Abstract
Thoracic endovascular aortic repair has emerged as a viable alternative for managing Sanford type B aortic dissection in adults. We report the first case of managing an acute and evolving communicating type B aortic dissection in an infant with endovascular aortic stenting.
Key Words: aortic dissection type B, endovascular stenting, pediatrics, TEVAR
Graphical Abstract
Aortic dissection (AD) is a rare disease, especially in children, that can be associated with life-threatening complications.1 There are no pediatric guidelines regarding the management of complicated AD. Thoracic endovascular aortic repair (TEVAR) is the treatment of choice in adult patients with Sanford type B AD complicated by malperfusion syndromes, including rupture tamponade, mesenteric or renal ischemia, and lower extremity ischemia.2
Take-Home Messages
-
•
Aortic dissection is a rare disease in children.
-
•
Percutaneous endovascular repair can be considered in high-risk scenarios without many alternative therapy options.
The TEVAR approach for AD type B in pediatrics is uncommon, and therefore, there is no gold standard management.3 In 2019, Hasjim et al4 reviewed the national trends in the management of 650 pediatric patients with blunt thoracic aortic injury (BTAI). The findings of these investigators revealed that TEVAR was associated with a shorter hospital length of stay compared with open thoracic aortic repair (OTAR), with no significant difference in mortality risk between the 2 procedures. Moreover, the rate of TEVAR in pediatric BTAI nearly doubled from 2007 to 2015, following the first use of thoracic stent grafts in adults in 2005. The youngest child treated with TEVAR in the review by Hasjim et al4 was 7 years old, and they did not report on the details and classifications of aortic injury. The current literature on the application of TEVAR in pediatric patients remains limited to case reports. As such, the generalizability of these findings may be restricted at present. To our knowledge, this technique has not been reported in infants. We report the use of covered stenting in the management of iatrogenic acute complicated AD type B in an infant patient.
History of Presentation
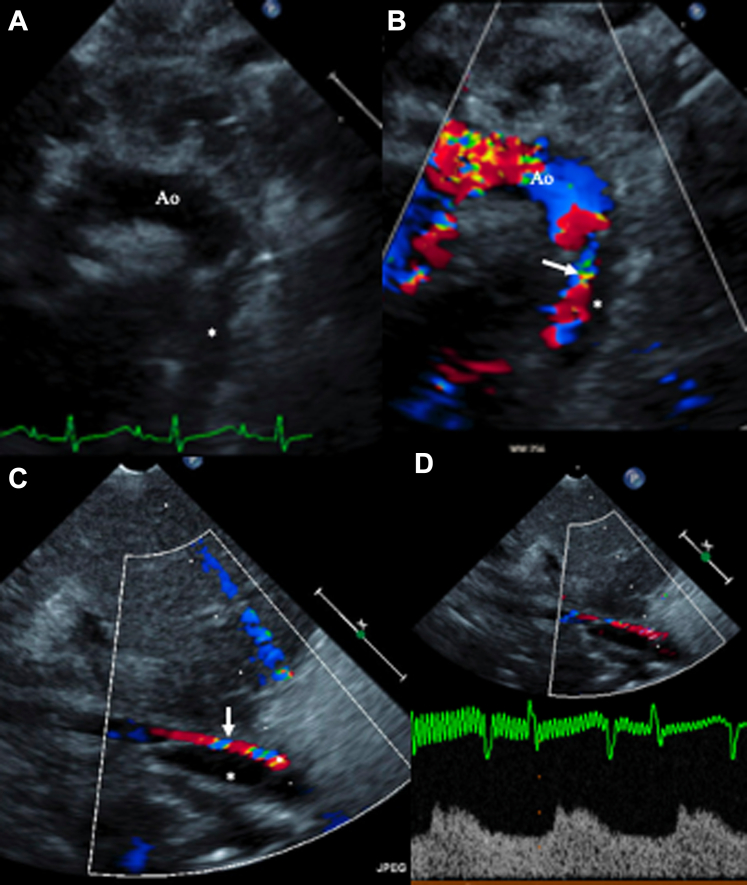
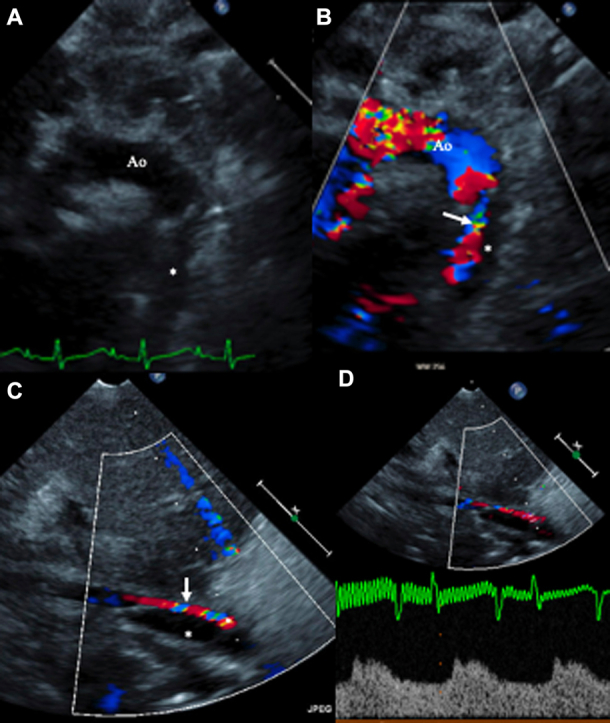
A 2-month-old female infant was admitted for a third redo sternotomy as a result of restenosis of the ascending aorta (AscAo) and right pulmonary artery (RPA). She underwent patching of the RPA and AscAo. During surgery, cannulation was performed in the right atrium (16-F) and transverse arch (8-F) during cardiopulmonary bypass. She tolerated the procedure well without any immediate complications. The next day, she developed hypoperfusion to the lower trunk, manifested by poor urine output and weak peripheral pulses. An echocardiogram confirmed thoracic aortic intimal dissection spanning the entire length of the thoracic aorta and extending into the abdominal aorta (AAo) above the renal arteries (Figures 1A to 1D).
Figure 1.
Echocardiogram at the Time of Diagnosis
(A and B) Suprasternal views demonstrating the dissection of the proximal descending aorta (Ao) distal to the origin of the left subclavian artery with the false lumen (asterisks) compressing the true aortic lumen (arrows), (C and D) Subcostal views demonstrating the false lumen (asterisks) compressing the true aortic lumen (arrows), thus resulting in significantly blunted abdominal aortic flow.
Past Medical History
The patient was born with an anomalous origin of the RPA from the AscAo. She had undergone translocation of the RPA to the main pulmonary artery and end-to-end anastomosis of the AscAo at 9 days of life. She underwent repeat sternotomy for patch augmentation of the AscAo and the aortic arch along with a LeCompte maneuver of the anastomosis of the RPA as a result of severe AscAo stenosis at 11 days of life.
Differential Diagnosis
A low cardiac output state post-bypass and arterial thrombus formation were the main differential diagnoses for our patient.
Investigations
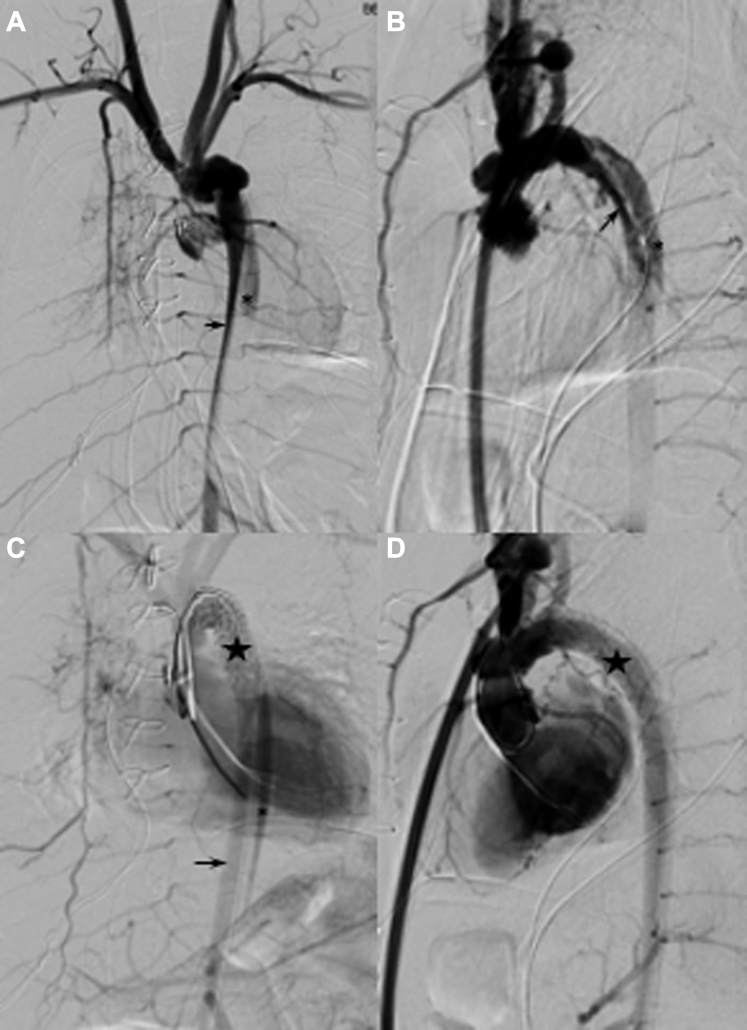
After an echocardiogram confirmed the diagnosis, the patient went to the cardiac catheterization laboratory on an emergency basis. A computed tomography (CT) angiogram was not performed before the intervention because of the critical illness, the presence of renal dysfunction, and the goal of limiting contrast medium dose. Angiography in the catheterization laboratory revealed AD that began proximally at the distal aortic arch right under the left subclavian arteries and extended down to the AAo below the renal arteries (Figures 2A and 2B). According to the anatomic classification of thoracic AD by the Society of Vascular Surgery and The Society of Thoracic Surgeons Reporting Standards published in 2020,5 our patient had a type B3,9 AD.5 The contrast medium was seen flowing sluggishly through the smaller-caliber true lumen that was compressed by the larger-caliber false lumen. There was delayed flow to the celiac trunk and no opacification of the mesenteric arteries.
Figure 2.
Baseline Digital Subtraction Angiogram of the Aorta
(A) Frontal and (B) lateral views demonstrating the extent of dissection with the false lumen (asterisks) compressing the true aortic lumen (arrows). (C and D) Angiographic improvement in the true lumen (arrow in C) after stent placement (stars) to cover the mouth of the dissection.
Management
After multidisciplinary discussion among the pediatric cardiac intensivist, pediatric cardiac and general surgeons, and pediatric cardiologists, it was decided that percutaneous intervention would be the best next approach. Subsequently, the patient was brought on an emergency basis to the catheterization laboratory with surgical backup. After discussion with vascular and cardiovascular surgery teams, the consensus approach was to block the entry point of the dissection into the false lumen by using a covered stent; the goal was to cover the dissection into a noncommunicating type so it could thrombose and prevent further extension. Given the location of the dissection, access was gained through the femoral arterial approach in the hope that this would aid entry into the true lumen. An 8 mm × 29 mm VBX stent (Gore Viabahn VBX, W.L. Gore & Associates) was deployed just distal to the left subclavian artery origin (Figures 2C and 2D). This procedure resulted in successful occlusion of the dissection with a residual small type 1a endoleak, improved filling of the true lumen and perfusion to the mesenteric arteries and decreased filling of the false lumen. Repeat dilation was performed with the same balloon with no resolution of the endoleak. Larger balloon angioplasty was not performed because of the risk of rupture and our inability to extend the stent proximally in view of the risk of covering the subclavian artery origin.
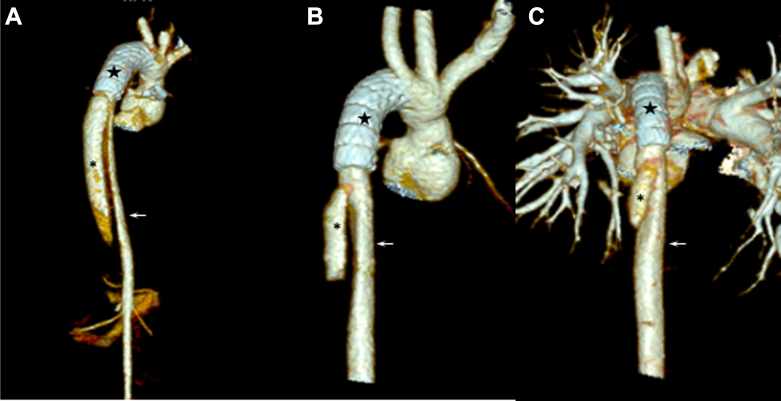
The procedure was complicated by intimal injury and dissection of the right external iliac artery (REIA) during an attempt to remove the 7-F right femoral arterial sheath secondary to arterial spasm. The sheath was finally removed with the use of various vasodilatory techniques (nitroglycerin injections through the sheath, lidocaine infusions, propofol, general anesthesia, and deep paralysis). Angiography of the right iliac artery showed complete REIA occlusion without any contrast extravasation, with reconstitution of distal femoral artery perfusion through collateral vessels arising from the internal iliac artery. At discharge, repeat CT angiography revealed improved true aortic lumen expansion (Figure 3A) and unobstructed perfusion of visceral vessels, including renal and iliac, except known REIA with reconstitution of the right femoral bifurcation.
Figure 3.
A 3-Dimensional Reconstruction of the Aorta on Computed Tomography Angiography After Thoracic Endovascular Aortic Repair
At (A) day 1, (B) 6 months, and (C) 18 months after the stent (stars) procedure, showing continued slow favorable remodeling with thrombosis of the false lumen (asterisks) and increasing dimension of the true lumen (arrows).
Follow-Up
At 10 months of age, the RPA had significant restenosis. The patient therefore underwent a fourth sternotomy with successful patch repair of the RPA. To minimize risks, this repair was accomplished without cardiopulmonary bypass. Repeat CT scans done at 6 and 18 months post-procedure continued to show false lumen thrombosis and favorable remodeling (Figures 3B and 3C). She remained on anticoagulation therapy for 8 months with enoxaparin, at which time an ultrasound scan demonstrated resolution of the REIA thrombus. Serial echocardiograms continue to demonstrate a normal abdominal aortic flow pattern. At the latest follow-up 3 years and 6 months post-procedure, she continues to do well, with unobstructed aortic flow on echocardiography.
Discussion
This is a case of successful percutaneous management of iatrogenic AD type B with a covered stent in an infant. In general, the principles of therapy in acute complicated type B AD are to prevent or treat aortic rupture, prevent retrograde or antegrade propagation of the dissection, and alleviate malperfusion syndrome.2 Endovascular therapies include TEVAR (with or without false lumen embolization) and false lumen fenestration.
In adult patients with uncomplicated acute type B AD, medical management or endovascular stent (EVS) therapy is recommended as initial treatment.2 Indications for EVS therapy as for type B AD include aortic rupture, intractable pain, uncontrolled hypertension, and extension of the dissection, resulting in complications such as organ malperfusion and branch artery occlusion.2 Our patient met the criteria for intervention because of the development of malperfusion syndrome. In addition, the small size of the patient and the 3 recent previous sternotomies added to the complexity of management.
TEVAR/EVS is a relatively new approach, especially in pediatrics; there have been only a few cases where children, adolescents, and young adults with AD type B were managed using this approach.3,6, 7, 8 Most reported cases have been in older children (aged 8-17 years). The benefits and risks of using balloon-expandable covered stents in the treatment of pediatric traumatic aortic injury have been described.9 Balloon-expandable covered stents provide the benefits of their smaller size profile and their ability to be safely redilated to keep up with somatic growth. The stent that we used can be safely dilated to 16 mm before affecting the covering and can be fractured with larger-diameter balloons. Stent therapy comes with inherent limitations of the need for repeat dilation and a limited postdilation potential. In our patient, the 8-mm Gore covered stent can be safely redilated to 16 mm with a future transcatheter approach.
This case report adds to the existing limited data on balloon-expandable covered stenting for the treatment of AD in children. For small children with AD, the percutaneous approach is a feasible alternative to open surgical repair. Follow-up imaging will be required given the patient’s somatic growth to monitor gradient development indicating repeat percutaneous stent dilation.10,11 Long-term follow-up with a special focus on endoleaks is necessary in these patients.
Conclusions
We report a case of successful treatment of type B AD in an infant by using a balloon-expandable covered stent with excellent 3.5-year follow-up. A percutaneous approach may be considered in select high-risk cases with limited alternative treatment options.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Zalzstein E., Hamilton R., Zucker N., et al. Aortic dissection in children and young adults: diagnosis, patients at risk, and outcomes. Cardiol Young. 2003;13(4):341–344. [PubMed] [Google Scholar]
- 2.Isselbacher E.M., Preventza O., Hamilton Black J., III, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;80(24):e223–e393. doi: 10.1016/j.jacc.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afzal M., Abdulreda Najar S., Baghazal H., et al. Endovascular treatment of a traumatic thoracic pseudo-aneurysm in a pediatric patient: a case report with review of literature. J Cardiothorac Surg. 2023;18(1):183. doi: 10.1186/s13019-023-02265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasjim B.J., Grigorian A., Barrios C., et al. National trends of thoracic endovascular aortic repair versus open thoracic aortic repair in pediatric blunt thoracic aortic injury. Ann Vasc Surg. 2019;59:150–157. doi: 10.1016/j.avsg.2018.12.094. [DOI] [PubMed] [Google Scholar]
- 5.Lombardi J.V., Hughes G.C., Appoo J.J., et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg. 2020;71(3):723–747. doi: 10.1016/j.jvs.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Petrov I., Kaneva-Nencheva A., Levunlieva E., et al. Successful endovascular treatment of type B aortic dissection in a 15-year-old child. Cor Vasa. 2017;59(2):e163–e170. doi: 10.1016/j.crvasa.2016.04.006. [DOI] [Google Scholar]
- 7.Kazimierczak A., Rynio P., Gutowski P., et al. Endovascular stenting of a complicated type B aortic dissection in an 11-year-old patient: case report. Medicine (Baltimore) 2018;97(14) doi: 10.1097/md.0000000000010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulsen F., Cantasdemir M., Ozluk E., et al. Endovascular stent-graft placement for ruptured dissecting aortic aneurysm in an adolescent patient with systemic lupus erythematosus: case report. Emerg Radiol. 2011;18(6):499–502. doi: 10.1007/s10140-011-0978-z. [DOI] [PubMed] [Google Scholar]
- 9.Hiremath G., Morgan G., Kenny D., et al. Balloon expandable covered stents as primary therapy for hemodynamically stable traumatic aortic injuries in children. Catheter Cardiovasc Interv. 2020;95(3):477–483. doi: 10.1002/ccd.28575. [DOI] [PubMed] [Google Scholar]
- 10.Blais B., Carr K., Sinha S.P., et al. Mechanical properties of low-diameter balloon expandable covered stents. Catheter Cardiovasc Interv. 2021;97(3):451–458. doi: 10.1002/ccd.29421. [DOI] [PubMed] [Google Scholar]
- 11.Mejia E.M., Kish C.E.R., Bocks L.M.M., et al. Stretched to the limit: comparing polytetrafluoroethylene-covered endovascular stents through serial dilations. J Soc Cardiovasc Angiogr Interv. 2022;1(2) doi: 10.1016/j.jscai.2022.100035. [DOI] [PMC free article] [PubMed] [Google Scholar]