOI] [PubMed] [Google Scholar]
- 9. Opichka MA, Rappelt MW, Gutterman DD, Grobe JL, McIntosh JJ. Vascular dysfunction in preeclampsia. Cells 10: 3055, 2021. doi: 10.3390/cells10113055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kraemer BF, Hennis I, Karge A, Kraemer AK, Dreyer TF, Kiechle M, Kuschel B, Bronger H. Platelet mitochondrial membrane depolarization reflects disease severity in patients with preeclampsia. Mol Med 28: 51, 2022. doi: 10.1186/s10020-022-00472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wójtowicz A, Zembala-Szczerba M, Babczyk D, Kołodziejczyk-Pietruszka M, Lewaczyńska O, Huras H. Early- and late-onset preeclampsia: a comprehensive cohort study of laboratory and clinical findings according to the new ISHHP criteria. Int J Hypertens 2019: 4108271, 2019. doi: 10.1155/2019/4108271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ 366: l2381, 2019. doi: 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 13. Kalafat E, Thilaganathan B. Cardiovascular origins of preeclampsia. Curr Opin Obstet Gynecol 29: 383–389, 2017. doi: 10.1097/GCO.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 14. Magee LA, Nicolaides KH, von Dadelszen P. Preeclampsia. N Engl J Med 386: 1817–1832, 2022. doi: 10.1056/NEJMra2109523. [DOI] [PubMed] [Google Scholar]
- 15. Chiarello DI, Abad C, Rojas D, Toledo F, Vázquez CM, Mate A, Sobrevia L, Marín R. Oxidative stress: normal pregnancy versus preeclampsia. Biochim Biophys Acta Mol Basis Dis 1866: 165354, 2020. doi: 10.1016/j.bbadis.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 16. Erez O, Romero R, Jung E, Chaemsaithong P, Bosco M, Suksai M, Gallo DM, Gotsch F. Preeclampsia and eclampsia: the conceptual evolution of a syndrome. Am J Obstet Gynecol 226: S786–S803, 2022. doi: 10.1016/j.ajog.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Redman CWG, Staff AC, Roberts JM. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol 226: S907–S927, 2022. doi: 10.1016/j.ajog.2020.09.047. [DOI] [PubMed] [Google Scholar]
- 18. Marín R, Chiarello DI, Abad C, Rojas D, Toledo F, Sobrevia L. Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim Biophys Acta Mol Basis Dis 1866: 165961, 2020. doi: 10.1016/j.bbadis.2020.165961. [DOI] [PubMed] [Google Scholar]
- 19. Jung E, Romero R, Yeo L, Gomez-Lopez N, Chaemsaithong P, Jaovisidha A, Gotsch F, Erez O. The etiology of preeclampsia. Am J Obstet Gynecol 226: S844–S866, 2022. doi: 10.1016/j.ajog.2021.11.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 10: e003497, 2017. doi: 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- 21. Hauge MG, Damm P, Kofoed KF, Ersbøll AS, Johansen M, Sigvardsen PE, Møller MB, Fuchs A, Kühl JT, Nordestgaard BG, Køber LV, Gustafsson F, Linde JJ. Early coronary atherosclerosis in women with previous preeclampsia. J Am Coll Cardiol 79: 2310–2321, 2022. doi: 10.1016/j.jacc.2022.03.381. [DOI] [PubMed] [Google Scholar]
- 22. Pruthi D, Khankin EV, Blanton RM, Aronovitz M, Burke SD, McCurley A, Karumanchi SA, Jaffe IZ. Exposure to experimental preeclampsia in mice enhances the vascular response to future injury. Hypertension 65: 863–870, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu X-Q, Zhang L. Mitochondrial dysfunction in the pathogenesis of preeclampsia. Curr Hypertens Rep 24: 157–172, 2022. doi: 10.1007/s11906-022-01184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yildirim RM, Ergun Y, Basar M. Mitochondrial dysfunction, mitophagy and their correlation with perinatal complications: preeclampsia and low birth weight. Biomedicines 10: 2539, 2022. doi: 10.3390/biomedicines10102539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol 226: S1019–S1034, 2022. doi: 10.1016/j.ajog.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melchiorre K, Giorgione V, Thilaganathan B. The placenta and preeclampsia: villain or victim? Am J Obstet Gynecol 226: S954–S962, 2022. doi: 10.1016/j.ajog.2020.10.024. [DOI] [PubMed] [Google Scholar]
- 27. Ward K, Taylor RN. Chapter 4—Genetic factors in the etiology of preeclampsia/eclampsia. In: Chesley’s Hypertensive Disorders in Pregnancy (4th ed.), edited by Taylor RN, Roberts JM, Cunningham FG, Lindheimer MD. San Diego, CA: Academic Press, 2015, p. 57–80. [Google Scholar]
- 28. Huppertz B. The critical role of abnormal trophoblast development in the etiology of preeclampsia. Curr Pharm Biotechnol 19: 771–780, 2018. doi: 10.2174/1389201019666180427110547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amaral LM, Wallace K, Owens M, LaMarca B. Pathophysiology and current clinical management of preeclampsia. Curr Hypertens Rep 19: 61, 2017. doi: 10.1007/s11906-017-0757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hecht JL, Zsengeller ZK, Spiel M, Karumanchi SA, Rosen S. Revisiting decidual vasculopathy. Placenta 42: 37–43, 2016. doi: 10.1016/j.placenta.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 31. Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW Jr, Wallace K, LaMarca B. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond) 130: 409–419, 2016. doi: 10.1042/CS20150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lamarca B. Endothelial dysfunction. An important mediator in the pathophysiology of hypertension during pre-eclampsia. Minerva Ginecol 64: 309–320, 2012. [PMC free article] [PubMed] [Google Scholar]
- 33. Tomimatsu T, Mimura K, Matsuzaki S, Endo M, Kumasawa K, Kimura T. Preeclampsia: maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int J Mol Sci 20: 4246, 2019. doi: 10.3390/ijms20174246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu M, Li J, Baker PN, Tong C. Revisiting preeclampsia: a metabolic disorder of the placenta. FEBS J 289: 336–354, 2022. doi: 10.1111/febs.15745. [DOI] [PubMed] [Google Scholar]
- 35. Schoots MH, Gordijn SJ, Scherjon SA, van Goor H, Hillebrands JL. Oxidative stress in placental pathology. Placenta 69: 153–161, 2018. doi: 10.1016/j.placenta.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 36. Skytte HN, Christensen JJ, Gunnes N, Holven KB, Lekva T, Henriksen T, Michelsen TM, Roland MCP. Metabolic profiling of pregnancies complicated by preeclampsia: a longitudinal study. Acta Obstet Gynecol Scand 102: 334–343, 2023. doi: 10.1111/aogs.14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feng Y, Lian X, Guo K, Zhang G, Huang X. A comprehensive analysis of metabolomics and transcriptomics to reveal major metabolic pathways and potential biomarkers of human preeclampsia placenta. Front Genet 13: 1010657, 2022. doi: 10.3389/fgene.2022.1010657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Youssef L, Crovetto F, Simoes RV, Miranda J, Paules C, Blasco M, Palomo M, Garcia-Caldero H, Tura-Ceide O, Dantas AP, Hernandez-Gea V, Herrero P, Canela N, Campistol JM, Garcia-Pagan JC, Diaz-Ricart M, Gratacos E, Crispi F. The interplay between pathophysiological pathways in early-onset severe preeclampsia unveiled by metabolomics. Life (Basel) 12: 86, 2022. doi: 10.3390/life12010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kelly RS, Croteau-Chonka DC, Dahlin A, Mirzakhani H, Wu AC, Wan ES, McGeachie MJ, Qiu W, Sordillo JE, Al-Garawi A, Gray KJ, McElrath TF, Carey VJ, Clish CB, Litonjua AA, Weiss ST, Lasky-Su JA. Integration of metabolomic and transcriptomic networks in pregnant women reveals biological pathways and predictive signatures associated with preeclampsia. Metabolomics 13: 7, 2017. doi: 10.1007/s11306-016-1149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stern C, Schwarz S, Moser G, Cvitic S, Jantscher-Krenn E, Gauster M, Hiden U. Placental endocrine activity: adaptation and disruption of maternal glucose metabolism in pregnancy and the influence of fetal sex. Int J Mol Sci 22: 12722, 2021. doi: 10.3390/ijms222312722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vaughan OR, Fowden AL. Placental metabolism: substrate requirements and the response to stress. Reprod Domest Anim 51, Suppl 2: 25–35, 2016. doi: 10.1111/rda.12797. [DOI] [PubMed] [Google Scholar]
- 42. Seyfried TN. Cancer as a mitochondrial metabolic disease. Front Cell Dev Biol 3: 43, 2015. doi: 10.3389/fcell.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39: 359–407, 2005. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol 8: 519–530, 1927. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karlstaedt A, Moslehi J, de Boer RA. Cardio-onco-metabolism: metabolic remodelling in cardiovascular disease and cancer. Nat Rev Cardiol 19: 414–425, 2022. doi: 10.1038/s41569-022-00698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Warburg OH , et al. Versuche an Überlebendem Carcinomgewebe. Klinische Wochenschrift 3: 1062–1064, 1923. [Google Scholar]
- 47. Colpman P, Dasgupta A, Archer SL. The role of mitochondrial dynamics and mitotic fission in regulating the cell cycle in cancer and pulmonary arterial hypertension: implications for dynamin-related protein 1 and mitofusin2 in hyperproliferative diseases. Cells 12: 1897, 2023. doi: 10.3390/cells12141897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu D, Dasgupta A, Read AD, Bentley RET, Motamed M, Chen KH, Al-Qazazi R, Mewburn JD, Dunham-Snary KJ, Alizadeh E, Tian L, Archer SL. Oxygen sensing, mitochondrial biology and experimental therapeutics for pulmonary hypertension and cancer. Free Radic Biol Med 170: 150–178, 2021. doi: 10.1016/j.freeradbiomed.2020.12.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dabral S, Muecke C, Valasarajan C, Schmoranzer M, Wietelmann A, Semenza GL, Meister M, Muley T, Seeger-Nukpezah T, Samakovlis C, Weissmann N, Grimminger F, Seeger W, Savai R, Pullamsetti SS. A RASSF1A-HIF1α loop drives Warburg effect in cancer and pulmonary hypertension. Nat Commun 10: 2130, 2019. doi: 10.1038/s41467-019-10044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. John AP. Dysfunctional mitochondria, not oxygen insufficiency, cause cancer cells to produce inordinate amounts of lactic acid: the impact of this on the treatment of cancer. Med Hypotheses 57: 429–431, 2001. doi: 10.1054/mehy.2001.1335. [DOI] [PubMed] [Google Scholar]
- 51. Burton GJ, Jauniaux E, Murray AJ. Oxygen and placental development; parallels and differences with tumour biology. Placenta 56: 14–18, 2017. doi: 10.1016/j.placenta.2017.01.130. [DOI] [PubMed] [Google Scholar]
- 52. Gou R, Zhang X. Glycolysis: a fork in the path of normal and pathological pregnancy. FASEB J 37: e23263, 2023. doi: 10.1096/fj.202301230R. [DOI] [PubMed] [Google Scholar]
- 53. Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update 13: 121–141, 2007. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- 54. Lala PK, Lee BP, Xu G, Chakraborty C. Human placental trophoblast as an in vitro model for tumor progression. Can J Physiol Pharmacol 80: 142–149, 2002. doi: 10.1139/y02-006. [DOI] [PubMed] [Google Scholar]
- 55. Armistead B, Johnson E, VanderKamp R, Kula-Eversole E, Kadam L, Drewlo S, Kohan-Ghadr HR. Placental regulation of energy homeostasis during human pregnancy. Endocrinology 161: bqaa076, 2020. doi: 10.1210/endocr/bqaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci 370: 20140066, 2015. doi: 10.1098/rstb.2014.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bax BE, Bloxam DL. Energy metabolism and glycolysis in human placental trophoblast cells during differentiation. Biochim Biophys Acta 1319: 283–292, 1997. doi: 10.1016/s0005-2728(96)00169-7. [DOI] [PubMed] [Google Scholar]
- 58. Burd LI, Jones MD Jr, Simmons MA, Makowski EL, Meschia G, Battaglia FC. Placental production and foetal utilisation of lactate and pyruvate. Nature 254: 710–711, 1975. doi: 10.1038/254710a0. [DOI] [PubMed] [Google Scholar]
- 59. Burton GJ, Jauniaux E. The human placenta: new perspectives on its formation and function during early pregnancy. Proc Biol Sci 290: 20230191, 2023. doi: 10.1098/rspb.2023.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 35: 851–905, 2014. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- 61. Zhu Y-Q, Yan X-Y, Li H, Zhang C. Insights into the pathogenesis of preeclampsia based on the features of placentation and tumorigenesis. Reprod Dev Med 5: 97–106, 2021. doi: 10.4103/2096-2924.320886. [DOI] [Google Scholar]
- 62. Bowman CE, Arany Z, Wolfgang MJ. Regulation of maternal-fetal metabolic communication. Cell Mol Life Sci 78: 1455–1486, 2021. doi: 10.1007/s00018-020-03674-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lebelo MT, Joubert AM, Visagie MH. Warburg effect and its role in tumourigenesis. Arch Pharm Res 42: 833–847, 2019. doi: 10.1007/s12272-019-01185-2. [DOI] [PubMed] [Google Scholar]
- 64. Fisher JJ, Bartho LA, Perkins AV, Holland OJ. Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy. Clin Exp Pharmacol Physiol 47: 176–184, 2020. doi: 10.1111/1440-1681.13172. [DOI] [PubMed] [Google Scholar]
- 65. Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol (Oxf) 57: 609–613, 2002. doi: 10.1046/j.1365-2265.2002.01638.x. [DOI] [PubMed] [Google Scholar]
- 66. Biondi C, Pavan B, Lunghi L, Fiorini S, Vesce F. The role and modulation of the oxidative balance in pregnancy. Curr Pharm Des 11: 2075–2089, 2005. doi: 10.2174/1381612054065747. [DOI] [PubMed] [Google Scholar]
- 67. Illsley NP, Caniggia I, Zamudio S. Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int J Dev Biol 54: 409–419, 2010. doi: 10.1387/ijdb.082798ni. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Picard M, Shirihai OS. Mitochondrial signal transduction. Cell Metab 34: 1620–1653, 2022. doi: 10.1016/j.cmet.2022.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aye I, Aiken CE, Charnock-Jones DS, Smith GCS. Placental energy metabolism in health and disease-significance of development and implications for preeclampsia. Am J Obstet Gynecol 226: S928–S944, 2022. doi: 10.1016/j.ajog.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 70. Martins Pinto M, Paumard P, Bouchez C, Ransac S, Duvezin-Caubet S, Mazat JP, Rigoulet M, Devin A. The Warburg effect and mitochondrial oxidative phosphorylation: friends or foes? Biochim Biophys Acta Bioenerg 1864: 148931, 2023. doi: 10.1016/j.bbabio.2022.148931. [DOI] [PubMed] [Google Scholar]
- 71. Sferruzzi-Perri AN, Higgins JS, Vaughan OR, Murray AJ, Fowden AL. Placental mitochondria adapt developmentally and in response to hypoxia to support fetal growth. Proc Natl Acad Sci USA 116: 1621–1626, 2019. doi: 10.1073/pnas.1816056116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hu X-Q, Zhang L. Hypoxia and the integrated stress response promote pulmonary hypertension and preeclampsia: implications in drug development. Drug Discov Today 26: 2754–2773, 2021. doi: 10.1016/j.drudis.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Higgins JS, Vaughan OR, Fernandez de Liger E, Fowden AL, Sferruzzi-Perri AN. Placental phenotype and resource allocation to fetal growth are modified by the timing and degree of hypoxia during mouse pregnancy. J Physiol 594: 1341–1356, 2016. doi: 10.1113/JP271057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta 30, Suppl A: S32–S37, 2009. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Della Rocca Y, Fonticoli L, Rajan TS, Trubiani O, Caputi S, Diomede F, Pizzicannella J, Marconi GD. Hypoxia: molecular pathophysiological mechanisms in human diseases. J Physiol Biochem 78: 739–752, 2022. doi: 10.1007/s13105-022-00912-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tal R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol Reprod 87: 134, 2012. doi: 10.1095/biolreprod.112.102723. [DOI] [PubMed] [Google Scholar]
- 77. van Patot MC, Ebensperger G, Gassmann M, Llanos AJ. The hypoxic placenta. High Alt Med Biol 13: 176–184, 2012. doi: 10.1089/ham.2012.1046. [DOI] [PubMed] [Google Scholar]
- 78. Fajersztajn L, Veras MM. Hypoxia: from placental development to fetal programming. Birth Defects Res 109: 1377–1385, 2017. doi: 10.1002/bdr2.1142. [DOI] [PubMed] [Google Scholar]
- 79. Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 69: 1–7, 2003. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 80. Barrientos G, Pussetto M, Rose M, Staff AC, Blois SM, Toblli JE. Defective trophoblast invasion underlies fetal growth restriction and preeclampsia-like symptoms in the stroke-prone spontaneously hypertensive rat. Mol Hum Reprod 23: 509–519, 2017. doi: 10.1093/molehr/gax024. [DOI] [PubMed] [Google Scholar]
- 81. Hu X, Zhang L. Uteroplacental circulation in normal pregnancy and preeclampsia: functional adaptation and maladaptation. Int J Mol Sci 22: 8622, 2021. doi: 10.3390/ijms22168622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huppertz B, Gauster M, Orendi K, Konig J, Moser G. Oxygen as modulator of trophoblast invasion. J Anat 215: 14–20, 2009. doi: 10.1111/j.1469-7580.2008.01036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pijnenborg R, Bland JM, Robertson WB, Dixon G, Brosens I. The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy. Placenta 2: 303–316, 1981. doi: 10.1016/s0143-4004(81)80027-6. [DOI] [PubMed] [Google Scholar]
- 84. Greenbaum S, Averbukh I, Soon E, Rizzuto G, Baranski A, Greenwald NF, Kagel A, Bosse M, Jaswa EG, Khair Z, Kwok S, Warshawsky S, Piyadasa H, Goldston M, Spence A, Miller G, Schwartz M, Graf W, Van Valen D, Winn VD, Hollmann T, Keren L, van de Rijn M, Angelo M. A spatially resolved timeline of the human maternal-fetal interface. Nature 619: 595–605, 2023. doi: 10.1038/s41586-023-06298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Moser G, Windsperger K, Pollheimer J, de Sousa Lopes SC, Huppertz B. Human trophoblast invasion: new and unexpected routes and functions. Histochem Cell Biol 150: 361–370, 2018. doi: 10.1007/s00418-018-1699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Staff AC, Benton SJ, von Dadelszen P, Roberts JM, Taylor RN, Powers RW, Charnock-Jones DS, Redman CW. Redefining preeclampsia using placenta-derived biomarkers. Hypertension 61: 932–942, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- 87. Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest 99: 2152–2164, 1997. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Alencar AKN, Swan KF, Pridjian G, Lindsey SH, Bayer CL. Connecting G protein-coupled estrogen receptor biomolecular mechanisms with the pathophysiology of preeclampsia: a review. Reprod Biol Endocrinol 21: 60, 2023. doi: 10.1186/s12958-023-01112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Matsubara K, Matsubara Y, Uchikura Y, Sugiyama T. Pathophysiology of preeclampsia: the role of exosomes. Int J Mol Sci 22: 2572, 2021. doi: 10.3390/ijms22052572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhao H, Wong RJ, Stevenson DK. The impact of hypoxia in early pregnancy on placental cells. Int J Mol Sci 22: 9675, 2021. doi: 10.3390/ijms22189675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell 21: 521–531, 2006. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J 31: 2448–2460, 2012. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Iriyama T, Wang W, Parchim NF, Song A, Blackwell SC, Sibai BM, Kellems RE, Xia Y. Hypoxia-independent upregulation of placental hypoxia inducible factor-1α gene expression contributes to the pathogenesis of preeclampsia. Hypertension 65: 1307–1315, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cheng L, Yu H, Yan N, Lai K, Xiang M. Hypoxia-inducible factor-1α target genes contribute to retinal neuroprotection. Front Cell Neurosci 11: 20, 2017. doi: 10.3389/fncel.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kosovic I, Prusac IK, Mestrovic Z, Berkovic A, Marusic J, Tomas SZ. HIF-1α immunohistochemical expression in decidual cells, villous and extravillous trophoblast in placentas from pregnancies complicated with preeclampsia. Pregnancy Hypertens 21: 176–178, 2020. doi: 10.1016/j.preghy.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 96. Ali LE, Salih MM, Elhassan EM, Mohmmed AA, Adam I. Placental growth factor, vascular endothelial growth factor, and hypoxia-inducible factor-1α in the placentas of women with pre-eclampsia. J Matern Fetal Neonatal Med 32: 2628–2632, 2019. doi: 10.1080/14767058.2018.1443066. [DOI] [PubMed] [Google Scholar]
- 97. Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 308: 1592–1594, 2005. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 98. Velicky P, Knofler M, Pollheimer J. Function and control of human invasive trophoblast subtypes: intrinsic vs. maternal control. Cell Adh Migr 10: 154–162, 2016. doi: 10.1080/19336918.2015.1089376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 123: 2856–2869, 2011. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Brosens I, Brosens JJ, Muter J, Puttemans P, Benagiano G. Preeclampsia: the role of persistent endothelial cells in uteroplacental arteries. Am J Obstet Gynecol 221: 219–226, 2019. doi: 10.1016/j.ajog.2019.01.239. [DOI] [PubMed] [Google Scholar]
- 101. McCarthy C, Kenny LC. Therapeutically targeting mitochondrial redox signalling alleviates endothelial dysfunction in preeclampsia. Sci Rep 6: 32683, 2016. doi: 10.1038/srep32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Myatt L. Review: reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 31, Suppl: S66–S69, 2010. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Aouache R, Biquard L, Vaiman D, Miralles F. Oxidative stress in preeclampsia and placental diseases. Int J Mol Sci 19: 1496, 2018. doi: 10.3390/ijms19051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ahmad IM, Zimmerman MC, Moore TA. Oxidative stress in early pregnancy and the risk of preeclampsia. Pregnancy Hypertens 18: 99–102, 2019. doi: 10.1016/j.preghy.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Han C, Huang P, Lyu M, Dong J. Oxidative stress and preeclampsia-associated prothrombotic state. Antioxidants (Basel) 9: 1139, 2020. doi: 10.3390/antiox9111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hu X-Q, Zhang L. Hypoxia and mitochondrial dysfunction in pregnancy complications. Antioxidants 10: 405, 2021. doi: 10.3390/antiox10030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Poinsignon L, Chissey A, Ajjaji A, Hernandez I, Vignaud M-L, Ferecatu I, Fournier T, Beaudeux J-L, Zerrad-Saadi A. Placental cartography of NADPH oxidase (NOX) family proteins: involvement in the pathophysiology of preeclampsia. Arch Biochem Biophys 749: 109787, 2023. doi: 10.1016/j.abb.2023.109787. [DOI] [PubMed] [Google Scholar]
- 108. Williamson RD, McCarthy FP, Khashan AS, Totorika A, Kenny LC, McCarthy C. Exploring the role of mitochondrial dysfunction in the pathophysiology of pre-eclampsia. Pregnancy Hypertens 13: 248–253, 2018. doi: 10.1016/j.preghy.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 109. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr Physiol 7: 113–170, 2016. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cowled P, Fitridge R. Pathophysiology of reperfusion injury. In: Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists, edited by Fitridge R, Thompson M. Adelaide, Australia: University of Adelaide Press, 2011. [PubMed] [Google Scholar]
- 111. Tenorio MB, Ferreira RC, Moura FA, Bueno NB, de Oliveira ACM, Goulart MOF. Cross-talk between oxidative stress and inflammation in preeclampsia. Oxid Med Cell Longev 2019: 8238727, 2019. doi: 10.1155/2019/8238727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hussain T, Murtaza G, Metwally E, Kalhoro DH, Kalhoro MS, Rahu BA, Sahito RGA, Yin Y, Yang H, Chughtai MI, Tan B. The role of oxidative stress and antioxidant balance in pregnancy. Mediators Inflamm 2021: 9962860, 2021. doi: 10.1155/2021/9962860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Haram K, Mortensen HJ, Myking O, Magann FE, Morrison CJ. The role of oxidative stress, adhesion molecules and antioxidants in preeclampsia. Curr Hypertens Rev 15: 105–112, 2019. doi: 10.2174/1573402115666190119163942. [DOI] [PubMed] [Google Scholar]
- 114. Manolis AS, Manolis AA, Manolis TA, Apostolaki NE, Apostolopoulos EJ, Melita H, Katsiki N. Mitochondrial dysfunction in cardiovascular disease: current status of translational research/clinical and therapeutic implications. Med Res Rev 41: 275–313, 2021. doi: 10.1002/med.21732. [DOI] [PubMed] [Google Scholar]
- 115. Prasun P. Mitochondrial dysfunction in metabolic syndrome. Biochim Biophys Acta Mol Basis Dis 1866: 165838, 2020. doi: 10.1016/j.bbadis.2020.165838. [DOI] [PubMed] [Google Scholar]
- 116. Smith AN, Wang X, Thomas DG, Tatum RE, Booz GW, Cunningham MW Jr.. The role of mitochondrial dysfunction in preeclampsia: causative factor or collateral damage? Am J Hypertens 34: 442–452, 2021. doi: 10.1093/ajh/hpab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Han C, Han L, Huang P, Chen Y, Wang Y, Xue F. Syncytiotrophoblast-derived extracellular vesicles in pathophysiology of preeclampsia. Front Physiol 10: 1236, 2019. doi: 10.3389/fphys.2019.01236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jahan F, Vasam G, Green AE, Bainbridge SA, Menzies KJ. Placental mitochondrial function and dysfunction in preeclampsia. Int J Mol Sci 24: 4177, 2023. doi: 10.3390/ijms24044177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Abu-Raya B, Michalski C, Sadarangani M, Lavoie PM. Maternal immunological adaptation during normal pregnancy. Front Immunol 11: 575197, 2020. doi: 10.3389/fimmu.2020.575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Apicella C, Ruano CSM, Méhats C, Miralles F, Vaiman D. The role of epigenetics in placental development and the etiology of preeclampsia. Int J Mol Sci 20: 2837, 2019. doi: 10.3390/ijms20112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders—a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis 1863: 1066–1077, 2017. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Maurizi G, Babini L, Della Guardia L. Potential role of microRNAs in the regulation of adipocytes liposecretion and adipose tissue physiology. J Cell Physiol 233: 9077–9086, 2018. doi: 10.1002/jcp.26523. [DOI] [PubMed] [Google Scholar]
- 123. Korkes HA, De Oliveira L, Sass N, Salahuddin S, Karumanchi SA, Rajakumar A. Relationship between hypoxia and downstream pathogenic pathways in preeclampsia. Hypertens Pregnancy 36: 145–150, 2017. doi: 10.1080/10641955.2016.1259627. [DOI] [PubMed] [Google Scholar]
- 124. Yu W, Gao W, Rong D, Wu Z, Khalil RA. Molecular determinants of microvascular dysfunction in hypertensive pregnancy and preeclampsia. Microcirculation 26: e12508, 2019. doi: 10.1111/micc.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Dilba P, Schoedl M, Hund M, Verlohren S. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med 374: 13–22, 2016. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 126. Lehnen H, Mosblech N, Reineke T, Puchooa A, Menke-Möllers I, Zechner U, Gembruch U. Prenatal clinical assessment of sFlt-1 (soluble fms-like tyrosine kinase-1)/PlGF (placental growth factor) ratio as a diagnostic tool for preeclampsia, pregnancy-induced hypertension, and proteinuria. Geburtshilfe Frauenheilkd 73: 440–445, 2013. doi: 10.1055/s-0032-1328601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rath G, Aggarwal R, Jawanjal P, Tripathi R, Batra A. HIF-1 α and placental growth factor in pregnancies complicated with preeclampsia: a qualitative and quantitative analysis. J Clin Lab Anal 30: 75–83, 2016. doi: 10.1002/jcla.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Albogami SM, Al-Kuraishy HM, Al-Maiahy TJ, Al-Buhadily AK, Al-Gareeb AI, Alorabi M, Alotaibi SS, De Waard M, Sabatier J-M, Saad HM, Batiha GE-S. Hypoxia-inducible factor 1 and preeclampsia: a new perspective. Curr Hypertens Rep 24: 687–692, 2022. doi: 10.1007/s11906-022-01225-1. [DOI] [PubMed] [Google Scholar]
- 129. Adu-Bonsaffoh K, Antwi DA, Obed SA, Gyan B. Nitric oxide dysregulation in the pathogenesis of preeclampsia among Ghanaian women. Integr Blood Press Control 8: 1–6, 2015. doi: 10.2147/IBPC.S68454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Holthe MR, Staff AC, Berge LN, Lyberg T. Leukocyte adhesion molecules and reactive oxygen species in preeclampsia. Obstet Gynecol 103: 913–922, 2004. doi: 10.1097/01.AOG.0000124806.39111.ba. [DOI] [PubMed] [Google Scholar]
- 131. LaMarca B, Wallace K, Granger J. Role of angiotensin II type I receptor agonistic autoantibodies (AT1-AA) in preeclampsia. Curr Opin Pharmacol 11: 175–179, 2011. doi: 10.1016/j.coph.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Campbell N, LaMarca B, Cunningham MW. The role of agonistic autoantibodies to the angiotensin II type 1 receptor (AT1-AA) in pathophysiology of preeclampsia. Curr Pharm Biotechnol 19: 781–785, 2018. doi: 10.2174/1389201019666180925121254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cunningham MW Jr, Vaka VR, McMaster K, Ibrahim T, Cornelius DC, Amaral L, Campbell N, Wallukat G, McDuffy S, Usry N, Dechend R, LaMarca B. Renal natural killer cell activation and mitochondrial oxidative stress; new mechanisms in AT1-AA mediated hypertensive pregnancy. Pregnancy Hypertens 15: 72–77, 2019. doi: 10.1016/j.preghy.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Deer E, Vaka VR, McMaster KM, Wallace K, Cornelius DC, Amaral LM, Cunningham MW, LaMarca B. Vascular endothelial mitochondrial oxidative stress in response to preeclampsia: a role for angiotension II type 1 autoantibodies. Am J Obstet Gynecol MFM 3: 100275, 2021. doi: 10.1016/j.ajogmf.2020.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tabacco S, Ambrosii S, Polsinelli V, Fantasia I, D'Alfonso A, Ludovisi M, Cecconi S, Guido M. Pre-eclampsia: from etiology and molecular mechanisms to clinical tools—a review of the literature. Curr Issues Mol Biol 45: 6202–6215, 2023. doi: 10.3390/cimb45080391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jardim LL, Rios DR, Perucci LO, de Sousa LP, Gomes KB, Dusse LM. Is the imbalance between pro-angiogenic and anti-angiogenic factors associated with preeclampsia? Clin Chim Acta 447: 34–38, 2015. doi: 10.1016/j.cca.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 137. Rios DRA, Alpoim PN, Godoi LC, Perucci LO, de Sousa LP, Gomes KB, Dusse LMS. Increased levels of sENG and sVCAM-1 and decreased levels of VEGF in severe preeclampsia. Am J Hypertens 29: 1307–1310, 2015. doi: 10.1093/ajh/hpv170. [DOI] [PubMed] [Google Scholar]
- 138. Hirashima C, Ohkuchi A, Matsubara S, Suzuki H, Takahashi K, Usui R, Suzuki M. Alteration of serum soluble endoglin levels after the onset of preeclampsia is more pronounced in women with early-onset. Hypertens Res 31: 1541–1548, 2008. doi: 10.1291/hypres.31.1541. [DOI] [PubMed] [Google Scholar]
- 139. Barrero JA, Villamil-Camargo LM, Imaz JN, Arciniegas-Villa K, Rubio-Romero JA. Maternal serum activin A, inhibin A and follistatin-related proteins across preeclampsia: insights into their role in pathogenesis and prediction. J Mother Child 27: 119–133, 2023. doi: 10.34763/jmotherandchild.20232701.d-23-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Keikkala E, Forstén J, Ritvos O, Stenman U-H, Kajantie E, Hämäläinen E, Räikkönen K, Villa PM, Laivuori H. Serum Inhibin-A and PAPP-A2 in the prediction of pre-eclampsia during the first and second trimesters in high-risk women. Pregnancy Hypertens 25: 116–122, 2021. doi: 10.1016/j.preghy.2021.05.024. [DOI] [PubMed] [Google Scholar]
- 141. Broumand F, Lak SS, Nemati F, Mazidi A. A study of the diagnostic value of Inhibin A Tests for occurrence of preeclampsia in pregnant women. Electron Physician 10: 6186–6192, 2018. doi: 10.19082/6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sunjaya AF, Sunjaya AP. Evaluation of serum biomarkers and other diagnostic modalities for early diagnosis of preeclampsia. J Family Reprod Health 13: 56–69, 2019. [PMC free article] [PubMed] [Google Scholar]
- 143. Karumanchi SA. Angiogenic factors in preeclampsia: from diagnosis to therapy. Hypertension 67: 1072–1079, 2016. doi: 10.1161/HYPERTENSIONAHA.116.06421. [DOI] [PubMed] [Google Scholar]
- 144. Wadhwani NS, Patil VV, Mehendale SS, Wagh GN, Gupte SA, Joshi SR. Increased homocysteine levels exist in women with preeclampsia from early pregnancy. J Matern Fetal Neonatal Med 29: 2719–2725, 2016. doi: 10.3109/14767058.2015.1102880. [DOI] [PubMed] [Google Scholar]
- 145. Şanlıkan F, Tufan F, Göçmen A, Kabadayı C, Şengül E. The evaluation of homocysteine level in patients with preeclampsia. Ginekol Pol 86: 287–291, 2015. doi: 10.17772/gp/2075. [DOI] [PubMed] [Google Scholar]
- 146. Aggarwal R, Jain AK, Mittal P, Kohli M, Jawanjal P, Rath G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J Clin Lab Anal 33: e22834, 2019. doi: 10.1002/jcla.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jancsura MK, Schmella MJ, Helsabeck N, Gillespie SL, Roberts JM, Conley YP, Hubel CA. Inflammatory markers are elevated in early pregnancy, but not late pregnancy, in women with overweight and obesity that later develop preeclampsia. Am J Reprod Immunol 90: e13763, 2023. doi: 10.1111/aji.13763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Bambrana V, Dayanand CD, Kotur P. Relationship between xanthine oxidase, ischemia modified albumin, nitric oxide with antioxidants in non pregnants, pre and post-delivery of normal pregnants and preeclampsia. Indian J Clin Biochem 32: 171–178, 2017. doi: 10.1007/s12291-016-0599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Onovughakpo-Sakpa EO, Onyeneke CE, Ayinbuomwan E, Atoe K. Antioxidant and malondialdehyde status in preeclampsia. Niger J Exp Clin Biosci 9: 110–116, 2021. doi: 10.4103/njecp.njecp_6_21. [DOI] [Google Scholar]
- 150. Freire VAF, Melo AD, Santos HL, Barros-Pinheiro M. Evaluation of oxidative stress markers in subtypes of preeclampsia: a systematic review and meta-analysis. Placenta 132: 55–67, 2023. doi: 10.1016/j.placenta.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 151. Omar M, Borg HM. Assessment of oxidative stress markers and level of antioxidant in preeclampsia. Indian J Obstet Gynecol Res 6: 268–275, 2019. doi: 10.18231/j.ijogr.2019.062. [DOI] [Google Scholar]
- 152. Borges VTM, Zanati SG, Peraçoli MTS, Poiati JR, Romão-Veiga M, Peraçoli JC, Thilaganathan B. Maternal left ventricular hypertrophy and diastolic dysfunction and brain natriuretic peptide concentration in early- and late-onset pre-eclampsia. Ultrasound Obstet Gynecol 51: 519–523, 2018. doi: 10.1002/uog.17495. [DOI] [PubMed] [Google Scholar]
- 153. Bakrania BA, George EM, Granger JP. Animal models of preeclampsia: investigating pathophysiology and therapeutic targets. Am J Obstet Gynecol 226: S973–S987, 2022. doi: 10.1016/j.ajog.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Morton A, Morton A. High sensitivity cardiac troponin I levels in preeclampsia. Pregnancy Hypertens 13: 79–82, 2018. doi: 10.1016/j.preghy.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 155. Gondek A, Jagodzińska A, Pietrzak B, Mamcarz A, Cudnoch-Jędrzejewska A. Relevance of the assessment of natriuretic peptide plasma concentrations in hypertensive pregnant women. Biomarkers 25: 449–457, 2020. doi: 10.1080/1354750X.2020.1795264. [DOI] [PubMed] [Google Scholar]
- 156. Marek-Iannucci S, Oliveros E, Brailovsky Y, Pirlamarla P, Roman A, Rajapreyar IN. Natriuretic peptide biomarkers in the imminent development of preeclampsia. Front Cardiovasc Med 10: 1203516, 2023. doi: 10.3389/fcvm.2023.1203516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Binder NK, Beard S, de Alwis N, Fato BR, Nguyen T-V, TuJ K-L, Hannan NJ. Investigating the effects of atrial natriuretic peptide on the maternal endothelium to determine potential implications for preeclampsia. Int J Mol Sci 24: 6182, 2023. doi: 10.3390/ijms24076182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lai M, Schachter A, Heard AJ, Grant J, Hagan J, Stafford IA. B-type natriuretic peptide (BNP) and hypertensive disorders in pregnancy [31H]. Obstet Gynecol 133: 92S–93S, 2019. doi: 10.1097/01.AOG.0000558770.17315.89. [DOI] [Google Scholar]
- 159. Grzeszczak K, Łanocha-Arendarczyk N, Malinowski W, Ziętek P, Kosik-Bogacka D. Oxidative stress in pregnancy. Biomolecules 13: 1768, 2023. doi: 10.3390/biom13121768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Xie JH, Li YY, Jin J. The essential functions of mitochondrial dynamics in immune cells. Cell Mol Immunol 17: 712–721, 2020. doi: 10.1038/s41423-020-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Deer E, Herrock O, Campbell N, Cornelius D, Fitzgerald S, Amaral LM, LaMarca B. The role of immune cells and mediators in preeclampsia. Nat Rev Nephrol 19: 257–270, 2023. doi: 10.1038/s41581-022-00670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Sakowicz A. The targeting of nuclear factor κ B by drugs adopted for the prevention and treatment of preeclampsia. Int J Mol Sci 23: 2881, 2022. doi: 10.3390/ijms23052881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol 63: 534–543, 2010. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 164. Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol 29: 151–162, 2007. doi: 10.1007/s00281-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 165. Seely EW, Tsigas E, Rich-Edwards JW. Preeclampsia and future cardiovascular disease in women: how good are the data and how can we manage our patients? Semin Perinatol 39: 276–283, 2015. doi: 10.1053/j.semperi.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 166. Cunningham MW, Castillo J, Ibrahim T, Cornelius DC, Campbell N, Amaral L, Vaka VR, Usry N, Williams JM, LaMarca B. AT1-AA (angiotensin II type 1 receptor agonistic autoantibody) blockade prevents preeclamptic symptoms in placental ischemic rats. Hypertension 71: 886–893, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Seki H. The role of the renin–angiotensin system in the pathogenesis of preeclampsia—new insights into the renin–angiotensin system in preeclampsia. Med Hypotheses 82: 362–367, 2014. doi: 10.1016/j.mehy.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 168. Garrido-Gimenez C, Mendoza M, Cruz-Lemini M, Galian-Gay L, Sanchez-Garcia O, Granato C, Rodriguez-Sureda V, Rodriguez-Palomares J, Carreras-Moratonas E, Cabero-Roura L, Llurba E, Alijotas-Reig J. Angiogenic factors and long-term cardiovascular risk in women that developed preeclampsia during pregnancy. Hypertension 76: 1808–1816, 2020. [Erratum in Hypertension 80: e74, 2023]. doi: 10.1161/HYPERTENSIONAHA.120.15830. [DOI] [PubMed] [Google Scholar]
- 169. Hammadah M, Georgiopoulou VV, Kalogeropoulos AP, Weber M, Wang X, Samara MA, Wu Y, Butler J, Tang WHW. Elevated soluble Fms-like tyrosine kinase-1 and placental-like growth factor levels are associated with development and mortality risk in heart failure. Circ Heart Fail 9: e002115, 2016. doi: 10.1161/CIRCHEARTFAILURE.115.002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bisson C, Dautel S, Patel E, Suresh S, Dauer P, Rana S. Preeclampsia pathophysiology and adverse outcomes during pregnancy and postpartum. Front Med (Lausanne) 10: 1144170, 2023. doi: 10.3389/fmed.2023.1144170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-β superfamily expression and actions in the endometrium and placenta. Reproduction 132: 217–232, 2006. doi: 10.1530/rep.1.01076. [DOI] [PubMed] [Google Scholar]
- 172. Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology 138: 4977–4988, 1997. doi: 10.1210/endo.138.11.5475. [DOI] [PubMed] [Google Scholar]
- 173. Bakrania BA, Hall ME, Shahul S, Granger JP. The Reduced Uterine Perfusion Pressure (RUPP) rat model of preeclampsia exhibits impaired systolic function and global longitudinal strain during pregnancy. Pregnancy Hypertens 18: 169–172, 2019. doi: 10.1016/j.preghy.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Naseem H, Dreixler J, Mueller A, Tung A, Dhir R, Chibber R, Fazal A, Granger JP, Bakrania BA, deMartelly V, Rana S, Shahul S. Antepartum Aspirin administration reduces activin A and cardiac global longitudinal strain in preeclamptic women. J Am Heart Assoc 9: e015997, 2020. doi: 10.1161/JAHA.119.015997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zheng Z, Liu L, Zhou K, Ding L, Zeng J, Zhang W. Anti-oxidant and anti-endothelial dysfunctional properties of nano-selenium in vitro and in vivo of hyperhomocysteinemic rats. Int J Nanomedicine 15: 4501–4521, 2020. doi: 10.2147/IJN.S255392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jensen MK, Bertoia ML, Cahill LE, Agarwal I, Rimm EB, Mukamal KJ. Novel metabolic biomarkers of cardiovascular disease. Nat Rev Endocrinol 10: 659–672, 2014. doi: 10.1038/nrendo.2014.155. [DOI] [PubMed] [Google Scholar]
- 177. Burgess A, Founds S. Cardiovascular implications of preeclampsia. MCN Am J Matern Child Nurs 41: 8–15, 2016. doi: 10.1097/NMC.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 178. Elliot CA, Hamlin MJ, Lizamore CA. Inter-operator reliability for measuring pulse wave velocity and augmentation index. Front Cardiovasc Med 7: 72, 2020. doi: 10.3389/fcvm.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Anthoulakis C, Mamopoulos A. Augmentation index and pulse wave velocity in normotensive versus preeclamptic pregnancies: a prospective case–control study using a new oscillometric method. Ann Med 54: 1–10, 2022. doi: 10.1080/07853890.2021.2014553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ospina-Tascón GA, Nieto Calvache AJ, Quiñones E, Madriñan HJ, Valencia JD, Bermúdez WF, Carvajal J, Escobar MF, de Backer D. Microcirculatory blood flow derangements during severe preeclampsia and HELLP syndrome. Pregnancy Hypertens 10: 124–130, 2017. doi: 10.1016/j.preghy.2017.07.140. [DOI] [PubMed] [Google Scholar]
- 181. Soullane S, Rhéaume M-A, Auger N. Preeclampsia and the retina. Curr Hypertens Rep 26: 169–174, 2023. doi: 10.1007/s11906-023-01290-0. [DOI] [PubMed] [Google Scholar]
- 182. Scioscia M, Siwetz M, Robillard P-Y, Brizzi A, Huppertz B. Placenta and maternal endothelium during preeclampsia: disruption of the glycocalyx explains increased inositol phosphoglycans and angiogenic factors in maternal blood. J Reprod Immunol 160: 104161, 2023. doi: 10.1016/j.jri.2023.104161. [DOI] [PubMed] [Google Scholar]
- 183. Matsubara K, Higaki T, Matsubara Y, Nawa A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int J Mol Sci 16: 4600–4614, 2015. doi: 10.3390/ijms16034600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Veerbeek JHW, Hermes W, Breimer AY, van Rijn BB, Koenen SV, Mol BW, Franx A, de Groot CJM, Koster MPH. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension 65: 600–606, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04850. [DOI] [PubMed] [Google Scholar]
- 185. Mohindra R, Agrawal DK, Thankam FG. Altered vascular extracellular matrix in the pathogenesis of atherosclerosis. J Cardiovasc Transl Res 14: 647–660, 2021. doi: 10.1007/s12265-020-10091-8. [DOI] [PubMed] [Google Scholar]
- 186. Diniz ALD, Paes M, Diniz AD. Analyzing preeclampsia as the tip of the iceberg represented by women with long-term cardiovascular disease, atherosclerosis, and inflammation. Curr Atheroscler Rep 22: 13, 2020. doi: 10.1007/s11883-020-0830-6. [DOI] [PubMed] [Google Scholar]
- 187. Chourdakis E, Oikonomou N, Fouzas S, Hahalis G, Karatza AA. Preeclampsia emerging as a risk factor of cardiovascular disease in women. High Blood Press Cardiovasc Prev 28: 103–114, 2021. doi: 10.1007/s40292-020-00425-7. [DOI] [PubMed] [Google Scholar]
- 188. Raia-Barjat T, Edebiri O, Ni Ainle F. Preeclampsia and venous thromboembolism: pathophysiology and potential therapy. Front Cardiovasc Med 9: 856923, 2022. doi: 10.3389/fcvm.2022.856923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Havers-Borgersen E, Butt JH, Johansen M, Petersen OB, Ekelund CK, Rode L, Olesen JB, Køber L, Fosbøl EL. Preeclampsia and long-term risk of venous thromboembolism. JAMA Netw Open 6: e2343804, 2023. [Erratum in JAMA Netw Open 7: e2354306, 2024]. doi: 10.1001/jamanetworkopen.2023.43804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol 75: 207–221, 2020. doi: 10.1016/j.jacc.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 191. Chen SN, Cheng CC, Tsui KH, Tang PL, Chern CU, Huang WC, Lin LT. Hypertensive disorders of pregnancy and future heart failure risk: a nationwide population-based retrospective cohort study. Pregnancy Hypertens 13: 110–115, 2018. doi: 10.1016/j.preghy.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 192. Yang C, Baker PN, Granger JP, Davidge ST, Tong C. Long-term impacts of preeclampsia on the cardiovascular system of mother and offspring. Hypertension 80: 1821–1833, 2023. doi: 10.1161/HYPERTENSIONAHA.123.21061. [DOI] [PubMed] [Google Scholar]
- 193. Ducat A, Doridot L, Calicchio R, Méhats C, Vilotte J-L, Castille J, Barbaux S, Couderc B, Jacques S, Letourneur F, Buffat C, Le Grand F, Laissue P, Miralles F, Vaiman D. Endothelial cell dysfunction and cardiac hypertrophy in the STOX1 model of preeclampsia. Sci Rep 6: 19196, 2016. doi: 10.1038/srep19196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Li X, Yang N, Wu Y, Wang X, Sun J, Liu L, Zhang F, Gong Y, Zhang Y, Li X, Du D, Ding B. Hypoxia regulates fibrosis-related genes via histone lactylation in the placentas of patients with preeclampsia. J Hypertens 40: 1189–1198, 2022. doi: 10.1097/HJH.0000000000003129. [DOI] [PubMed] [Google Scholar]
- 195. Miralles F, Collinot H, Boumerdassi Y, Ducat A, Duché A, Renault G, Marchiol C, Lagoutte I, Bertholle C, Andrieu M, Jacques S, Méhats C, Vaiman D. Long-term cardiovascular disorders in the STOX1 mouse model of preeclampsia. Sci Rep 9: 11918, 2019. doi: 10.1038/s41598-019-48427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Akhter T, Wikström AK, Larsson M, Larsson A, Wikström G, Naessen T. Association between angiogenic factors and signs of arterial aging in women with pre-eclampsia. Ultrasound Obstet Gynecol 50: 93–99, 2017. doi: 10.1002/uog.15981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Verlohren S, Perschel FH, Thilaganathan B, Dröge LA, Henrich W, Busjahn A, Khalil A. Angiogenic markers and cardiovascular indices in the prediction of hypertensive disorders of pregnancy. Hypertension 69: 1192–1197, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09256. [DOI] [PubMed] [Google Scholar]
- 198. Ramadan H, Rana S, Mueller A, Bajracharya S, Zhang D, Salahuddin S, Nasim R, Perdigao JL, Minhaj M, Tung A, Arany Z, Shahul S. Myocardial performance index in hypertensive disorders of pregnancy: the relationship between blood pressures and angiogenic factors. Hypertens Pregnancy 36: 161–167, 2017. doi: 10.1080/10641955.2017.1280048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Thilaganathan B, Kalafat E. Cardiovascular system in preeclampsia and beyond. Hypertension 73: 522–531, 2019. doi: 10.1161/HYPERTENSIONAHA.118.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Perry H, Khalil A, Thilaganathan B. Preeclampsia and the cardiovascular system: an update. Trends Cardiovasc Med 28: 505–513, 2018. doi: 10.1016/j.tcm.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 201. Miller EC, Vollbracht S. Neurology of preeclampsia and related disorders: an update in neuro-obstetrics. Curr Pain Headache Rep 25: 40, 2021. doi: 10.1007/s11916-021-00958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Cipolla MJ, Tremble SM, DeLance N, Johnson AC. Worsened stroke outcome in a model of preeclampsia is associated with poor collateral flow and oxidative stress. Stroke 54: 354–363, 2023. doi: 10.1161/STROKEAHA.122.041637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Lackovic M, Nikolic D, Jankovic M, Rovcanin M, Mihajlovic S. Stroke vs. preeclampsia: dangerous liaisons of hypertension and pregnancy. Medicina 59: 1707, 2023. doi: 10.3390/medicina59101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. León-Reyes G, Maida-Claros RF, Urrutia-Medina AX, Jorge-Galarza E, Guzmán-Grenfell AM, Fuentes-García S, Medina-Navarro R, Moreno-Eutimio MA, Muñoz-Sánchez JL, Hicks JJ, Torres-Ramos YD. Oxidative profiles of LDL and HDL isolated from women with preeclampsia. Lipids Health Dis 16: 90, 2017. doi: 10.1186/s12944-017-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Mushenkova NV, Bezsonov EE, Orekhova VA, Popkova TV, Starodubova AV, Orekhov AN. Recognition of oxidized lipids by macrophages and its role in atherosclerosis development. Biomedicines 9: 915, 2021. doi: 10.3390/biomedicines9080915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Parasher A, Jhamb R. Posterior reversible encephalopathy syndrome (PRES): presentation, diagnosis and treatment. Postgrad Med J 96: 623–628, 2020. doi: 10.1136/postgradmedj-2020-137706. [DOI] [PubMed] [Google Scholar]
- 207. Thombarapu U, Dwarampudi AD, Kodey PD. Posterior reversible encephalopathy syndrome in preeclampsia. Int J Reprod Contracept Obstet Gynecol 9: 358–363, 2019. doi: 10.18203/2320-1770.ijrcog20196048. [DOI] [Google Scholar]
- 208. Martínez-Martínez MM, Fernández-Travieso J, Gómez Muñoz N, Varela Mezquita B, Almarcha-Menargues ML, Miralles Martínez A. Cerebral hemodynamics and vasoconstriction in preeclampsia: from diagnosis to resolution. Pregnancy Hypertens 26: 42–47, 2021. doi: 10.1016/j.preghy.2021.08.114. [DOI] [PubMed] [Google Scholar]
- 209. Shah A, Patel J, Isath A, Hassan Virk H, Jneid H, Zelop C, Mehta-Lee S, Economy K, Gulati M, Krittanawong C. Cardiovascular complications in pregnancy. Curr Treat Options Cardio Med 25: 391–414, 2023. doi: 10.1007/s11936-023-01000-8. [DOI] [Google Scholar]
- 210. Kerley RN, McCarthy C. Biomarkers of glomerular dysfunction in pre-eclampsia—a systematic review. Pregnancy Hypertens 14: 265–272, 2018. doi: 10.1016/j.preghy.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 211. Kattah A. Preeclampsia and kidney disease: deciphering cause and effect. Curr Hypertens Rep 22: 91, 2020. doi: 10.1007/s11906-020-01099-1. [DOI] [PubMed] [Google Scholar]
- 212. Covella B, Vinturache AE, Cabiddu G, Attini R, Gesualdo L, Versino E, Piccoli GB. A systematic review and meta-analysis indicates long-term risk of chronic and end-stage kidney disease after preeclampsia. Kidney Int 96: 711–727, 2019. doi: 10.1016/j.kint.2019.03.033. [DOI] [PubMed] [Google Scholar]