Abstract
An unguarded mitral valve orifice is a rare condition characterized by a thinned, hypocontractile left ventricle on fetal echocardiogram. This is the first report of an unguarded mitral valve orifice with a double-outlet right ventricle and intact ventricular septum diagnosed prenatally from these typical ultrasound features.
Key Words: fetal echocardiogram, prenatal diagnosis, thinned left ventricle, unguarded mitral valve orifice
Graphical Abstract
A 31-year-old woman, gravida 2, para 0, naturally conceived monochorionic diamniotic twins. She was referred to our hospital at 29 weeks of gestation with a diagnosis of heterotaxy and a double-outlet right ventricle (DORV) in 1 of the twins.
Learning Objectives
-
•
To recognize the ultrasound features of the unguarded MV orifice during the fetal stage.
-
•
To better understand the cardiac embryology and fetal circulation with the spectrum of MV abnormalities.
Past Medical History
Her past medical history was noncontributory.
Differential Diagnosis
The differential diagnosis included unguarded mitral valve (MV) orifice or Ebstein’s disease of the MV.
Investigations
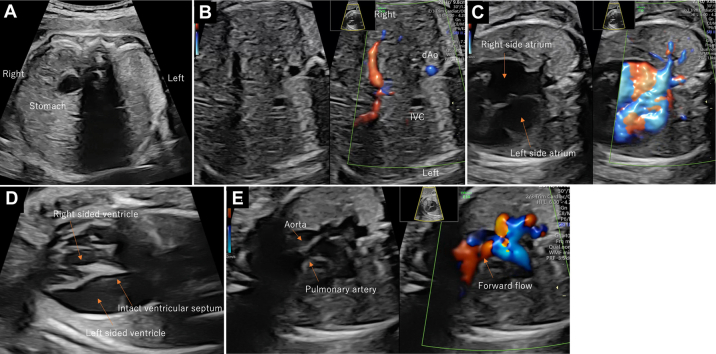
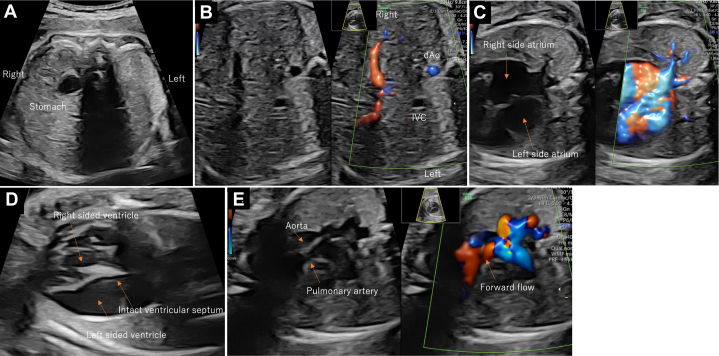
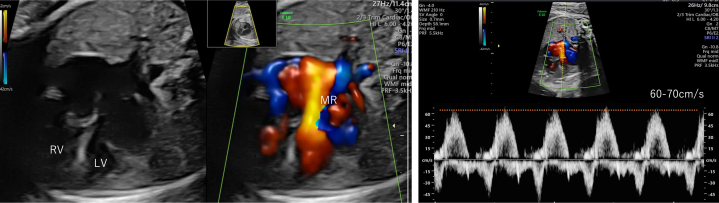
On the fetal echocardiogram, the stomach was on the right, and the cardiac axis showed a leftward shift, indicating heterotaxy (Figure 1A). The fetal inferior vena cava directly drained into the left-sided atrium and the pulmonary veins drained into the right-sided atrium (Figures 1B and 1C). On the 4-chamber view (Video 1), thinning of the left ventricle located on the left was prominent, and the papillary muscles could not be identified (Figure 1D). The noticeably thin left ventricle mimicked the presence of a third atrium. MV regurgitation was present, with a low-pressure gradient of 60 to 70 cm/s (Figure 2, Video 2). Both great arteries originated from the right ventricle, with the aorta arising anteriorly (Figure 1E). Ventricular septal defects (VSD) were not detected. The aorta was 6.9 mm (Z score 2.8), and the pulmonary artery was 2.9 mm (Z score −5.9) with forward flow; the reversed flow in the ductus arteriosus indicated severe pulmonary valve stenosis. The retrograde flow from the ductus arteriosus continued toward the right pulmonary artery. The left pulmonary artery originated from the main pulmonary trunk. No signs of fetal hydrops or extracardiac anomalies were detected, and fetal growth was normal. On the basis of these features, the fetal diagnosis was heterotaxy syndrome with an unguarded MV orifice, DORV without VSD, severe pulmonary stenosis, and a right-sided aortic arch. The cardiac function of the fetus remained stable until delivery.
Figure 1.
Fetal Echocardiogram at 24 Weeks of Gestation
Abdominal situs shows heterotaxy (A, B), pulmonary veins draining into the right-sided atrium (C), 4-chamber view with thinning left ventricle (D), and relationship of great arteries (E).
Figure 2.
4-Chamber View With Mitral Regurgitation (Video 2)
Mitral regurgitation at a low pressure of 60-70 cm/s.
Management: Medical Interventions
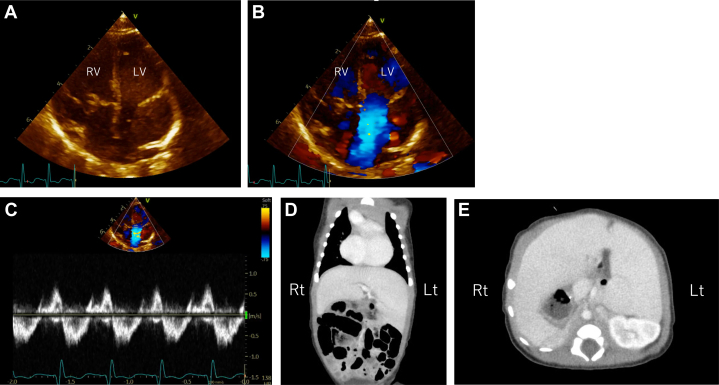
At 37+0 weeks of gestation, 2 female neonates were born by elective cesarean section; the healthy neonate weighing 2,678 g with an Apgar score of 9/10 points (1-minute/5-minutes, respectively) and the other with an unguarded MV weighing 2,565 g with Apgar scores of 7/9 points (1-minute/5-minutes, respectively). Echocardiography after birth confirmed the absence of the MV leaflets, chordae tendineae, and papillary muscles (Figures 3A to 3C). Additional findings included pulmonary valve atresia and stenosis of the right pulmonary artery fed by a patent ductus arteriosus. Postnatal computed tomography revealed left isomerism (Figures 3D and 3E), and postnatal enhanced computed tomography revealed clear visualization of the arterial relationships (Figure 4). Prostaglandins were administered after birth because pulmonary blood flow depended on the ductus arteriosus. At 28 days of age, a left Blalock-Taussig shunt was placed, and the patent ductus arteriosus was clipped. Intraoperatively, the thinning left ventricle dilatated, and ventricular tachycardia occurred. Extracorporeal membrane oxygenation support was administered to stabilize the circulation for 3 days, then withdrawn on the 13th postoperative day. The affected infant was discharged at 3 months of age. After discharge, she gained weight, with no worsening of cardiomegaly or arrhythmia. At 11 months of age, she underwent bidirectional Glenn surgery with mitral valve closure. She is currently 15 months old, weighs 8,700 g with an approximate cardiothoracic ratio of 60%, has only mild tricuspid regurgitation, and does not require oxygenation at home. Surgery aiming for total cavopulmonary bypass connection has been scheduled for when she is approximately 2 years of age.
Figure 3.
Postnatal Ultrasound Findings and Abdominal Computed Tomography
Ultrasound confirms absence of mitral valve leaflets, chordae tendineae, and papillary muscles (A). Mitral valve regurgitation with color Doppler (B) reveals low pressure (C). Computed tomography reveals polysplenia (D, E).
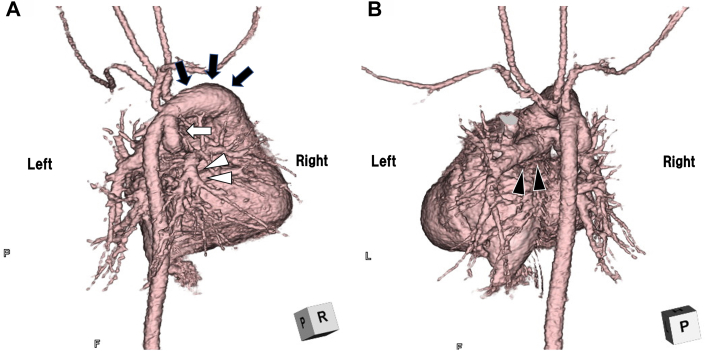
Figure 4.
Postnatal-Enhanced Computed Tomography of Heart
Images show the aorta arising from anterior to main pulmonary artery (dark arrows), right pulmonary artery (white arrowheads), continuing to ductus arteriosus (white arrow) (A), and left pulmonary artery (dark arrowheads) arises from the main pulmonary trunk (B).
Discussion
An unguarded MV orifice is rarely reported, as shown in Table 1.1, 2, 3, 4, 5, 6, 7, 8 This is the first patient to receive a diagnosis prenatally and to survive infancy. Prenatal diagnosis and appropriate postnatal treatment contributed to her survival.
Table 1.
Reports About Fetal and Neonatal Unguarded Mitral Valve Orifice
| Case | Year | Sex | Findings (Except for Severe MR) | Prenatal Diagnosis | Treatment | Clinical Course |
|---|---|---|---|---|---|---|
| Yasukochi-11 | 1999 | Male | DORV, PS, ASD, dilated LV, pinhole-shaped VSD, heterotaxy | No | Blalock-Taussig shunt at 15 months old | Died at 17 years old due to CHF and arrhythmia |
| Yasukochi-21 | 1999 | Male | DORV, PA, ASD, small VSD, small LV, heterotaxy | No | Blalock-Taussig shunt at day 28, coronary resection at 5 months old (bowel perforation) | Died at 13 months old due to CHF and VF |
| Earing2 | 2003 | Male | DORV, PA, ASD, thin and poor contractive LV, heterotaxy | No | Mee operation (aAo-mPA anastomosis) at day 7, Glenn shunt at 6 months old | Scheduled for Fontan |
| Hwang3 | 2010 | Male | DORV, PA, ASD, thin and dilated LV, heterotaxy | No | Blalock-Taussig shunt at day 33, CHF at 2.5 months old | Scheduled for Fontan |
| Su4 | 2015 | Male | HLHS, AA | No | NA | Neonatal period survival |
| Kishi5 | 2016 | Male | DORV, PS, TV dysplastic, heterotaxy | No | NA | Died at day 2 due to heart failure |
| Banerji6 | 2019 | NA | DORV, PA, small VSD, normal atrial arrangement, heterotaxy | No | Palliative care | NA |
| Subramanian7 | 2019 | Male | DORV, IAA, ASD, hypoplastic LV, heterotaxy | No | Palliative care | NA |
| Howley8 | 2021 | Male | Normal AV, VA connection, dilatation LV, AA, arrhythmia in fetal period (AF) | Yes | ECMO, cardioversion | Died at day 26 due to bradycardia and hypotension |
| This case | 2022 | Female | DORV, PS, ASD, thin and dilated LV, heterotaxy | Yes | Blalock-Taussig shunt at day 28, bidirectional Glenn surgery with mitral valve closure at 11 months old | Ongoing (15 months), scheduled for total cavopulmonary bypass connection |
Summary of the ultrasound findings, treatment, and course of 9 previously reported cases and this case.
AA = aortic atresia; aAo = ascending aorta; AF = atrial flutter; ASD = atrial septal defect; CHF = chronic heart failure; ECMO = extracorporeal membrane oxygenation; HLHS = hypoplastic left heart syndrome; IAA = interrupted aortic arch; LV = left ventricle; mPA = main pulmonary artery; MR = mitral regurgitation; NA = not applicable; PA = pulmonary atresia; PS = pulmonary stenosis; RAI = right atrial isomerism; TR = tricuspid regurgitation; TV = tricuspid valve; VF = ventricular fibrillation; VSD = ventricular septal defect.
The following 2 points are reported in this case: prenatal diagnosis with fetal echocardiogram and the anatomical features of this rare condition, and consideration of the complicated structural changes affecting fetal and neonatal circulation in this condition.
First, the impression of the thinning left ventricle on the fetal echocardiogram mimicked a third atrium. This ventricular wall thinning is vital to diagnosing a congenital unguarded MV orifice. The normal ventricular formation is undermined and forms tethering cords.9 Normal development of the MV leads to the usual smooth ventricular septum and apical trabeculation.10 Hence, we believe that the unguarded MV orifice led to the thin ventricular wall of the left ventricle, one of the key features in diagnosing this disease with fetal echocardiography.
Second, focusing on the relationship between the systemic chamber and outflow tract, 7 previous cases were complicated by DORV and had ventriculoarterial discordance. Both patients in the 2 normal VA concordance cases4,8 had a hypoplastic aortic arch. DORV is commonly associated with VSD; however, DORV without VSD is often observed in cases of unguarded MV orifices. Three of the 7 patients with DORV had small VSDs, whereas the remaining 4 were without VSD, leading to the consideration that survival would not be possible with a large VSD to maintain fetal circulation. According to past reports, some cases resulted in heart failure even though the patients received systemic to pulmonary artery shunt surgery in the neonatal period,1,3,5 which suggests that the enlarged left ventricle may inhibit right ventricular movement, as in Ebstein disease. Arrhythmia is another poor prognostic factor, possibly secondary to a dilatated ventricle.1,8 Preserving right ventricular function and preventing arrhythmia are crucial.
Follow-Up
When the infant was 11 months old, bidirectional Glenn surgery with mitral valve closure was performed, and her cardiac function became stable. Surgery aiming for total cavopulmonary bypass connection has been scheduled for when she is approximately 2 years old.
Conclusions
Thinning of the left ventricle with low-pressure mitral regurgitation is a feature in diagnosis.
Similar to the Starnes procedure involving tricuspid valve closure and right ventricular plication in Ebstein disease, mitral valve closure may be a key for survival to prevent heart failure and arrhythmias.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the patient and her family for their participation in this study. They also thank Editage (https://www.editage.jp) for editing a draft of this manuscript.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
4-Chamber View
Thinning of the left ventricle was prominent, and the papillary muscles could not be identified.
4-Chamber View With Color Doppler
Mitral valve regurgitation was present.
References
- 1.Yasukochi S., Satomi G., Park I., Ando M., Momma K. Unguarded mitral orifice, mirror-imaged atrial arrangement, and discordant atrioventricular connections. Cardiol Young. 1999;9(5):478–483. doi: 10.1017/s1047951100005382. [DOI] [PubMed] [Google Scholar]
- 2.Earing M.G., Edwards W.D., Puga F.J., Cabalka A.K. Unguarded mitral orifice associated with discordant atrioventricular connection, double-outlet right ventricle, and pulmonary atresia. Pediatr Cardiol. 2003;24(5):490–492. doi: 10.1007/s00246-002-0389-8. [DOI] [PubMed] [Google Scholar]
- 3.Hwang M.S., Chang Y.S., Chu J.J., Lin W.S., Su W.J. A potential new constellation of defects: unguarded mitral orifice associated with double-outlet right ventricle {I, D, D} and pulmonary atresia/stenosis. Int J Cardiol. 2011;148(3):354–357. doi: 10.1016/j.ijcar.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Su J.A., Ho J., Wong P.C. Unguarded mitral orifice associated with hypoplastic left heart syndrome. Cardiol Young. 2015;25(5):1002–1005. doi: 10.1017/S1047951114001334. [DOI] [PubMed] [Google Scholar]
- 5.Kishi K., Katayama H., Ozaki N., et al. Fatal cardiac anomaly of unguarded mitral orifice with asplenia syndrome. J Cardiol Cases. 2017;15(1):6–9. doi: 10.1016/j.jccase.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerji N., Krishna M., Kumar R., Anderson R. Caught-off guard: unguarded mitral valve orifice in usual atrial arrangement with discordant atrioventricular connections and pulmonary atresia. Ann Pediatr Cardiol. 2020;13(1):84–86. doi: 10.4103/apc.APC_4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramanian A., Bharath A.P., Jayaranganath M. Unguarded left atrioventricular orifice: an unusual cause of hypoplastic left ventricle and double-outlet right ventricle with intact ventricular septum. Ann Pediatr Cardiol. 2019;12(2):153–155. doi: 10.4103/apc.APC_124_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howley L.W., Strasburger J., Maleszewski J.J., et al. Fetal unguarded mitral valve orifice, aortic atresia, and severe left heart enlargement. JACC Case Rep. 2021;3(2):206–211. doi: 10.1016/j.jaccas.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdulla R., Blew G.A., Holterman M.J. Cardiovascular embryology. Pediatr Cardiol. 2004;25(3):191–200. doi: 10.1007/s00246-003-0585-1. [DOI] [PubMed] [Google Scholar]
- 10.Anderson R.H. 3rd ed. Churchill Livingstone; 2010. 2009, Paediatric Cardiology; pp. 22–26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
4-Chamber View
Thinning of the left ventricle was prominent, and the papillary muscles could not be identified.
4-Chamber View With Color Doppler
Mitral valve regurgitation was present.