Abstract
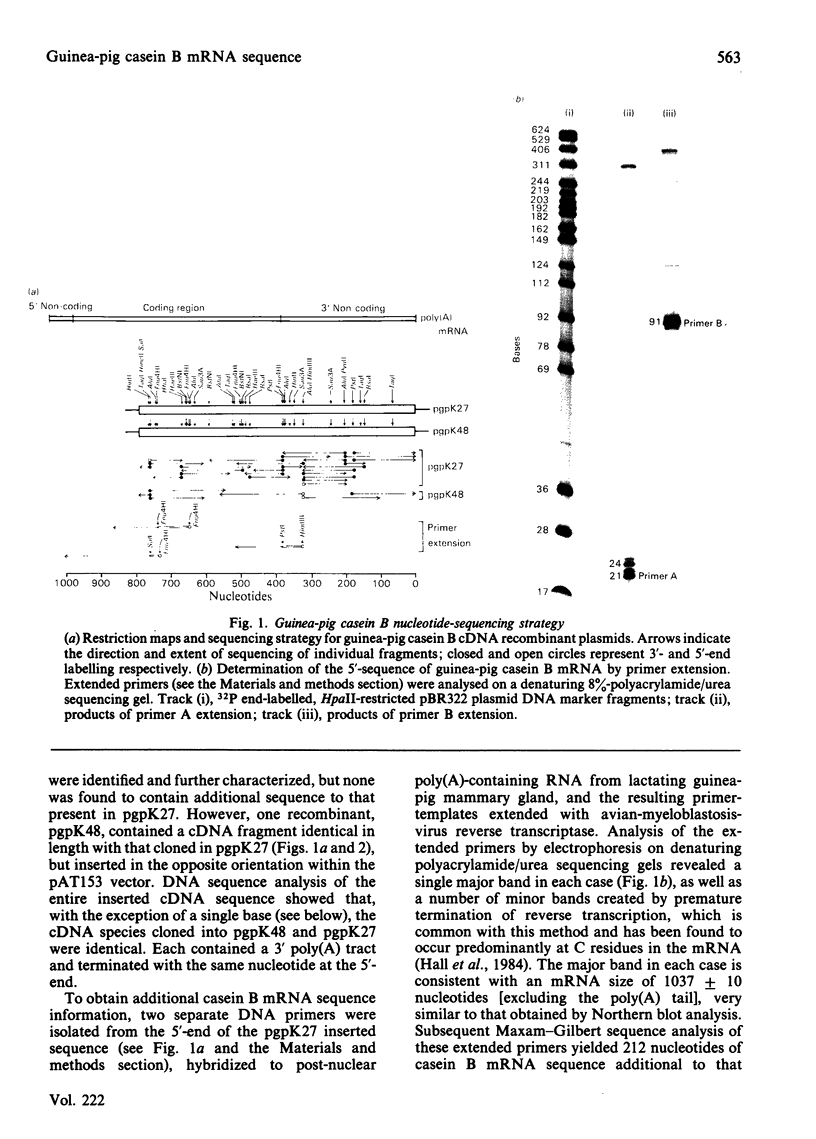
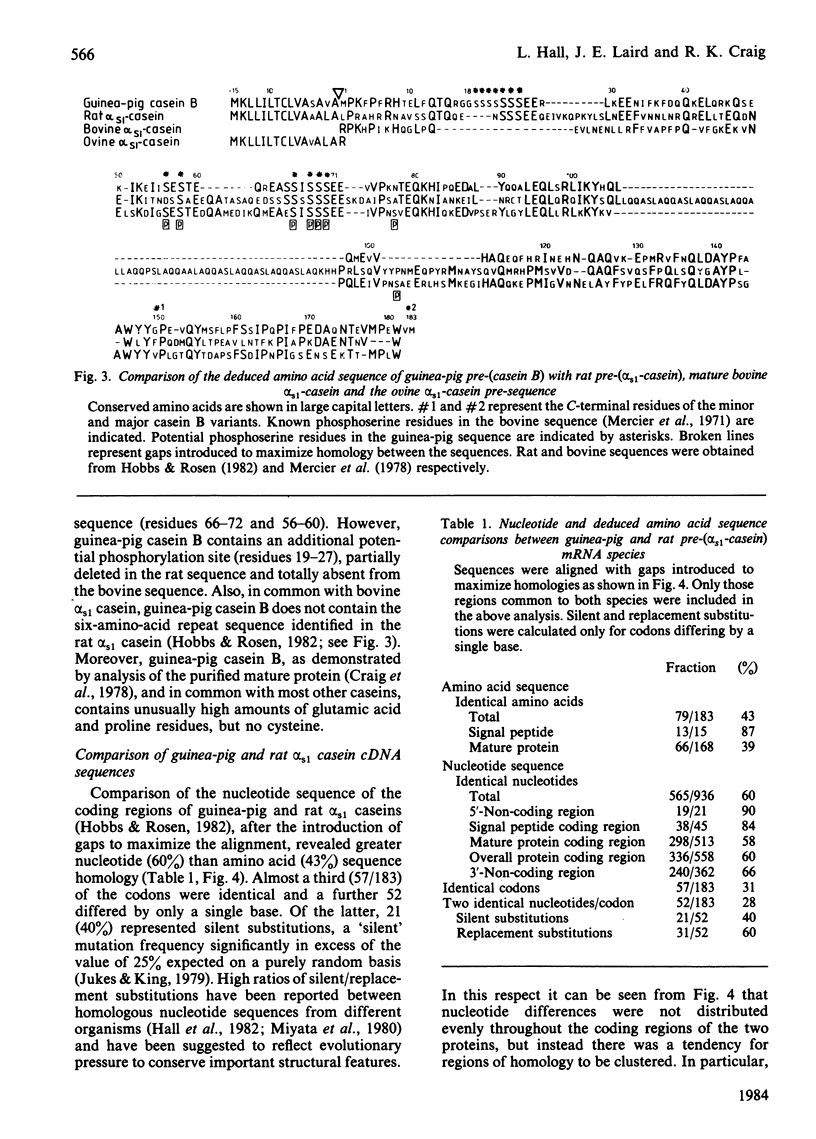
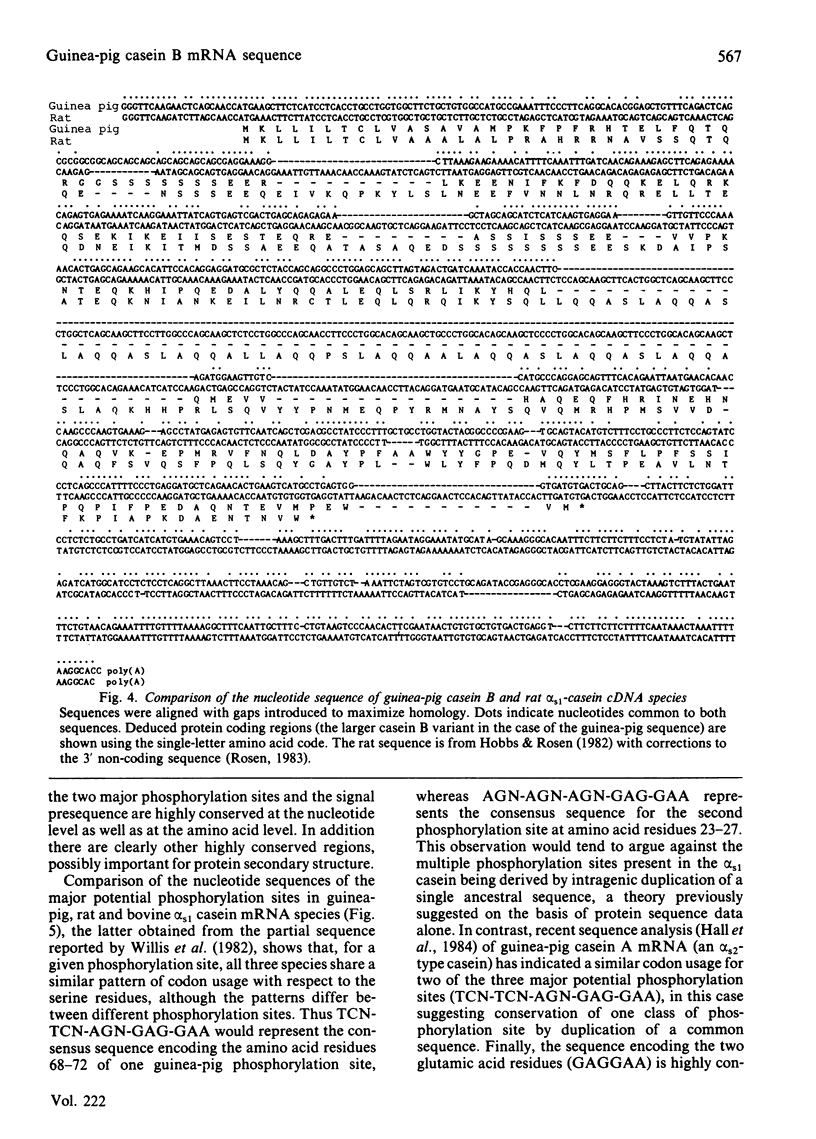
Nucleotide sequence analysis of cloned guinea-pig casein B cDNA sequences has identified two casein B variants related to the bovine and rat alpha s1 caseins. Amino acid homology was largely confined to the known bovine or predicted rat phosphorylation sites and within the 'signal' precursor sequence. Comparison of the deduced nucleotide sequence of the guinea-pig and rat alpha s1 casein mRNA species showed greater sequence conservation in the non-coding than in the coding regions, suggesting a functional and possibly regulatory role for the non-coding regions of casein mRNA. The results provide insight into the evolution of the casein genes, and raise questions as to the role of conserved nucleotide sequences within the non-coding regions of mRNA species.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn D. E., Hobbs A. A., Rosen J. M. Rat beta casein cDNA: sequence analysis and evolutionary comparisons. Nucleic Acids Res. 1982 Apr 10;10(7):2295–2307. doi: 10.1093/nar/10.7.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignon G., Ribadeau Dumas B., Mercier J. C., Pelissier J. P., Das B. C. Complete amino acid sequence of bovine alphaS2-casein. FEBS Lett. 1977 Apr 15;76(2):274–279. doi: 10.1016/0014-5793(77)80167-1. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Boulton A. P., Harrison O. S., Parker D., Campbell P. N. Studies on the intracellular segregation of polyribosome-associated messenger ribonucleic acid species in the lactating guinea-pig mammary gland. Biochem J. 1979 Sep 1;181(3):737–756. doi: 10.1042/bj1810737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Brown P. A., Harrison O. S., McIlreavy D., Campbell P. N. Guinea-pig milk-protein synthesis. Isolation and characterization of messenger ribonucleic acids from lactating mammary gland and identification of caseins and pre-alpha-lactalbumin as translation products in heterologous cell-free systems. Biochem J. 1976 Oct 15;160(1):57–74. doi: 10.1042/bj1600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Hall L., Parker D., Campbell P. N. The construction, identification and partial characterization of plasmids containing guinea-pig milk protein complementary DNA sequences. Biochem J. 1981 Mar 15;194(3):989–998. doi: 10.1042/bj1940989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., McIlreavy D., Hall R. L. Separation and partial characterization of guinea-pig caseins. Biochem J. 1978 Aug 1;173(2):633–641. doi: 10.1042/bj1730633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon W. G., Groves M. L., Greenberg R., Jones S. B., Kalan E. B., Peterson R. F., Townend R. E. Probable identification of -, TS-, R- and S-caseins as fragments of -casein. J Dairy Sci. 1972 Feb;55(2):261–263. doi: 10.3168/jds.s0022-0302(72)85469-9. [DOI] [PubMed] [Google Scholar]
- Grosclaude F., Mahé M. F., Ribadeau-Dumas B. Structure primaire de la caséine alphasl et de la caséine beta bovines. Correctif. Eur J Biochem. 1973 Dec 3;40(1):323–324. doi: 10.1111/j.1432-1033.1973.tb03199.x. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Hall L., Craig R. K., Edbrooke M. R., Campbell P. N. Comparison of the nucleotide sequence of cloned human and guinea-pig pre-alpha-lactalbumin cDNA with that of chick pre-lysozyme cDNA suggests evolution from a common ancestral gene. Nucleic Acids Res. 1982 Jun 11;10(11):3503–3515. doi: 10.1093/nar/10.11.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L., Laird J. E., Pascall J. C., Craig R. K. Guinea-pig casein A cDNA. Nucleotide sequence analysis and comparison of the deduced protein sequence with that of bovine alpha s2 casein. Eur J Biochem. 1984 Feb 1;138(3):585–589. doi: 10.1111/j.1432-1033.1984.tb07954.x. [DOI] [PubMed] [Google Scholar]
- Hennighausen L. G., Steudle A., Sippel A. E. Nucleotide sequence of cloned cDNA coding for mouse epsilon casein. Eur J Biochem. 1982 Sep 1;126(3):569–572. doi: 10.1111/j.1432-1033.1982.tb06818.x. [DOI] [PubMed] [Google Scholar]
- Hobbs A. A., Rosen J. M. Sequence of rat alpha- and gamma-casein mRNAs: evolutionary comparison of the calcium-dependent rat casein multigene family. Nucleic Acids Res. 1982 Dec 20;10(24):8079–8098. doi: 10.1093/nar/10.24.8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes T. H., King J. L. Evolutionary nucleotide replacements in DNA. Nature. 1979 Oct 18;281(5732):605–606. doi: 10.1038/281605a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Brignon G., Ribadeau-Dumas B. Structure primaire de la caséine kappa B bovine. Séquence complète. Eur J Biochem. 1973 Jun;35(2):222–235. doi: 10.1111/j.1432-1033.1973.tb02829.x. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Gaye P. Study of secretory lactoproteins: primary structures of the signals and enzymatic processing. Ann N Y Acad Sci. 1980;343:232–251. doi: 10.1111/j.1749-6632.1980.tb47255.x. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Grosclaude F., Ribadeau-Dumas B. Structure primaire de la caséine s1 -bovine. Séquence complète. Eur J Biochem. 1971 Nov 11;23(1):41–51. doi: 10.1111/j.1432-1033.1971.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Haze G., Gaye P., Hue D. Amino terminal sequence of the precursor of ovine beta-lactoglobulin. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1236–1245. doi: 10.1016/0006-291x(78)90320-0. [DOI] [PubMed] [Google Scholar]
- Mercier J. C. Phosphorylation of caseins, present evidence for an amino acid triplet code posttranslationally recognized by specific kinases. Biochimie. 1981 Jan;63(1):1–17. doi: 10.1016/s0300-9084(81)80141-1. [DOI] [PubMed] [Google Scholar]
- Miyata T., Yasunaga T., Nishida T. Nucleotide sequence divergence and functional constraint in mRNA evolution. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7328–7332. doi: 10.1073/pnas.77.12.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. The complete sequence of a chromosomal mouse alpha--globin gene reveals elements conserved throughout vertebrate evolution. Cell. 1979 Nov;18(3):875–882. doi: 10.1016/0092-8674(79)90139-9. [DOI] [PubMed] [Google Scholar]
- Ordahl C. P., Cooper T. A. Strong homology in promoter and 3'-untranslated regions of chick and rat alpha-actin genes. Nature. 1983 May 26;303(5915):348–349. doi: 10.1038/303348a0. [DOI] [PubMed] [Google Scholar]
- Pascall J. C., Boulton A. P., Parker D., Hall L., Craig R. K. Heterogeneity of guinea-pig caseins synthesized and sequestered by cell-free protein-synthesizing systems. Biochem J. 1981 May 15;196(2):567–574. doi: 10.1042/bj1960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. Sequence at the 3' end of globin mRNA shows homology with immunoglobulin light chain mRNA. Nature. 1974 Nov 29;252(5482):359–362. doi: 10.1038/252359a0. [DOI] [PubMed] [Google Scholar]
- Ribadeau Dumas B., Brignon G., Grosclaude F., Mercier J. C. Structure primaire de la caséine beta bovine. Séquence complète. Eur J Biochem. 1972 Feb;25(3):505–514. doi: 10.1111/j.1432-1033.1972.tb01722.x. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis I. M., Stewart A. F., Caputo A., Thompson A. R., Mackinlay A. G. Construction and identification by partial nucleotide sequence analysis of bovine casein and beta-lactoglobulin cDNA clones. DNA. 1982;1(4):375–386. doi: 10.1089/dna.1982.1.375. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]



