Abstract
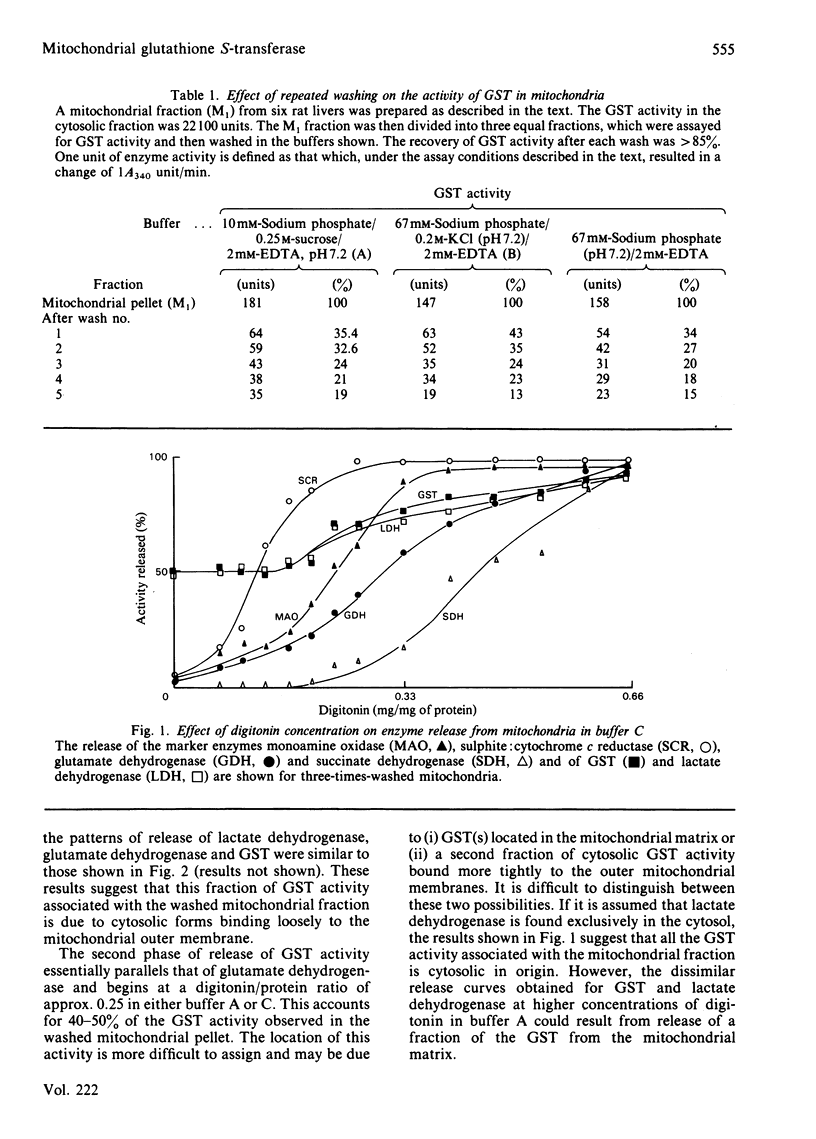
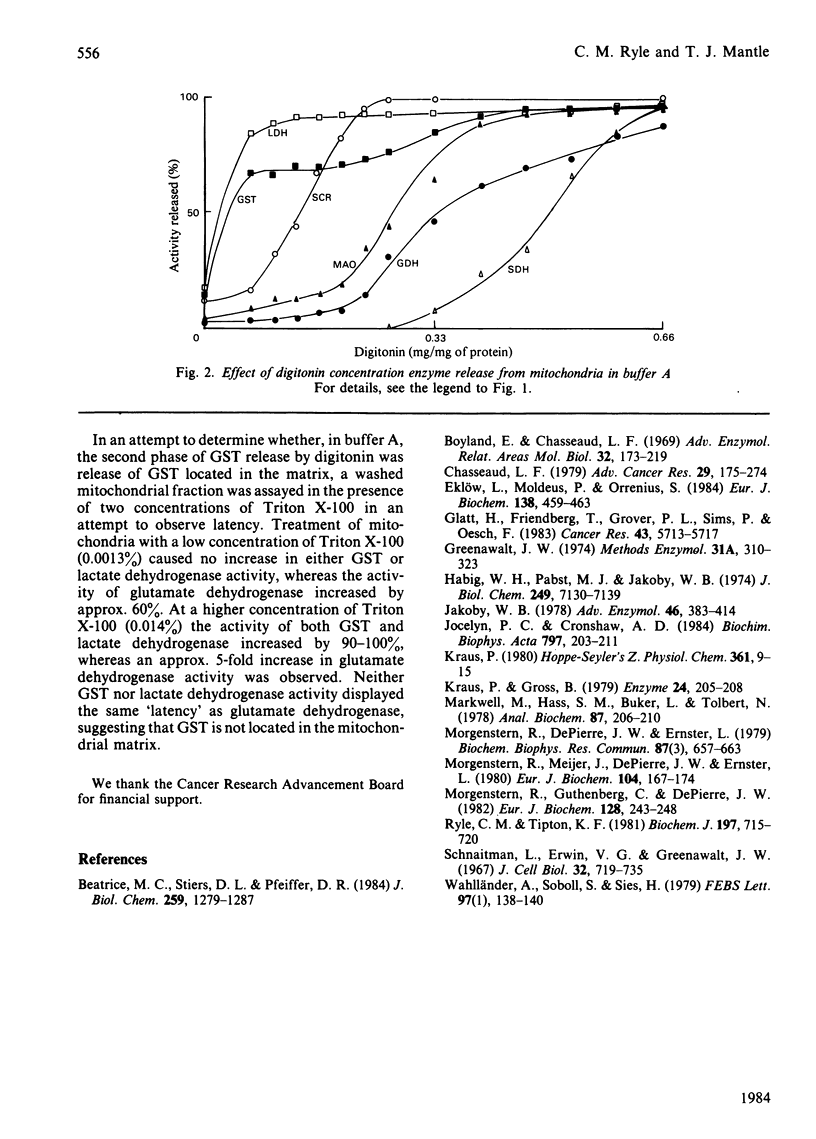
The major proportion of rat liver glutathione S-transferase is cytosolic. Carefully washed mitochondria contain 0.25-0.47% of the cytosolic activity. Subfractionation of washed mitochondria using digitonin treatment revealed that glutathione S-transferase release did not parallel that of any of the mitochondrial marker enzymes. Glutathione S-transferase release paralleled that of lactate dehydrogenase, suggesting that these 'mitochondrial' activities are due to loosely bound cytoplasmic forms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatrice M. C., Stiers D. L., Pfeiffer D. R. The role of glutathione in the retention of Ca2+ by liver mitochondria. J Biol Chem. 1984 Jan 25;259(2):1279–1287. [PubMed] [Google Scholar]
- Boyland E., Chasseaud L. F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:173–219. doi: 10.1002/9780470122778.ch5. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Eklöw L., Moldéus P., Orrenius S. Oxidation of glutathione during hydroperoxide metabolism. A study using isolated hepatocytes and the glutathione reductase inhibitor 1,3-bis(2-chloroethyl)-1-nitrosourea. Eur J Biochem. 1984 Feb 1;138(3):459–463. doi: 10.1111/j.1432-1033.1984.tb07938.x. [DOI] [PubMed] [Google Scholar]
- Glatt H., Friedberg T., Grover P. L., Sims P., Oesch F. Inactivation of a diol-epoxide and a K-region epoxide with high efficiency by glutathione transferase X. Cancer Res. 1983 Dec;43(12 Pt 1):5713–5717. [PubMed] [Google Scholar]
- Greenawalt J. W. The isolation of outer and inner mitochondrial membranes. Methods Enzymol. 1974;31:310–323. doi: 10.1016/0076-6879(74)31033-6. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Jocelyn P. C., Cronshaw A. D. The reduction of disulphides by rat liver mitochondria. Biochim Biophys Acta. 1984 Feb 14;797(2):203–211. doi: 10.1016/0304-4165(84)90123-5. [DOI] [PubMed] [Google Scholar]
- Kraus P., Gross B. Particle-bound glutathione-S-transferases. Enzyme. 1979;24(3):205–208. doi: 10.1159/000458656. [DOI] [PubMed] [Google Scholar]
- Kraus P. Resolution, purification and some properties of three glutathione transferases from rat liver mitochondria. Hoppe Seylers Z Physiol Chem. 1980 Jan;361(1):9–15. doi: 10.1515/bchm2.1980.361.1.9. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., DePierre J. W., Ernster L. Activation of microsomal glutathione S-transferase activity by sulfhydryl reagents. Biochem Biophys Res Commun. 1979 Apr 13;87(3):657–663. doi: 10.1016/0006-291x(79)92009-6. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., Guthenberg C., Depierre J. W. Microsomal glutathione S-transferase. Purification, initial characterization and demonstration that it is not identical to the cytosolic glutathione S-transferases A, B and C. Eur J Biochem. 1982 Nov;128(1):243–248. [PubMed] [Google Scholar]
- Morgenstern R., Meijer J., Depierre J. W., Ernster L. Characterization of rat-liver microsomal glutathione S-transferase activity. Eur J Biochem. 1980 Feb;104(1):167–174. doi: 10.1111/j.1432-1033.1980.tb04412.x. [DOI] [PubMed] [Google Scholar]
- Ryle C. M., Tipton K. F. Subcellular localization of aldehyde reductase activities in ox brain. Biochem J. 1981 Sep 1;197(3):715–720. doi: 10.1042/bj1970715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlländer A., Soboll S., Sies H., Linke I., Müller M. Hepatic mitochondrial and cytosolic glutathione content and the subcellular distribution of GSH-S-transferases. FEBS Lett. 1979 Jan 1;97(1):138–140. doi: 10.1016/0014-5793(79)80069-1. [DOI] [PubMed] [Google Scholar]


