Abstract
A 72-year-old man with cirrhosis had undiagnosed hepatocellular carcinoma with distant metastasis occupying nearly the entire right atrium. He was a poor surgical candidate because of his bleeding risks and advanced liver cirrhosis. He successfully underwent urgent large-bore aspiration thrombectomy under simultaneous echocardiography and fluoroscopy, thus leading to a diagnosis of metastatic malignant disease.
Key Words: FlowTriever, Inari, intracardiac, large-bore aspiration thrombectomy, mechanical thrombectomy, right atrium, tumor thrombus
Graphical Abstract
History of Presentation
A 72-year-old man presented to the emergency department with a 3-day history of progressive shortness of breath. On arrival, the patient was normotensive but hypoxic with an oxygen saturation SpO2 of 70% on room air. Relevant laboratory values included the following: lactic acid, >11.60 mmol/L; creatinine, 6.17 mg/dL; platelets, 48 μL; albumin, 2.4 g/dL; international normalized ratio, 2.1; total bilirubin, 2.1 mg/dL; and B-type natriuretic peptide, 346 pg/mL. Serial troponin results were negative. The electrocardiogram was unremarkable. Computed tomography angiography (CTA) of the chest showed a 6.6 cm × 5.9 cm × 4.2 cm filling defect in the right atrium without extension into the pulmonary artery (Figure 1). Review of systems and physical examination were otherwise negative.
Learning Objectives
-
•
To use LBAT for novel applications beyond bland thrombus.
-
•
To consider tumor metastasis in the differential diagnosis of a right atrial mass.
Figure 1.
Coronal Computed Tomography Angiography
The image demonstrates a right atrial filling 6.6 cm × 5.9 cm × 4.2 cm defect (green arrow) without extension into the pulmonary artery.
Past Medical History
The patient had a history of liver cirrhosis, end-stage renal disease, and previous cauterization of a cecal arteriovenous malformation for a recent episode of gastrointestinal bleeding.
Differential Diagnosis
Differential considerations included a bland thrombus in transit and a primary cardiac mass. Metastatic tumor thrombus was not a consideration.
Investigations
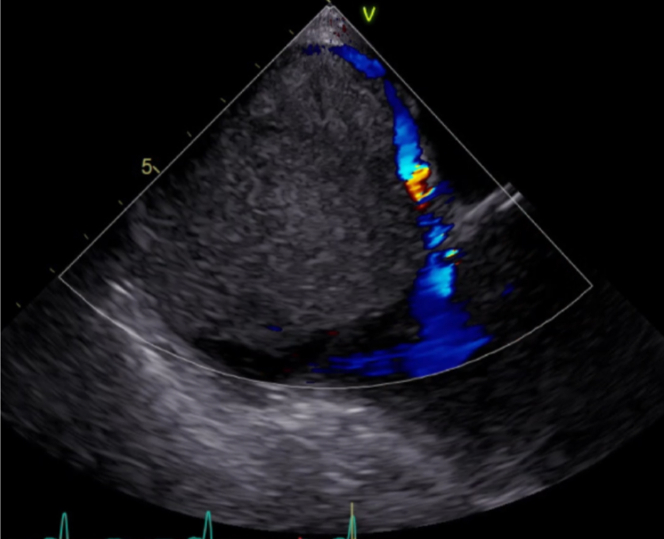
The abnormal finding on chest CTA prompted further imaging assessment with abdomen/pelvis CTA, which showed a 4.0 cm × 3.9 cm hypervascular lesion in the anterior segment of the right hepatic lobe on a background of liver cirrhosis concerning for hepatocellular carcinoma (HCC). There was no direct extension of this lesion into the inferior vena cava or right atrium. Evaluation with a transesophageal echocardiogram (TEE) showed a solid, nonmobile echogenic mass adhered to the posterior wall of a dilated right atrium and occupying almost 95% of the right atrial cavity with circumferential flow (Figure 2). The right ventricular size and function were normal, and the left ventricular ejection fraction (LVEF) was 60%.
Figure 2.
Pretreatment Transesophageal Echocardiography
The image shows the large, 6.6 cm × 5.9 cm × 4.2 cm solid, nonmobile mass with circumferential color Doppler flow.
Management
The patient was admitted to the intensive care unit (ICU) with a heparin infusion. The uncertain origin of the intra-atrial mass and the potential bleeding risks precluded the use of intravenous thrombolytic agents.
Cardiothoracic surgery was consulted on the same day for surgical embolectomy, but the surgeons considered the patient to be a nonsurgical candidate because of his increased bleeding risk and advanced liver and renal disease.
Given the high mortality risk from cardiac decompensation and/or pulmonary embolism (PE), the care team decided to perform an urgent large-bore aspiration thrombectomy (LBAT) using the 24-F FlowTriever thrombectomy system (Inari Medical) with simultaneous TEE for intra-atrial guidance. General endotracheal anesthesia (GETA) was used despite its potential downfalls (eg, circulatory collapse) in this situation, because the procedure was performed with a large-bore TEE and had an unknown lengthy procedural time.
This exclusively endovascular procedure was formulated after an extensive discussion with the patient and his family, including an explanation of the high-risk nature of his condition and the treatment plan, the risks of which included massive PE, stroke given inconclusive findings for possible patent foramen ovale, and death. Informed consent was obtained.
On the following day, the patient underwent GETA with intraprocedural TEE and a simultaneous heparin infusion.
Percutaneous access was gained through the right common femoral vein. A 24-F Intri sheath (Inari Medical) was inserted, and a caval venogram was performed, showing sluggish cephalad venous flow resulting from the right atrial obstructing mass. A soft angled Glidewire (Terumo) was used to cross the mass into the right internal jugular vein as a “safety buddy wire,” and subsequently an angled glide catheter was passed over a 0.035-inch exchange Advantage wire (Terumo) into the right atrium. Over the wire and into the sheath, a 24-F FlowTriever aspiration catheter was advanced into the right atrium (Video 1). Multiple attempts were made to aspirate the clot/mass but were unsuccessful. Therefore, the Advantage Glidewire floppy tip was used to probe gently into the mass and create microchannels to render the mass more amenable to aspiration.
Repeat aspiration thrombectomy was then performed with satisfactory extraction. A total of 25 aspirations were made, and extensive mass was removed; samples were sent for pathologic examination (Figure 3). Two liters of aspirated blood were filtered through 4 FlowSaver blood return filters (Inari Medical) and readministered into the patient’s circulation; the total blood loss was approximately 600 mL. The endovascular debulking was successful, with approximately 90% of the original mass successfully extracted by visual estimation (Figure 4, Videos 2 and 3). The residual 10% was adherent to the wall of the right atrium and thus was not easily retrievable. Post-thrombectomy right ventricular tricuspid annular plane systolic excursion was adequate. There were no procedural complications, and hemostasis was achieved with a figure-8 2-0 silk suture.
Figure 3.
Samples of the Extracted Right Atrial Mass
Figure 4.
Postprocedural Transesophageal Echocardiography
The image shows a marked decrease (∼90%) in the original right atrial mass size.
A follow-up echocardiogram performed the next day demonstrated a normal-sized right atrium with a significant reduction in mass burden and an LVEF of 65%. The pathology report confirmed a diagnosis of HCC metastasis.
Outcome and Follow-Up
The patient was extubated in the angiography suite and taken to the ICU for 24 hours of observation. He was subsequently transferred to the medical-surgical floor the following day on room air. His postprocedural hospital stay was uneventful, and he was discharged home on day 5 with a follow-up appointment with oncology for management of his metastatic HCC. Follow-up unenhanced CT of the chest performed 18 days later showed no gross heart or pulmonary arterial dilatation.
Discussion
LBAT has become an established technique for removal of acute or chronic bland thrombi from the pulmonary arteries, lower extremity veins, and other vascular beds.1, 2, 3
Although intracardiac LBAT has been previously described in emergency cases, our case demonstrates LBAT utility in an urgent, exclusively endovascular approach to debulking metastatic tumor thrombus.4,5 We were able to extract/debulk a large malignant tumor thrombus from the right atrium that could have led to the patient’s death. This unconventional application of an existing technology provided immediate clinical improvement and upgraded the patient’s prognosis from poor to stable. In addition, LBAT established the diagnosis of advanced/metastatic HCC instead of the previously assumed bland thrombus in transit or primary atrial tumor, thereby altering the patient’s oncologic treatment plan.
In the event of intraprocedural embolization into the pulmonary arterial circulation, we had the proper equipment and position to aspirate the emboli;4, 5, 6 however, no clinically significant embolization occurred during this procedure. Pulmonary microemboli occur in up to 26% of patients with solid malignant tumors and are not of hemodynamic significance.7,8
Despite a wide array of options that are available to treat bland thrombus, options are often limited when it comes to tumor thrombus. In our case, systemic or catheter-directed thrombolysis would have been without benefit given the tumor tissue composition, and it would have potentially increased bleeding risks.
Surgical embolectomy remains an option but was not feasible in our patient because of his high mortality and morbidity risks. Furthermore, surgical embolectomy necessitates extracorporeal membrane oxygenation infrastructure, which is not available in most hospitals.
LBAT for tumor thrombus is a feasible procedure that can be viewed as the endovascular counterpart to surgical excision or resection in a variety of conditions. Possible areas of investigation for its use as an adjunct tool could include endovascular aspiration for retrieval of misplaced coils or foreign bodies, evaluation of suspicious endoluminal lesions for diagnosis and cancer staging, debridement of pancreatic pseudocysts and necrotic tissue, and evacuation of cholelithiasis or nephrolithiasis.
Conclusions
Although a favorable outcome was achieved in our patient, we acknowledge the limitations of a single case report. Nevertheless, this case highlights that expanding the scope of this minimally invasive endovascular technique for new clinical applications is feasible.
By thinking outside of the box, we can offer catheter aspiration technology to treat a myriad of clinical conditions that have been addressed until now by using outdated algorithms.
Funding Support and Author Disclosures
Drs Ghaleb and Sardar have served as consultants for Inari Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Fluoroscopic Video Loop Showing TEE Probe and 24-F FlowTriever (Inari Medical) Aspiration Catheter Being Advanced Into the Right Atrium
Preprocedural Intraoperative TEE Demonstrating the Size of a Right Atrial Metastasis
Postprocedural Intraoperative TEE Demonstrating Significant Reduction in the Size of a Right Atrial Metastasis
References
- 1.Tu T., Toma C., Tapson V.F., et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc Interv. 2019;12(9):859–869. doi: 10.1016/j.jcin.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenberg M.K.W., Stahlhoff S., Młyńczak K., et al. Endovascular mechanical thrombectomy versus thrombolysis in patients with iliofemoral deep vein thrombosis - a systematic review and meta-analysis. Vasa. 2021;50(1):59–67. doi: 10.1024/0301-1526/a0008753. [DOI] [PubMed] [Google Scholar]
- 3.Milioglou I., Farmakis I., Wazirali M., et al. Percutaneous thrombectomy in patients with intermediate- and high-risk pulmonary embolism and contraindications to thrombolytics: a systematic review and meta-analysis. J Thromb Thrombolysis. 2023;55(2):228–242. doi: 10.1007/s11239-022-02750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gayed A., Stringfellow S., Yamada R., Johnson T., Wright-Void C., Hannegan C. Percutaneous large bore aspiration thrombectomy of tumor embolism causing massive pulmonary embolism. JACC Case Rep. 2022;4(6):359–363. doi: 10.1016/j.jaccas.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pallister Z.S., Montero-Baker M., Mills J.L., Chung J. Percutaneous suction thrombectomy of large tumor thrombus causing massive pulmonary embolism. J Vasc Surg Cases Innov Tech. 2018;4(3):244–247. doi: 10.1016/j.jvscit.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nezami N., Latich I., Murali N., et al. Right atrial and massive pulmonary artery mechanical thrombectomy under echocardiography guidance using the FlowTriever system. EJVES Short Rep. 2019;45:22–25. doi: 10.1016/j.ejvssr.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuji Y., Goto A., Hara I., et al. Renal cell carcinoma with extension of tumor thrombus into the vena cava: surgical strategy and prognosis. J Vasc Surg. 2001;33(4):789–796. doi: 10.1067/mva.2001.111996. [DOI] [PubMed] [Google Scholar]
- 8.Konstantinides S.V., Torbicki A., Agnelli G., et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069k. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluoroscopic Video Loop Showing TEE Probe and 24-F FlowTriever (Inari Medical) Aspiration Catheter Being Advanced Into the Right Atrium
Preprocedural Intraoperative TEE Demonstrating the Size of a Right Atrial Metastasis
Postprocedural Intraoperative TEE Demonstrating Significant Reduction in the Size of a Right Atrial Metastasis