Abstract
We have previously observed that the synthetic immunomodulator Murabutide inhibits human immunodeficiency virus type 1 (HIV-1) replication at multiple levels in macrophages and dendritic cells. The present study was designed to profile the activity of Murabutide on CD8-depleted phytohemagglutinin-activated lymphocytes from HIV-1-infected subjects and on the outcome of HIV-1 infection in severe combined immunodeficiency mice reconstituted with human peripheral blood leukocytes (hu-PBL-SCID mice). Maintaining cultures of CD8-depleted blasts from 36 patients in the presence of Murabutide produced dramatically reduced levels of viral p24 protein in the supernatants. This activity correlated with reduced viral transcripts and proviral DNA, was evident in cultures harboring R5, X4-R5, or X4 HIV-1 isolates, was not linked to inhibition of cellular DNA synthesis, and did not correlate with β-chemokine release. Moreover, c-myc mRNA expression was down-regulated in Murabutide-treated cells, suggesting potential interference of the immunomodulator with the nuclear transport of viral preintegration complexes. On the other hand, daily treatment of HIV-1-infected hu-PBL-SCID mice with Murabutide significantly reduced the viral loads in plasma and the proviral DNA content in human peritoneal cells. These results are the first to demonstrate that a clinically acceptable synthetic immunomodulator with an ability to enhance the host's nonspecific immune defense mechanisms against infections can directly regulate cellular factors in infected lymphocytes, leading to controlled HIV-1 replication.
Recent developments in antiretroviral therapy have led to substantial advances in the management of human immunodeficiency virus type 1 (HIV-1)-infected patients. However, it is becoming evident that potent antiretrovirals do not seem to be sufficiently efficient either in targeting the pool of cells that supports low levels of virus replication (44) or in eliminating latently infected T cells (50). Additional strategies including immune-based interventions are now believed to be essential for the long-term control and eradication of HIV infection (44, 60). Recently, structured treatment interruptions have been suggested as a means of enhancing immune control of the virus after early treatment of acute infection (54). Nevertheless, the efficacy of this approach in producing clinical benefit and in restoring durable virus-specific T-cell responses in chronically infected patients has not been observed (10, 24). Furthermore, viral rebound after cessation of therapy has revealed the existence of HIV reservoirs, other than latently infected CD4+ T cells, that prompt the rapid emergence of plasma viremia (15). Taken together, these recent findings underscore the necessity to develop new strategies aimed at controlling virus replication in multiple populations of reservoir cells.
Based on the considerable immune dysfunction caused by HIV-1 infection in cells implicated in innate and acquired immunity (37, 58, 75), it is rather obvious that immunotherapeutic strategies should address both compartments of the immune system. The use of a variety of HIV-specific and nonspecific immunotherapeutic agents has been aimed to enhance the control of HIV-1 replication in different cell populations, to restore the functionality of antigen-presenting cells (APCs), to increase CD4+ cell counts and lymphocyte responses, and to potentiate immune mechanisms known to contribute to a higher resistance against infections (16, 20, 33, 42, 45, 56). Today, limited success has been achieved and the link between immunological effects and clinical benefit has yet to be demonstrated. In an effort to identify clinically acceptable immunomodulators with potential application in the immunotherapy of HIV disease, we have previously evaluated the capacity of Murabutide, a safe synthetic muramyl dipeptide (MDP) derivative, to activate APCs and to suppress viral replication (17). Independent of its ability to induce HIV-suppressive β-chemokines and in the absence of a direct effect on viral enzymes, this immunomodulator was found to regulate cellular pathways in macrophages and in dendritic cells, leading to potent inhibition of proviral DNA integration and of virus transcription. Unlike most other exogenous immunomodulators, Murabutide is apyrogenic, does not induce inflammatory responses, and is well tolerated by humans (5, 13, 68, 76). Furthermore, this molecule maintains a capacity to enhance resistance against viral infections and to potentiate the antiviral and antitumor activities of therapeutic cytokines (6, 7, 14). The clinical tolerance and the immunopharmacological profile of Murabutide may justify its clinical evaluation for the nonspecific immunotherapy of HIV disease. However, prior to seriously considering the use of this immunomodulator in HIV-infected subjects, the potential effects on CD4 lymphocyte activation and on virus replication in this cell population need to be addressed. Moreover, it would be highly reassuring to observe a virus-inhibiting activity in vitro translated into a therapeutic effect in an in vivo model.
The biological effects of immunomodulators of bacterial origin, including muramyl peptides, peptidoglycans, and lipopolysaccharide, have been associated mostly with the activation of APCs and the induction of cytokine and chemokine release (4, 32, 71). However, it is becoming evident that some of these compounds may act on other cell types, including lymphocytes (38, 69), and can regulate the expression of multiple cellular genes other than those of cytokines (67, 71). To address whether Murabutide could target HIV-1-infected lymphocytes, we first evaluated its activity, in vitro, on CD8-depleted phytohemagglutinin (PHA)-activated lymphocytes from HIV-1 patients and then profiled the in vivo effects of Murabutide on viral replication in HIV-infected severe combined immunodeficiency mice reconstituted with human peripheral blood leukocytes (hu-PBL-SCID mice). We demonstrate, in both tested models, a potent virus-suppressive activity of the immunomodulator in lymphocytes. Moreover, we provide data to show that this activity is equally evident on R5 and X4 virus isolates, is targeting proviral DNA integration and virus transcription, and is devoid of inhibitory effects on cellular proliferation. Finally, our findings correlate the virus-suppressive effects of Murabutide with a capacity to regulate the expression of cellular genes, including c-Myc, that are necessary for the completion of different steps in the virus life cycle.
MATERIALS AND METHODS
Patients.
Thirty-six HIV-1-seropositive adults attending the Infectious Diseases clinic at Hôpital Dron in Tourcoing, France, were investigated. There were 11 asymptomatics, 18 symptomatics with no AIDS-defining events, and 7 AIDS patients. All subjects were receiving treatment either with two reverse transcriptase (RT) inhibitors (13 patients) or with a three-drug regimen including 1 protease inhibitor (23 patients). At the time of blood sampling, patients had a mean CD4+ count of 607 cells/mm3 and a median plasma viral load of 2,103 copies/ml. Moreover, plasma viral loads below the detection limit of 200 copies/ml were observed for three asymptomatics, three symptomatics, and one AIDS patient. The study and blood sampling were approved by the local ethics committee (Lille, France).
Reagents.
Murabutide (N-acetyl-muramyl-l-alanyl-d-glutamine-n-butyl ester) was provided by ISTAC S.A. (Lille, France) and was prepared as described elsewhere (13). The compound was dissolved in phosphate-buffered saline (PBS) at 5 mg/ml, and the absence of endotoxin contamination (<6 × 10−2 endotoxin U/ml) was verified by the Limulus amebocyte lysate assay (BioWhittaker, Fontenay-sous-bois, France). Fluorescein isothiocyanate, R-phycoerythrin, or R-phycoerythrin–cyanin 5.1-labeled monoclonal antibodies (MAbs) directed against human CD3, CD4, CD8, CD25, CD45, CC chemokine receptor 5 (CCR5), and CXC chemokine receptor 4 (CXCR4) and their isotype-matched controls were purchased from Pharmingen (Becton Dickinson, Rungis, France) or Immunotech (Beckman Coulter, Marseille, France). Normal goat immunoglobulin G (IgG) and neutralizing goat IgG Abs against alpha macrophage inflammatory protein 1 (MIP-1α), MIP-1β, and the protein regulated upon activation normal T expressed and secreted (RANTES) were obtained from R&D Systems (Abingdon, United Kingdom).
Culture conditions.
Venous blood was collected into heparinized tubes and processed within 5 h of collection as previously described (2). Briefly, peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque centrifugation as recommended by the manufacturer (Pharmacia Biotech AB, Uppsala, Sweden), washed thoroughly to remove platelets, and depleted of CD8+ cells by using anti-CD8 antibody-coated electromagnetic beads (Dynal, Oslo, Norway). The depleted PBMCs obtained by following the manufacturer's protocol were found to contain <3% CD8+ cells by flow cytometry. Cells were then cultured with 5 μg of PHA (Difco, Elancout, France)/ml in endotoxin-free RPMI 1640 (Gibco, Courbevoie, France) containing 10% fetal calf serum (FCS), 2 mM l-glutamine, penicillin (100 U/μl), streptomycin (100 U/μl), and gentamicin (10 μg/ml). After 2 to 3 days, nonadherent cells were collected, washed, and cultured in 24-well plates at 5 × 105 cells/ml in medium supplemented with 10 U of interleukin-2 (IL-2) (Boehringer Mannheim, Meylan, France)/ml. On culture initiation and throughout the 14-day culture period, duplicate or triplicate wells were maintained in the absence or presence of different concentrations of Murabutide. Cultures were fed twice a week by replacing half of the supernatant in each well with the same volume of freshly prepared medium containing the respective concentrations of the immunomodulator.
Assays of p24 and cytokine release.
Viral replication was evaluated by measuring p24 antigen levels in culture supernatants by use of an HIV-1 p24 antigen assay kit (Coulter, Miami, Fla.) and by following the manufacturer's instructions. Quantitation in culture supernatants of the levels of tumor necrosis factor alpha, gamma interferon (IFN-γ), interleukin-6 (IL-6), IL-10, IL-16, MIP-1α, MIP-1β, and RANTES was done by use of commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems and Biosource, Camarillo, Calif.).
Analysis of HIV-1 RNA expression and viral DNA content.
Total cellular RNA was extracted using RNAzol (Bioprobe Systems, Montreuil, France) and was amplified using rTth Polymerase (Perkin-Elmer, Norwalk, Conn.) in the presence of the GAG06-GAG04 primer pair to detect the HIV-1 unspliced Gag-Pol mRNA and the BSS-KPNA primer pair to detect the singly spliced mRNA, as described elsewhere (2, 17, 41, 57). To measure proviral DNA levels, total cellular DNA was extracted and subjected to 20 or 40 repeated rounds of amplification with Amplitaq Gold DNA polymerase (Perkin-Elmer). PCR amplification of β-globin or β-actin sequences was performed to standardize for cell equivalence. HIV-1 proviral DNA in each sample was measured by using the GAG06-GAG04 primer pair (17, 46). All PCR products were separated on acrylamide gels and visualized by ethidium bromide staining. Using imaging systems (Image Master ID prime; Pharamacia Biotech AB), the percentage of inhibition of HIV-1 DNA and RNA expression was deduced after normalization to the levels of the corresponding internal standards as previously described (2, 17). The viral DNA levels were also evaluated in peritoneal or spleen cells from hu-PBL-SCID mice, and the quantification was done by calculating the number of viral copies per nanogram of human DNA, using 8E5 cell extracts to construct standard curves (18).
Flow cytometry analysis.
To assess surface receptor expression in CD8-depleted blasts or in peritoneal cells from hu-PBL-SCID mice, 2 × 105 cells were incubated for 30 min at 4°C with specific MAbs or with isotype-matched controls diluted 1:100 in PBS containing 2% FCS. Following two washes with PBS, cells were resuspended and fixed in 1% paraformaldehyde and analyzed with a FACSCalibur flow cytometer by using the CellQuest software (Becton Dickinson). Live cells were gated on their forward and side light scatter characteristics, and the mean percentage of positive cells as well as the mean fluorescence intensity (MFI) were recorded. In some experiments, exclusion of dead cells was verified by propidium iodide staining.
Cell proliferation assay.
PHA-blasts of endogenously infected CD8-depleted cells were washed, resuspended in medium containing 10 U of IL-2/ml, and seeded at 105 cells/well in 96-well microtiter plates (Falcon, Le Pont de Claix, France). Cultures were left unstimulated or were stimulated with Murabutide, in quadriplicate, for another 4 days. The level of DNA synthesis was measured after a 16-h pulse with 0.5 μCi of [3H]thymidine (Amersham Pharmacia Biotech)/well, and cells were harvested on a filter mat for scintillation counting (Skatron, Lier, Norway). Radioactivity was read using a Tricard 1600LR liquid scintillation β-counter (Packard, Downers Grove, Ill.).
Recovery of primary HIV-1 isolates and analysis of coreceptor specificity.
CD8-depleted blasts from HIV-1 patients were cocultured with PHA-activated PBMCs from healthy controls in RPMI 1640 supplemented with FCS and 10 U of IL-2/ml. Cultures were fed twice a week by removing half of the culture content (cells and medium) and adding an equal volume of fresh medium. Supernatants were monitored for p24 content over a 3-week period, and those presenting high p24 levels (>10 μg/ml) were collected, filtered, and frozen at −70°C. These supernatants served as virus stocks to determine the coreceptor usage of each isolate by using the GHOST cell infectivity assay (11). Briefly, virus isolates were grown on GHOST cells transfected with CXCR4, CCR5, or CCR1 and a reporter construct encoding enhanced green fluorescent protein. These cell lines were obtained through the National Institute of Allergy and Infectious Diseases/National Institutes of Health AIDS Research and Reference Reagent Program. Cells were infected overnight with undiluted virus stocks (100 μl/well), free virus was removed by washing, and cultures were maintained for an additional 4 to 5 days. Prior to analysis by flow cytometry, cells were washed and fixed with 2% formaldehyde. Background infectibility was determined on the basis of the mean number of fluorescent GHOST-CCR1 cells, as previously reported (11).
Analysis of c-Myc mRNA expression.
CD8-depleted blasts from HIV-1 patients were cultured for 6 and 24 h in the absence or presence of 10 μg of Murabutide/ml. Total cellular RNA was extracted and subjected to RT-PCR amplification using c-Myc-specific primers (19). Amplification of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene by employing a set of specific primers (17) was carried out in parallel to standardize for cell equivalence. All PCR products were separated on 2% agarose gels and visualized by ethidium bromide staining. Detection of c-Myc protein was examined in cell lysates of CD8-depleted blasts following a 24- and a 48-h culture with or without Murabutide. Western blot analysis was performed identically as described elsewhere (65), using overnight incubation at 4°C with primary Phospho-c-Myc polyclonal antibody (New England Biolabs, Hitchin, United Kingdom) and a 1-h incubation at room temperature with horseradish peroxidase-conjugated second antibodies (Sigma-Aldrich, St-Quentin-Fallavier, France).
hu-PBL-SCID mouse model.
CB17 scid/scid female mice (Iffa Credo, L'Abresle, France) were used at 4 to 5 weeks of age and kept under pathogen-free conditions. SCID mice were housed in microisolator cages, and all food, water, and bedding were autoclaved before use. Human PBLs were obtained from the peripheral blood of healthy donors (screened for HIV-1 and hepatitis before donation) by using Ficoll-Paque density gradient centrifugation. Then, 20 × 106 freshly isolated PBMCs were suspended in 0.5 ml of PBS and injected intraperitoneally into non-leaky phenotype mice. On day 12, mice were bled and sera were examined for the levels of human IgG as described below. Only human IgG-positive mice were used for the infection studies. On day 14 after reconstitution, mice were infected intraperitoneally with HIV-1Ba-L or HIV-1LAI at a dose corresponding to 50 kcpm of virus RT activity. This dose was determined in preliminary experiments to be 10-fold the virus inoculum needed to result in measurable infection on day 7 in 50% of the hu-PBL-SCID mice. Groups of infected mice received 2 h after infection and daily for the following 13 days an intraperitoneal injection with 400 μl of either PBS or Murabutide (10 mg/kg of body weight). Unless otherwise indicated, mice were bled 24 h after the last administration and were then killed by cervical dislocation, and peritoneal cell suspension was obtained by washing with ice-cold PBS. In some experiments, spleens were also removed and cell suspensions were prepared for DNA extraction. All procedures for injection and maintenance of the hu-PBL-SCID mice were performed in a biosafety level 3 facility.
Measurement of human IgG.
An ELISA system was used to quantitate human IgG in sera or plasma of reconstituted mice by using Fc-specific goat anti-human IgG (Sigma-Aldrich) as capture antibody and peroxidase-conjugated anti-human IgG (Fab specific) as secondary antibody. Purified human IgG was used to construct standard curves. All ELISAs were performed in duplicate, and sera from nonreconstituted SCID mice were used as negative controls.
Assay for detection of HIV-1 RNA in plasma.
Plasma samples from untreated or Murabutide-treated mice were aliquoted and stored at −70°C. HIV-1 RNA was measured using PCR (Amplicor Monitor HIV-1; Hoffmann-La Roche, Basel, Switzerland) according to the manufacturer's instructions. The limit of detection of this assay is 200 RNA copies/ml. When levels below this cutoff were found, an arbitrary number of 100 copies/ml was attributed to the test sample.
Statistical analysis.
The nonparametric Wilcoxon matched-pair test and Mann-Whitney U rank test were used to determine the statistical significance of all reported results unless otherwise mentioned. P values of <0.05 were considered statistically significant.
RESULTS
Murabutide inhibits HIV-1 replication in naturally infected cells.
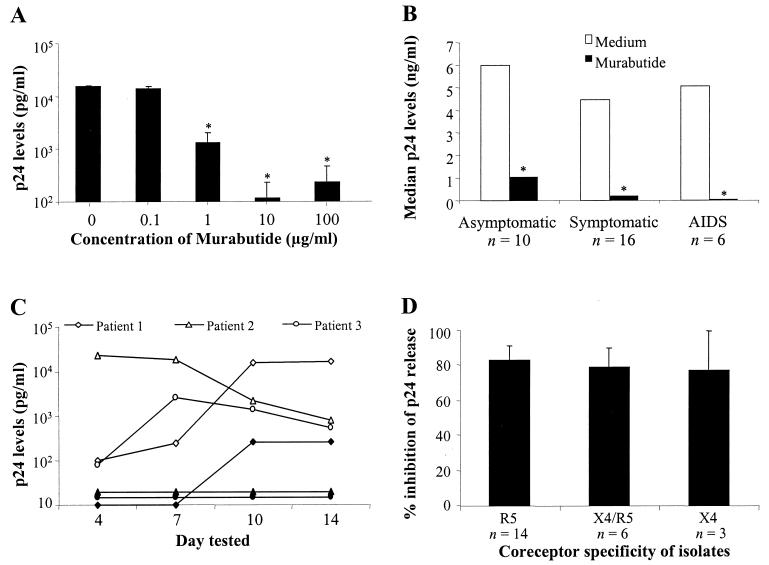
The effect of different concentrations of Murabutide was examined on HIV-1 replication in CD8-depleted PHA-activated lymphocytes from four seropositive subjects. As shown in Fig. 1A, 90% inhibition of p24 release was observed with 1 μg of Murabutide/ml and >97% inhibition was achieved with 10 or 100 μg/ml. We then extended these studies, using the 10-μg/ml concentration, to 32 patients at different stages of the disease. The Murabutide-suppressive activity on viral replication was equally evident in cultures from asymptomatics, symptomatics, and AIDS patients (Fig. 1B). Moreover, the observed effects were detectable as early as 4 days after culture initiation and were maintained throughout the 14-day culture period, as evidenced in the kinetic study performed on cells from three separate patients (Fig. 1C). Recovery of virus isolates from the coculture system and analysis of coreceptor specificity of the isolated strains, using the GHOST cell infectivity assay, were performed on samples from 23 patients. Analysis of the Murabutide effect in relation to virus coreceptor specificity revealed a similar level of inhibition of HIV-1 replication in cultures harboring R5, X4-R5, or X4 isolates (Fig. 1D). This demonstrates that the HIV-suppressive activity of Murabutide is independent of virus tropism. To further ensure that the effect of Murabutide was directly targeting CD4+ lymphocytes, cells from two patients were subjected to an additional step of purification by positive selection with anti-CD4 antibody-coated magnetic beads. Maintaining the highly purified CD4+ cultures (>99% CD4+ and <1% CD14+) with Murabutide resulted in 94 and 100% inhibition of p24 release. This clearly indicates that the observed effect of the immunomodulator on viral replication is not dependent on the presence of accessory cells in culture.
FIG. 1.
Murabutide suppresses HIV-1 replication in CD8-depleted lymphocytes from HIV-1 patients. CD8-depleted PBMCs from 36 patients were activated with PHA for 2 to 3 days, and nonadherent lymphocytes were then collected, washed, and cultured in medium containing 10 U of IL-2/ml in the absence or presence of Murabutide. Culture supernatants were harvested over a 2-week period and were tested for p24 Ag secretion by ELISA. (A) Cultures were left unstimulated or were stimulated with 0.1 to 100 μg of Murabutide/ml, and supernatants were tested 10 days later for p24 content. Results are means ± standard errors of the mean of values of samples from four different patients. (B) Levels of p24 release in 8- to 10-day cultures from 10 asymptomatics, 16 symptomatics, and 6 AIDS patients are shown as median values. (C) Kinetics of p24 release over a 14-day culture period was evaluated in unstimulated (white symbols) and Murabutide-stimulated (black symbols) cultures from three different patients. (D) The mean percent inhibition (± standard errors of the mean) by Murabutide (10 μg/ml) is presented for p24 release in 8- to 10-day cultures harboring X4, X4-R5, and R5 HIV-1 isolates. The recovery and analysis of coreceptor specificity for each of the 23 tested isolates are explained in Materials and Methods. ∗, significantly lower levels (P < 0.05; Wilcoxon matched-pair test) for treated cultures than for untreated (Medium) cultures.
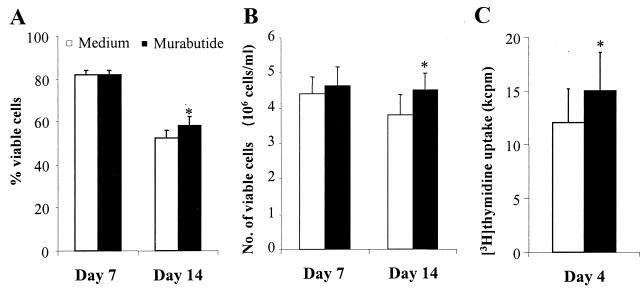
Murabutide has no inhibitory effect on cell viability or cellular proliferation.
To rule out a potential toxic effect of Murabutide on patients' cells, the percentage and the total number of viable cells were evaluated by trypan blue exclusion in cultures from 13 different subjects 7 and 14 days after culture initiation. No differences whatsoever could be noted on parameters of cell viability between cultures maintained for 7 days in the absence or presence of Murabutide (Fig. 2A and B). Moreover, for Murabutide-treated cells versus untreated cells, a significantly higher mean percentage (P = 0.0382 by Wilcoxon matched-pair test) and mean number of viable cells per milliliter (P = 0.0077) could be observed after 14 days in culture. These data, indicating the lack of cell toxicity by Murabutide, were further confirmed by measuring [3H]thymidine uptake in cultures from all 13 HIV-1 patients. Following a 4-day culture period, the level of [3H]thymidine incorporation was significantly higher (P = 0.0015) in the presence of the immunomodulator (Fig. 2C). This demonstrates that the observed HIV-suppressive activity of Murabutide is not linked to an inhibitory effect on cellular DNA synthesis.
FIG. 2.
Murabutide enhances cell viability and cellular proliferation. CD8-depleted PHA-activated lymphocytes from 13 HIV-1 patients were cultured at 5 × 105 cells/ml in the absence or presence of Murabutide (10 μg/ml). The percent (A) and the number (B) of viable cells were evaluated by trypan blue exclusion 7 and 14 days after culture initiation. (C) CD8-depleted blasts were cultured at 105 cells/well and were maintained for a period of 4 days. During the last 16 h prior to harvesting, cultures were pulsed with 0.5 μCi of [3H]thymidine/well, and the amount of radioactivity incorporated into the DNA of dividing cells was measured using a β-counter. Results are means ± standard errors of the mean. ∗, significantly higher levels (P < 0.05; Wilcoxon matched-pair test) for treated cultures than for untreated (Medium) cultures.
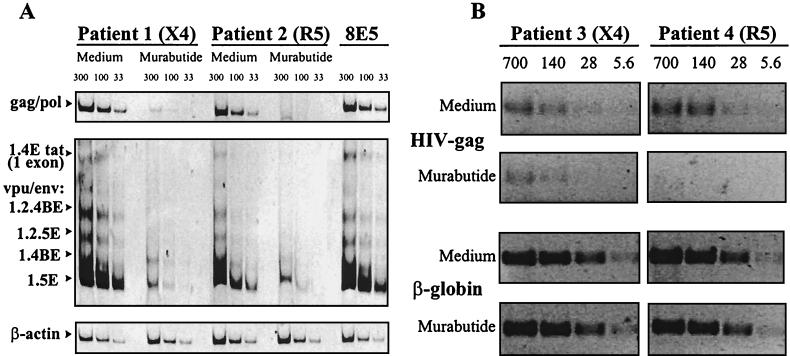
Murabutide inhibits HIV-1 mRNA expression and viral DNA.
After 3 days in culture, the levels of unspliced and singly spliced viral mRNAs were examined in cells from two HIV-1 patients, one producing an X4 isolate and the other producing an R5 isolate. Cultures maintained in the presence of Murabutide presented dramatically reduced levels (>92% inhibition) of both forms of HIV-1 mRNAs (Fig. 3A). This was further verified by the findings of 84 to 98% inhibition of viral transcripts, after a 7-day stimulation period, in cultures from three additional patients harboring X4-R5 and R5 isolates (data not shown). These results point to the process of virus transcription as a target for the HIV-suppressive activity of Murabutide. However, the number of infected cells and the levels of expression of the endogenous virus in the CD8-depleted blasts are usually low, so the measured virus output after 1 to 2 weeks in culture is primarily a result of amplification by virus spread to new targets (29, 39, 59). Thus, it is possible that the Murabutide effect could also target the earlier process of proviral DNA formation and/or its nuclear transport in the newly infected cells (17). To address this issue, we examined, after a 1- or 5-day culture period, the cellular viral DNA content in cultures from two additional HIV-1 patients, one harboring an X4 isolate and the other an R5 isolate. Identical viral DNA levels were detected on day 1 in unstimulated and Murabutide-stimulated cells (data not shown), suggesting the absence of an effect on already integrated provirus. However, results shown in Fig. 3B demonstrate that, following a 5-day culture period, significantly reduced viral DNA levels were detected in Murabutide-treated cells. The calculated mean percent inhibition in the X4 and R5 cultures was 69 and 100%, respectively. Nevertheless, the inhibition of p24 release by Murabutide was identical (92 to 93%) in supernatants from both cultures. These results of reduced viral DNA levels after 5 days but not after 1 day of culture initiation strongly indicate that Murabutide is targeting a process anterior to complete virus integration in newly infected cells.
FIG. 3.
Murabutide inhibits HIV-1 gene expression and viral DNA content in endogenously infected CD8-depleted blasts. Following PHA activation, CD8-depleted blasts from four HIV-1 patients were cultured in the absence or presence of 10 μg of Murabutide/ml. (A) Total RNA was extracted after a 3-day culture period, and samples (33, 100, and 300 ng) were subjected to RT-PCR amplification with primer pair GAG06-GAG04 to detect unspliced Gag or Pol mRNA and with primer pair BSS-KPNA to detect intermediate-size singly spliced viral transcripts. These mRNAs were named on the basis of the exons they contain and the proteins they produce (41). Constitutively expressed β-actin mRNA in the same samples was also amplified. An equivalent amount of RNA from the 8E5 cell line was used as positive control for RT-PCR amplification. The HIV-1 isolates in cultures from patients 1 and 2 were X4 and R5 tropic, respectively. (B) Total DNA was extracted after 5 days of culture in the absence or presence of Murabutide, and various concentrations (5.6, 28, 140, and 700 ng) were subjected to PCR amplification with primer pair GAG06-GAG04 to detect the HIV-1 gag gene. Cell equivalence was determined by amplification of the β-globin housekeeping gene. HIV-1 isolates from cultures of patients 3 and 4 presented X4 and R5 tropism, respectively.
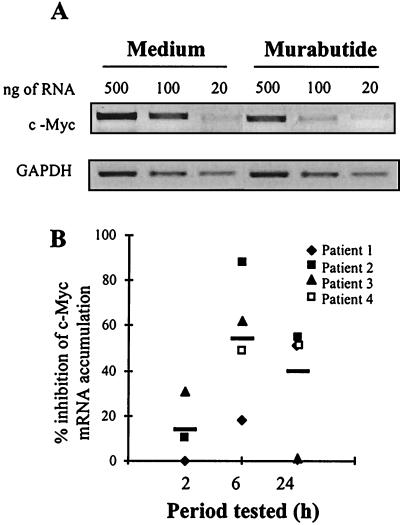
Murabutide down-regulates c-Myc expression.
It has been reported that the proto-oncogene c-Myc is necessary for efficient HIV-1 DNA nuclear import in activated T cells (65). Therefore, we examined the possibility that the Murabutide-induced inhibition of proviral DNA levels may have been mediated, at least partly, by regulating the expression of this cellular gene which is required for successful proviral DNA integration. Analysis by RT-PCR of the levels of c-Myc mRNA expression was performed with CD8-depleted blasts from four patients following 2, 6, and 24 h of stimulation with Murabutide. Results shown in Fig. 4 demonstrate an important inhibition of c-Myc mRNA accumulation after 6 and 24 h of treatment with Murabutide. This suggests that, by inhibiting c-Myc expression, the immunomodulator could block efficient nuclear import of viral preintegration complexes (PICs). Attempts to analyze the intracellular protein levels of c-Myc by Western blottings on lysates from either untreated or Murabutide-treated cells were totally unsuccessful. This is not surprising since c-Myc protein is difficult to detect in lymphocytes and costimulation with anti-CD3 and anti-CD28 was found necessary to induce sufficient protein levels to be revealed by Western blotting (65).
FIG. 4.
Murabutide suppresses c-Myc mRNA accumulation. PHA-activated CD8-depleted lymphocytes from four HIV-1 patients were cultured in the absence or presence of Murabutide (10 μg/ml). After 2, 6, and 24 h, total RNA was extracted and various concentrations (20, 100, and 500 ng) were subjected to RT-PCR amplification using c-Myc- or GAPDH-specific primer pairs. (A) The intensity of the PCR products revealed by ethidium bromide staining is shown for a sample from one representative patient after a 6-h stimulation period. (B) The percent inhibition by Murabutide of c-Myc expression in cultures from four patients is presented at the different time points tested. Horizontal bars reflect the mean percent inhibition.
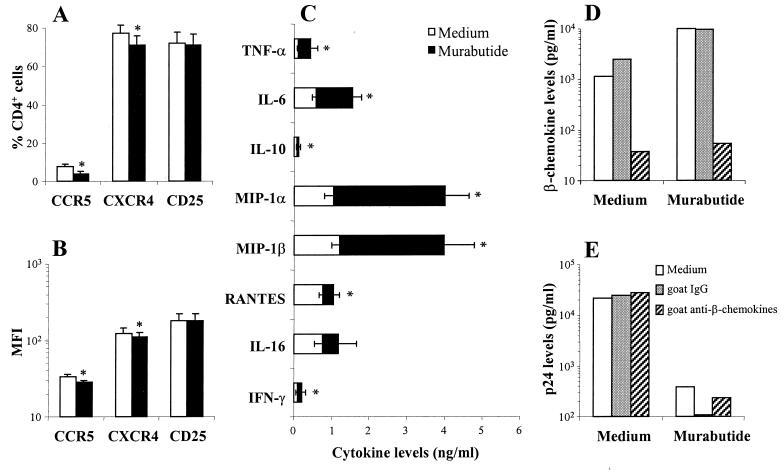
Regulation by Murabutide of chemokine receptor expression and cytokine release.
To address the immunoregulatory effects of Murabutide, we first analyzed the changes in CCR5, CXCR4, and CD25 expression in CD8-depleted blasts from 13 HIV-1 patients. Evaluations were done 2, 4, and 7 days after culture initiation, and maximal effects were observed on day 4. The great majority of cells either in untreated (mean ± standard deviation, 93% ± 4.4%) or in Murabutide-treated (93% ± 3.8%) cultures were CD3+ lymphocytes containing <3% CD8+ T cells. Stimulation with Murabutide had no effect on the level of expression of either CD3 or CD4, as measured by the MFI of positive cells (data not shown). Using three-color flow cytometry, a significant down-regulation of CXCR4 and CCR5, but not of CD25, was noted in Murabutide-stimulated CD4+/CD3+ lymphocytes (Fig. 5A and B). The effect was most evident on the mean percentage of CCR5+ cells, which was reduced from 7.5 to 3.9%. However, these changes are unlikely to account for the dramatic HIV-suppressive activity of the compound and may be explained by an increased release of chemokines. To verify this point, we then profiled the secreted levels of eight different cytokines and chemokines in 48-h cultures from 11 to 26 patients. Although the release of most of the tested cytokines (except for IL-16) was significantly but modestly enhanced by Murabutide, a higher induction level of MIP-1α and MIP-1β was noted in stimulated cultures from the majority of tested subjects (Fig. 5C). Nevertheless, the induced levels of β-chemokines in cultures from patients with R5 isolates did not correlate with the level of inhibition of virus replication (n = 8, r = −0.2196, and P = 0.578; Spearman rank order correlation). Moreover, although the presence of a mixture of neutralizing Abs to MIP-1α, MIP-1β, and RANTES abrogated the detectable β-chemokines in the supernatants (Fig. 5D), it did not block the inhibitory effect of Murabutide on the replication of R5 isolates (Fig. 5E).
FIG. 5.
HIV-1 coreceptor expression and cytokine release are regulated by Murabutide in CD8-depleted lymphocyte cultures. PHA-activated CD8-depleted lymphocytes from 13 HIV-1 patients were cultured for 4 days in the absence or presence of Murabutide (10 μg/ml). Cells were then washed, and the CD4+/CD3+ population was analyzed by three-color flow cytometry for the level of expression of either CCR5, CXCR4, or CD25. Results are shown as means (± standard errors of the mean) of the percent of positive cells (A) and MFI (B). ∗, significantly lower (P < 0.05; Wilcoxon matched-pair test) receptor expression for treated cultures than for untreated (Medium) cultures. (C) CD8-depleted cultures from 26 HIV-1 patients were maintained for 2 days with or without 10 μg of Murabutide/ml. Supernatants were then analyzed for the content of the indicated cytokines and chemokines by using commercially available ELISA kits. Levels of secreted IFN-γ and IL-16 were evaluated only on samples from 11 and 17 patients, respectively. Values shown are means ± standard errors of the means. ∗, significantly higher (P < 0.05, Wilcoxon matched-pair test) cytokine levels for treated cultures than for untreated (Medium) cultures. (D and E) Untreated and Murabutide-treated CD8-depleted cultures from HIV-1 patients harboring R5 isolates were maintained in the absence or presence of 150 μg of normal goat IgG/ml or of 50 μg of each of the three neutralizing goat IgG Abs/ml against MIP-1α, MIP-1β, and RANTES. The sums of the levels of the three β-chemokines in the supernatants (D) and of viral p24 protein (E) were analyzed by ELISA kits after a 3- and 7-day culture period, respectively. Results are representative of those of three patients.
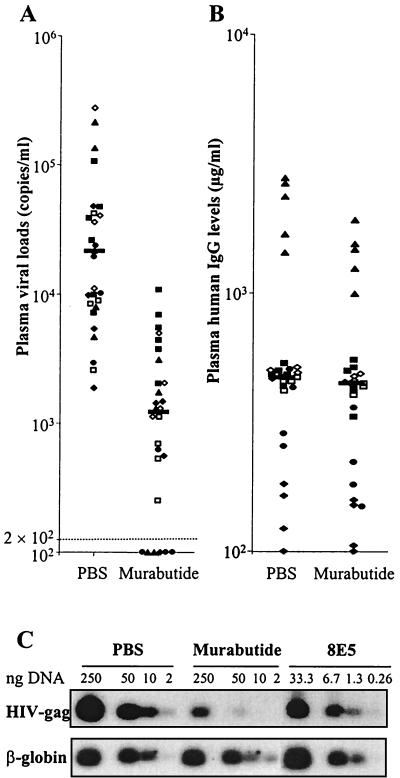
Murabutide reduces plasma viral loads and viral DNA in hu-PBL-SCID mice.
HIV-1Ba-L-infected hu-PBL-SCID mice presented, 14 days after infection, detectable but variable levels of plasma viral loads ranging from 1,854 to 276,692 copies/ml (median, 21,146). Daily treatment with Murabutide significantly reduced the viral loads (P < 0.0001, Mann-Whitney U rank test) in six separate experiments on a total of 27 mice per group (Fig. 6A). The median plasma viral copies per milliliter in Murabutide-treated mice was 1,208 (range, 100 to 10,723), and 6 out of the 27 treated mice had viral loads below the detection limit (P = 0.01146 versus controls; Fisher's exact test). Untreated (PBS) and Murabutide-treated mice presented similar levels of plasma human IgG (Fig. 6B), indicating the absence of Murabutide toxicity on human cells. This was further verified, in four out of the six experiments, by evaluating the percentage of human CD4+ and CD45+ cells recovered from peritoneal lavages. Using flow cytometry, the mean (± standard deviation) of human CD4+ cells from untreated mice was 4.7% ± 2.8%, and this was similar to that detected in Murabutide-treated mice (5.9% ± 5.6%). Similarly, no difference could be observed in the percentage of CD45+ cells recovered from the control (26% ± 4%) and from the Murabutide group (34% ± 7%). Furthermore, analysis of the viral DNA levels in peritoneal cells recovered 14 days after infection revealed highly reduced levels (>95%) in cells from Murabutide-treated mice (Fig. 6C). Similar results were also obtained when viral DNA content was examined in spleen cells of the hu-PBL-SCID mice, and >70% inhibition (range, 73 to 95%) could be detected in three separate experiments following treatment with Murabutide (data not shown).
FIG. 6.
Treatment with Murabutide suppresses plasma viral loads and viral DNA levels in HIV-1-infected hu-PBL-SCID mice. Two weeks after repopulating SCID mice with hu-PBL, HIV-1Ba-L was administered intraperitoneally at a dose equivalent to 50 kcpm of viral RT activity. Starting 2 h after infection and for the following 13 days, groups of mice were treated daily with Murabutide (10 mg/kg of body weight) or with the excipient (PBS) by injecting a volume of 400 μl intraperitoneally. (A) Viral loads were quantified in plasma samples 24 h after the last administration by using Amplicor Monitor HIV-1 kits. (B) The levels of human IgG (B) in the same samples were evaluated by ELISA. Results are values for individual mice (four to six per group) from six independent experiments and are represented by a different symbol for each experiment. Horizontal bars reflect the median values of pooled data from all experiments. (C) Peritoneal cells from PBS- or Murabutide-treated mice were collected 24 h after the last injection, and an equivalent number of cells were pooled from all mice in each group. Total DNA was extracted, and various concentrations (2, 10, 50, and 250 ng) were subjected to PCR amplification with primer pair GAG06-GAG04 to detect the HIV-1 gag gene. Cell equivalence was determined by amplification of the human β-globin housekeeping gene, and DNA extracts from 8E5 cells were used as PCR standards. Since the 8E5 cells contain one integrated provirus copy per cell, the limit of detection of our PCR was 40 copies (or 40 cells) in 0.26 ng of human DNA. Results are representative of four experiments.
The magnitude of the effect on proviral DNA levels suggested that Murabutide could effectively reduce virus spread and that the observed inhibition of viral loads should also be detectable at time points extending beyond the treatment period. To address this issue, we compared, in four independent experiments, the plasma viral loads at the end of treatment (week 2) and then 2 weeks later (week 4). Results shown in Table 1 clearly demonstrate that Murabutide activity was still maintained 2 weeks after the end of treatment and that viral rebound was slow or absent. Since viral turnover in this model and in humans has been reported to be similar (48), the persistent effect of Murabutide on viral loads suggests an induced state of low cell permissiveness to HIV-1 replication. Finally, using a similar protocol, infection of hu-PBL-SCID mice was also attempted with the T-tropic strain HIV-1LAI. In three separate experiments and on a total of 17 mice per group, viral loads in the PBS-treated controls were generally low (median copies/ml = 1,462), though detectable in all infected mice (range, 288 to 69,529), when examined 2 weeks after challenge. Treatment with Murabutide resulted in significant reduction in viral loads (median copies/ml = 459; P = 0.0183 by Mann-Whitney U rank test), and 8 of the 17 treated mice presented plasma viral copies per milliliter below the detection limit (P = 0.00134 versus PBS-treated mice; Fisher's exact test). In contrast, the levels of plasma human IgG were identical in both groups, with median IgG values of 527 and 516 μg/ml in the PBS-treated and Murabutide-treated groups, respectively. However, the HIV-1LAI infection model was much less productive than that of HIV-1Ba-L, and viral loads were extremely low or undetectable, irrespective of treatment, after 4 weeks of challenge with the T-tropic strain.
TABLE 1.
Effect of 2 weeks of treatment with Murabutide on plasma viral loads in hu-PBL-SCID micea
| Expt no. | No. of mice/group | Plasma viral loads at week 2 of treatment
|
Plasma viral loads at week 4 of treatment
|
||
|---|---|---|---|---|---|
| PBS | Murabutide | PBS | Murabutide | ||
| 1 | 4 | 13,456 (13,077 ± 5,625) | 100 (747 ± 647) | 43,599 (38,134 ± 7,566) | 1,535 (1,531 ± 594) |
| 2 | 4 | 7,590 (16,187 ± 10,633) | 987 (886 ± 335) | 8,243 (21,774 ± 14,830) | 472 (1,080 ± 752) |
| 3 | 5 | 21,508 (77,134 ± 42,271) | 1,208 (1,247 ± 561) | 26,854 (212,989 ± 128,824) | 1,677 (2,398 ± 1,037) |
| 4 | 4 | 8,578 (15,346 ± 8,898) | 604 (657 ± 194) | 2,443 (3,254 ± 1,563) | 100 (206 ± 105) |
Two hours following HIV-1Ba-L infection of hu-PBL-SCID mice, groups (four or five mice per group) were treated for 13 consecutive days with PBS or with Murabutide (10 mg/kg). Plasma viral loads were evaluated at week 2 (end of treatment) and at week 4. Data shown are median plasma viral copies/milliliter, and the numbers in parentheses reflect the means ± standard errors of the mean.
DISCUSSION
For nearly 2 decades, muramyl peptides were evaluated mostly as nonspecific enhancers of host resistance against microbial infections and as vaccine adjuvants (32, 73). The biological activities of these immunomodulators were also linked to their capacity to activate macrophages, and few studies have addressed the direct effects on T lymphocytes (4, 63). However, in the last few years, potent immunomodulating activities for some of these synthetic molecules have been reported, including the suppression of antigen-specific IgE responses (3) and the inhibition of tissue IL-4 and tumor necrosis factor alpha mRNA expression (34, 77). The availability of safe MDP analogues, retaining selected biological activities without associated prohibiting toxicity, has prompted the investigation of their potential use as immunomodulators in combination with antibiotics (43), with antivirals (23), and with therapeutic cytokines (6, 7, 30). One selected and safe derivative, Murabutide, has been shown to retain the capacity of inhibiting IgE responses (3), of down-regulating inflammatory reactions (5, 7), and of inducing colony-stimulating and antiviral activities (22, 49, 74). This immunomodulator could also enhance lymphoproliferative responses, increase CD26 and CD71 expression on T cells (51), and synergize with IL-2 or IFN-α to drive T-helper 1 cytokine release (6, 7). Based on these properties and on a capacity to suppress HIV-1 replication in APCs (17), it was our intention to profile the effects of Murabutide on HIV-1-infected CD4+ T cells.
Using CD8-depleted blasts from endogenously infected PBMCs, we were able to demonstrate a highly potent HIV-suppressive activity of Murabutide, irrespective of virus tropism. The effects of the immunomodulator were detectable both at the level of p24 release and of virus transcription. The findings of reduced unspliced and singly spliced viral mRNA transcripts after a short period of culture (3 days), strongly argue for a direct effect of Murabutide on HIV-1 expression in already-activated virus producing CD4+ blasts. Furthermore, dramatically reduced viral mRNA levels were also detected after longer incubation periods, and this effect could be attributed, at least in part, to interference with proviral DNA formation in newly infected cells. Although we did not address in this study the exact mechanism of inhibition of viral DNA by Murabutide, we were able to correlate the observed activity with inhibition of c-Myc expression. Such an effect has been previously reported to block the nuclear transport of viral PICs in activated T cells (65). These findings, together with the previously described ability of Murabutide to inhibit the nuclear transport of PICs in macrophages (17), strongly argue for a similar mechanism operating in activated CD4+ cells. Although inhibition of c-Myc expression might play a critical role in mediating the Murabutide effect on proviral DNA, we cannot exclude the involvement of other cellular factors, such as high mobility group 1 protein or virion-associated matrix-interacting protein, in this process (28, 36). Nevertheless, based on the lack of inhibition of CD25 expression by Murabutide, it is quite unlikely that the effect of the immunomodulator on proviral DNA could involve the down-regulation of phosphodiesterase 4 expression, a phenomenon reported to block simultaneously the nuclear transport of viral PICs and CD25 expression in memory T cells (66). Taking into account that Murabutide has not demonstrated any direct effect on viral enzymes or on virus entry (17), and although we cannot totally rule out a potential effect of Murabutide on virus entry in endogenously infected lymphocytes, it is most likely that the immunomodulator exerts its HIV-suppressive activity by regulating the expression of multiple cellular genes implicated in the processes of virus integration and transcription. This conclusion is receiving substantial support from our preliminary findings, using differential display RT-PCR analysis, on the capacity of Murabutide to regulate the expression of multiple cellular genes coding for transcription factors, DNA binding proteins, and molecules involved in RNA processing (C. Cocude, M.-J. Truong, and G. M. Bahr, unpublished data). Among the Murabutide-regulated cellular genes, some have been previously implicated in the control of virus expression and few are of unmatched sequences in the data banks. Ongoing studies in our laboratory will dissect the role of already described as well as new cellular factors in the Murabutide-induced inhibition of HIV expression.
The HIV-suppressive activity of Murabutide in APCs (17) and in CD4+ lymphocytes (present work) has not been linked either to increased cell death or to the inhibition of cellular DNA synthesis. This differentiates the effects of the immunomodulator from those reported with other pharmacologic agents for which the capacity to suppress viral replication has been associated with an antiproliferative activity (9) or with the induction of apoptosis in activated CD4+ T cells (12). On the other hand, stimulation of CD8-depleted blasts with Murabutide resulted in the release of multiple cytokines and β-chemokines. However, we could not establish a direct role for the induced β-chemokines in mediating the virus-suppressive effects of the immunomodulator. This was evident by the fact that neutralization of the released chemokines with polyclonal antibodies did not reverse the Murabutide-induced inhibition of p24 release. Moreover, the replication of CXCR4-dependent T-tropic isolates was equally suppressed by the immunomodulator, as that of CCR5-dependent isolates. These findings are in agreement with the reported effects of Murabutide on HIV-1 replication in APCs (17) and support previous results demonstrating a lack of correlation between the level of β-chemokines and that of virus replication in CD4+ lymphocyte cultures (26). Similarly, activation of PBMCs with influenza virus has been shown to inhibit HIV-1 replication through mechanisms independent of the high-level induction of β-chemokine release (47). Nevertheless, the ability of Murabutide to induce the secretion of β-chemokines may still have relevance to the control of HIV-1 replication in vivo. In addition, the released β-chemokines in culture could explain the reduced expression of CCR5 in treated cells (31) and may participate in the protection of neighboring cells against infection. Finally, our results cannot exclude the implication of yet-unidentified factor(s), released after stimulation of CD4+ cells with Murabutide, in contributing toward the HIV-suppressive activity of the immunomodulator. Such factors have been recently reported to be produced by endogenously infected CD4+ lymphocytes following alloantigenic stimulation and to be capable of inhibiting the replication of R5 and X4 HIV-1 isolates independently of an effect on cellular proliferation (27).
Experiments with the hu-PBL-SCID mice were conducted to determine whether the in vitro effects of Murabutide on viral replication would be reflected in studies in vivo. Daily injection of the immunomodulator, starting 2 h after challenge with HIV-1Ba-L, resulted in a 20-fold decrease in plasma viremia and in dramatically reduced proviral DNA levels in peritoneal and spleen cells. To our knowledge, this is the first demonstration of the efficacy of a nonspecific synthetic immunomodulator to result in a significant suppression of viral replication in hu-PBL-SCID mice. It is of interest to note that the observed HIV-suppressive activity could not be attributed to a rapid elimination of human cells and was maintained even after cessation of treatment. This would suggest that a state of low cell permissiveness to HIV-1 infection and replication had been generated following treatment with Murabutide. Moreover, the absence of a rapid and considerable viral rebound after the treatment period clearly suggests that the immunotherapeutic approach does not result in the selection of mutant or resistant viruses. Unlike the reported effects with antiretrovirals (62), with β-chemokines (40), or with neutralizing antibodies (48), regulating cellular susceptibility to the virus can avoid the pressure which normally leads to the emergence of variants with drug-resistant mutations or altered coreceptor specificity. The ability of Murabutide to inhibit plasma viremia was also assessed for hu-PBL-SCID mice infected with the T-tropic HIV-1LAI. In agreement with previous reports (21, 53), infection with the X4 virus 2 weeks after the reconstitution of SCID mice resulted in low levels and short-lived viral loads. This has been attributed to the weak activation state of PBLs at the time of infection and to the low pathogenicity of X4 isolates in this model (21). Nevertheless, administration of Murabutide was still effective in reducing viral loads and resulted in a high percentage of mice with undetectable viremia. These results confirm the observations made in vitro on the ability of Murabutide to inhibit the replication of X4 HIV-1 isolates and further demonstrate that the virus-suppressive activity of the immunomodulator is not strictly related to its capacity to induce β-chemokine release.
The administration of certain adjuvants into hu-PBL-SCID mice has been correlated with high mortality or with a dramatic elevation in human serum IgG levels (55). While Murabutide has been found to present an important adjuvant effect (13, 68), the repeated injection of this molecule into hu-PBL-SCID mice did not induce any detectable toxicity or increase in serum IgG. This confirms the absence of polyclonal B-cell activation by Murabutide of human cells (4) and points to major differences in the mechanism of action between different molecules presenting adjuvant activity. In this respect, it is of interest to note that whereas several adjuvant-active bacterial cell wall structures and synthetic MDP derivatives were found to induce fever and enhance slow wave sleep, the adjuvant-active but safe analogue Murabutide was reported to be devoid of such effects (35, 52). Furthermore, in contrast to the effects of endotoxin or of other muramyl peptides, the administration of Murabutide to humans was found to be associated with the induction of anti-inflammatory cytokines without any detectable release of inflammatory mediators (5). These differences in the biological activities among different muramyl peptides may be explained by the presence of multiple cell surface and intracellular receptors for this family of molecules (64, 70, 72). Alternatively, the affinity of different analogues to a defined receptor may drastically change depending on the introduced chemical modification. Thus, it is quite tempting to suggest that by modifying the isoglutamine residue of MDP to become a butyl ester-bearing glutamine (as in Murabutide), this could result in the loss of binding to brain cells regulating temperature and sleep but in enhanced binding affinity to intracellular receptors present in lymphocytes and macrophages (64). A recent study has identified histones as receptors for muramyl peptides and showed that binding to these nuclear proteins is determined by certain chemical modifications of the analogues (25). Thus, it would be reasonable to predict that Murabutide retains an ability to bind histones and, consequently, to interfere with histone acetylation, which is required for the transcriptional activation of the chromatin-associated HIV-1 provirus (8, 61). This could partly explain the capacity of Murabutide to inhibit HIV-1 gene expression and the replication of virus isolates with different tropism. Studies are presently ongoing to verify the binding of Murabutide to histones and the implications of this interaction on HIV-1 replication.
In conclusion, our findings strongly argue in favor of the use of nonspecific immunotherapy as adjunct to antiretrovirals in order to maintain strict control of virus replication. While Murabutide is not the only immunomodulator of bacterial origin to inhibit HIV-1 replication in lymphocytes and macrophages (1), its potential use for the immunotherapy of HIV-1 infection presents several advantages. First, the administration of Murabutide to humans is associated with no prohibiting toxicity, and this has been recently verified in phase I studies on 30 HIV-1 patients receiving potent antiretroviral therapy (C. Amiel, Y. Mouton, and G. M. Bahr, unpublished data). Second, the HIV-suppressive activity of the immunomodulator, verified on isolates with different tropism and in different cell populations, is targeting host cellular factors and not directly viral enzymes; this activity would therefore be highly complementary to the therapeutic effects of antiretrovirals. Third, the profile of cell activation induced by Murabutide is associated with the release of HIV-inhibiting factors which could protect, in vivo, against the spread of infection to new target cells. Fourth, by virtue of its immunomodulating properties, the administration of Murabutide into HIV-1 patients would enhance resistance against microbial infections, including that against HIV-1, and would be expected to participate in correcting the virus-disrupted immune homeostasis. Currently ongoing clinical trials in HIV-1 patients, employing 6 weeks of immunotherapy with Murabutide as adjunct to potent antiretrovirals, will soon determine the validity of this approach for enhancing immune recovery and the control of HIV-1 replication.
ACKNOWLEDGMENTS
This work was supported by grants from the Agence National pour la Valorisation et l'Avancement de la Recherche (ANVAR) and from the Association Stop Sida (Lille, France).
We thank all the patients who participated in this study and who agreed to give blood samples on repeated occasions. We also thank J. P. Kusnierz for assistance in the flow cytometry experiments and N. Béthencourt for help in the preparation of the manuscript.
REFERENCES
- 1.Alfano M, Schmidtmayerova H, Bukrinsky M. Bacterial lipopolysaccharide is a potent inhibitor of HIV-1 replication in T lymphocytes and macrophages. AIDS. 1998;12:1724–1726. [PubMed] [Google Scholar]
- 2.Amiel C, Darcissac E, Truong M J, Dewulf J, Loyens M, Mouton Y, Capron A, Bahr G M. Interleukin-16 (IL-16) inhibits human immunodeficiency virus replication in cells from infected subjects, and serum IL-16 levels drop with disease progression. J Infect Dis. 1999;179:83–91. doi: 10.1086/314550. [DOI] [PubMed] [Google Scholar]
- 3.Auci D L, Carucci J A, Chice S M, Smith M C, Dukor P, Durkin H G. Control of IgE responses. 4. Isotype-specific suppression of peak BPO-specific IgE antibody-forming cell responses and of BPO-specific IgE in serum by muramyldipeptide or murabutide after administration to mice by gavage. Int Arch Allergy Immunol. 1993;101:167–176. doi: 10.1159/000236515. [DOI] [PubMed] [Google Scholar]
- 4.Bahr G M, Chedid L. Immunological activities of muramyl peptides. Fed Proc. 1986;45:2541–2544. [PubMed] [Google Scholar]
- 5.Bahr G M, Darcissac E, Bevec D, Dukor P, Chedid L. Immunopharmacological activities and clinical development of muramyl peptides with particular emphasis on murabutide. Int J Immunopharmacol. 1995;17:117–131. doi: 10.1016/0192-0561(94)00094-5. [DOI] [PubMed] [Google Scholar]
- 6.Bahr G M, Darcissac E, Pouillart P R, Chedid L A. Synergistic effects between recombinant interleukin-2 and the synthetic immunomodulator murabutide: selective enhancement of cytokine release and potentiation of antitumor activity. J Interferon Cytokine Res. 1996;16:169–178. doi: 10.1089/jir.1996.16.169. [DOI] [PubMed] [Google Scholar]
- 7.Bahr G M, Pouillart P R, Chedid L A. Enhancement in vivo of the antiinflammatory and antitumor activities of type I interferon by association with the synthetic immunomodulator murabutide. J Interferon Cytokine Res. 1996;16:297–306. doi: 10.1089/jir.1996.16.297. [DOI] [PubMed] [Google Scholar]
- 8.Benkirane M, Chun R F, Xiao H, Ogryzko V V, Howard B H, Nakatani Y, Jeang K T. Activation of integrated provirus requires histone acetyltransferase. p300 and P/CAF are coactivators for HIV-1 Tat. J Biol Chem. 1998;273:24898–24905. doi: 10.1074/jbc.273.38.24898. [DOI] [PubMed] [Google Scholar]
- 9.Borvak J, Chou C S, Van Dyke G, Rosenwirth B, Vitetta E S, Ramilo O. The use of cyclosporine, FK506, and SDZ NIM811 to prevent CD25-quiescent peripheral blood mononuclear cells from producing human immunodeficiency virus. J Infect Dis. 1996;174:850–853. doi: 10.1093/infdis/174.4.850. [DOI] [PubMed] [Google Scholar]
- 10.Carcelain G, Tubiana R, Samri A, Calvez V, Delaugerre C, Agut H, Katlama C, Autran B. Transient mobilization of human immunodeficiency virus (HIV)-specific CD4 T-helper cells fails to control virus rebounds during intermittent antiretroviral therapy in chronic HIV type 1 infection. J Virol. 2001;75:234–241. doi: 10.1128/JVI.75.1.234-241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cecilia D, KewalRamani V N, O'Leary J, Volsky B, Nyambi P, Burda S, Xu S, Littman D R, Zolla-Pazner S. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J Virol. 1998;72:6988–6996. doi: 10.1128/jvi.72.9.6988-6996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapuis A G, Paolo Rizzardi G, D'Agostino C, Attinger A, Knabenhans C, Fleury S, Acha-Orbea H, Pantaleo G. Effects of mycophenolic acid on human immunodeficiency virus infection in vitro and in vivo. Nat Med. 2000;6:762–768. doi: 10.1038/77489. [DOI] [PubMed] [Google Scholar]
- 13.Chedid L A, Parant M A, Audibert F M, Riveau G J, Parant F J, Lederer E, Choay J P, Lefrancier P L. Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect Immun. 1982;35:417–424. doi: 10.1128/iai.35.2.417-424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chomel J J, Simon-Lavoine N, Thouvenot D, Valette M, Choay J, Chedid L, Aymard M. Prophylactic and therapeutic effects of murabutide in OF1 mice infected with influenza A/H3N2 (A/Texas/1/77) virus. J Biol Response Modif. 1988;7:581–586. [PubMed] [Google Scholar]
- 15.Chun T W, Davey R T, Ostrowski M, Shawn Justement J, Engel D, Mullins J I, Fauci A S. Relationship between preexisting viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat Med. 2000;6:757–761. doi: 10.1038/77481. [DOI] [PubMed] [Google Scholar]
- 16.Clerici M, Piconi S, Balotta C, Trabattoni D, Capetti A, Fusi M L, Ruzzante S, Longhi R, Colombo M C, Moroni M, Milazzo F. Pentoxifylline improves cell-mediated immunity and reduces human immunodeficiency virus (HIV) plasma viremia in asymptomatic HIV-seropositive persons. J Infect Dis. 1997;175:1210–1215. doi: 10.1086/593570. [DOI] [PubMed] [Google Scholar]
- 17.Darcissac E C, Truong M J, Dewulf J, Mouton Y, Capron A, Bahr G M. The synthetic immunomodulator murabutide controls human immunodeficiency virus type 1 replication at multiple levels in macrophages and dendritic cells. J Virol. 2000;74:7794–7802. doi: 10.1128/jvi.74.17.7794-7802.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Real G, Llorente M, Bosca L, Hortelano S, Serrano A, Lucas P, Gomez L, Toran J L, Redondo C, Martinez C. Suppression of HIV-1 infection in linomide-treated SCID-hu-PBL mice. AIDS. 1998;12:865–872. doi: 10.1097/00002030-199808000-00008. [DOI] [PubMed] [Google Scholar]
- 19.el Khyari S, Bourgarel V, Barra Y, Braguer D, Briand C. Pretreatment by tubulin agents decreases C-MYC induction in human colon carcinoma cell line HT29–D4. Biochem Biophys Res Commun. 1997;231:751–754. doi: 10.1006/bbrc.1997.6187. [DOI] [PubMed] [Google Scholar]
- 20.Emery S, Capra W B, Cooper D A, Mitsuyasu R T, Kovacs J A, Vig P, Smolskis M, Saravolatz L D, Lane H C, Fyfe G A, Curtin P T. Pooled analysis of 3 randomized, controlled trials of interleukin-2 therapy in adult human immunodeficiency virus type 1 disease. J Infect Dis. 2000;182:428–434. doi: 10.1086/315736. [DOI] [PubMed] [Google Scholar]
- 21.Fais S, Lapenta C, Santini S M, Spada M, Parlato S, Logozzi M, Rizza P, Belardelli F. Human immunodeficiency virus type 1 strains R5 and X4 induce different pathogenic effects in hu-PBL-SCID mice, depending on the state of activation/differentiation of human target cells at the time of primary infection. J Virol. 1999;73:6453–6459. doi: 10.1128/jvi.73.8.6453-6459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galelli A, Lefrancier P, Chedid L. Colony-stimulating activity induced by synthetic muramyl peptides: variation with chemical structure and association with anti-infectious activity. Infect Immun. 1984;46:495–500. doi: 10.1128/iai.46.2.495-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gangemi J D, Nachtigal M, Barnhart D, Krech L, Jani P. Therapeutic efficacy of liposome-encapsulated ribavirin and muramyl tripeptide in experimental infection with influenza or herpes simplex virus. J Infect Dis. 1987;155:510–517. doi: 10.1093/infdis/155.3.510. [DOI] [PubMed] [Google Scholar]
- 24.Garcia F, Plana M, Vidal C, Cruceta A, O'Brien W A, Pantaleo G, Pumarola T, Gallart T, Miro J M, Gatell J M. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS. 1999;13:F79–F86. doi: 10.1097/00002030-199907300-00002. [DOI] [PubMed] [Google Scholar]
- 25.Golovina T, Fattakhova G, Swiderek K, Makarov E, Bovin N, Shively J, Nesmeyanov V. Specific binding of glucosaminylmuramyl peptides to histones. FEBS Lett. 1999;454:152–156. doi: 10.1016/s0014-5793(99)00689-4. [DOI] [PubMed] [Google Scholar]
- 26.Greco G, Barker E, Levy J A. Differences in HIV replication in CD4+ lymphocytes are not related to beta-chemokine production. AIDS Res Hum Retrovir. 1998;14:1407–1411. doi: 10.1089/aid.1998.14.1407. [DOI] [PubMed] [Google Scholar]
- 27.Grene E, Pinto L A, Cohen S S, Mac Trubey C, Trivett M T, Simonis T B, Liewehr D J, Steinberg S M, Shearer G. Generation of alloantigen-stimulated anti-human immunodeficiency virus activity is associated with HLA-A*02 expression. J Infect Dis. 2001;183:409–416. doi: 10.1086/318085. [DOI] [PubMed] [Google Scholar]
- 28.Gupta K, Ott D, Hope T J, Siliciano R F, Boeke J D. A human nuclear shuttling protein that interacts with human immunodeficiency virus type 1 matrix is packaged into virions. J Virol. 2000;74:11811–11824. doi: 10.1128/jvi.74.24.11811-11824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haffar O K, Smithgall M D, Popov S, Ulrich P, Bruce A G, Nadler S G, Cerami A, Bukrinsky M I. CNI-H0294, a nuclear importation inhibitor of the human immunodeficiency virus type 1 genome, abrogates virus replication in infected activated peripheral blood mononuclear cells. Antimicrob Agents Chemother. 1998;42:1133–1138. doi: 10.1128/aac.42.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hockertz S, Franke G, Paulini I, Lohmann-Matthes M L. Immunotherapy of murine visceral leishmaniasis with murine recombinant interferon-gamma and MTP-PE encapsulated in liposomes. J Interferon Res. 1991;11:177–185. doi: 10.1089/jir.1991.11.177. [DOI] [PubMed] [Google Scholar]
- 31.Hornung F, Scala G, Lenardo M J. TNF-alpha-induced secretion of C-C chemokines modulates C-C chemokine receptor 5 expression on peripheral blood lymphocytes. J Immunol. 2000;164:6180–6187. doi: 10.4049/jimmunol.164.12.6180. [DOI] [PubMed] [Google Scholar]
- 32.Johannsen L. Biological properties of bacterial peptidoglycan. APMIS. 1993;101:337–344. doi: 10.1111/j.1699-0463.1993.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 33.Kemper C A, Bermudez L E, Deresinski S C. Immunomodulatory treatment of Mycobacterium avium complex bacteremia in patients with AIDS by use of recombinant granulocyte-macrophage colony-stimulating factor. J Infect Dis. 1998;177:914–920. doi: 10.1086/515249. [DOI] [PubMed] [Google Scholar]
- 34.Kricek F, Zunic M, Ruf C, De Jong G, Dukor P, Bahr G M. Suppression of in vivo IgE and tissue IL-4 mRNA induction by SDZ 280.636, a synthetic muramyl dipeptide derivative. Immunopharmacology. 1997;36:27–39. doi: 10.1016/s0162-3109(96)00151-8. [DOI] [PubMed] [Google Scholar]
- 35.Krueger J M, Walter J, Karnovsky M L, Chedid L, Choay J P, Lefrancier P, Lederer E. Muramyl peptides. Variation of somnogenic activity with structure. J Exp Med. 1984;159:68–76. doi: 10.1084/jem.159.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Yoder K, Hansen M S, Olvera J, Miller M D, Bushman F D. Retroviral cDNA integration: stimulation by HMG I family proteins. J Virol. 2000;74:10965–10974. doi: 10.1128/jvi.74.23.10965-10974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macatonia S E, Lau R, Patterson S, Pinching A J, Knight S C. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990;71:38–45. [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuguchi T, Takagi K, Musikacharoen T, Yoshikai Y. Gene expressions of lipopolysaccharide receptors, toll-like receptors 2 and 4, are differently regulated in mouse T lymphocytes. Blood. 2000;95:1378–1385. [PubMed] [Google Scholar]
- 39.Moran P A, Diegel M L, Sias J C, Ledbetter J A, Zarling J M. Regulation of HIV production by blood mononuclear cells from HIV-infected donors. I. Lack of correlation between HIV-1 production and T cell activation. AIDS Res Hum Retrovir. 1993;9:455–464. doi: 10.1089/aid.1993.9.455. [DOI] [PubMed] [Google Scholar]
- 40.Mosier D E, Picchio G R, Gulizia R J, Sabbe R, Poignard P, Picard L, Offord R E, Thompson D A, Wilken J. Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants. J Virol. 1999;73:3544–3550. doi: 10.1128/jvi.73.5.3544-3550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neumann M, Harrison J, Saltarelli M, Hadziyannis E, Erfle V, Felber B K, Pavlakis G N. Splicing variability in HIV type 1 revealed by quantitative RNA polymerase chain reaction. AIDS Res Hum Retrovir. 1994;10:1531–1542. doi: 10.1089/aid.1994.10.1531. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen S D, Afzelius P, Dam-Larsen S, Nielsen C, Nielsen J O, Mathiesen L, Hansen J E. Effect of granulocyte colony-stimulating factor (G-CSF) in human immunodeficiency virus-infected patients: increase in numbers of naive CD4 cells and CD34 cells makes G-CSF a candidate for use in gene therapy or to support antiretroviral therapy. J Infect Dis. 1998;177:1733–1736. doi: 10.1086/517434. [DOI] [PubMed] [Google Scholar]
- 43.O'Reilly T, Zak O. Enhancement of the effectiveness of antimicrobial therapy by muramyl peptide immunomodulators. Clin Infect Dis. 1992;14:1100–1109. doi: 10.1093/clinids/14.5.1100. [DOI] [PubMed] [Google Scholar]
- 44.Pantaleo G. How immune-based interventions can change HIV therapy. Nat Med. 1997;3:483–486. doi: 10.1038/nm0597-483. [DOI] [PubMed] [Google Scholar]
- 45.Patterson B K, Carlo D J, Kaplan M H, Marecki M, Pawha S, Moss R B. Cell-associated HIV-1 messenger RNA and DNA in T-helper cell and monocytes in asymptomatic HIV-1-infected subjects on HAART plus an inactivated HIV-1 immunogen. AIDS. 1999;13:1607–1611. doi: 10.1097/00002030-199909100-00002. [DOI] [PubMed] [Google Scholar]
- 46.Piatak M, Luk K C, Williams B, Lifson J D. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. BioTechniques. 1993;14:70–81. [PubMed] [Google Scholar]
- 47.Pinto L A, Blazevic V, Patterson B K, Mac Trubey C, Dolan M J, Shearer G M. Inhibition of human immunodeficiency virus type 1 replication prior to reverse transcription by influenza virus stimulation. J Virol. 2000;74:4505–4511. doi: 10.1128/jvi.74.10.4505-4511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poignard P, Fouts T, Naniche D, Moore J P, Sattentau Q J. Neutralizing antibodies to human immunodeficiency virus type-1 gp120 induce envelope glycoprotein subunit dissociation. J Exp Med. 1996;183:473–484. doi: 10.1084/jem.183.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pouillart P R, Audibert F M, Chedid L A, Lefrancier P L, Bahr G M. Enhancement by muramyl peptides of the protective response of interferon-alpha/beta against encephalomyocarditis virus infection. Int J Immunopharmacol. 1996;18:183–192. doi: 10.1016/0192-0561(96)00005-7. [DOI] [PubMed] [Google Scholar]
- 50.Ramratnam B, Mittler J E, Zhang L, Boden D, Hurley A, Fang F, Macken C A, Perelson A S, Markowitz M, Ho D D. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6:82–85. doi: 10.1038/71577. [DOI] [PubMed] [Google Scholar]
- 51.Riveau G J, Brunel-Riveau B G, Audibert F M, Chedid L A. Influence of a muramyl dipeptide on human blood leukocyte functions and their membrane antigens. Cell Immunol. 1991;134:147–156. doi: 10.1016/0008-8749(91)90338-c. [DOI] [PubMed] [Google Scholar]
- 52.Riveau G J, Chedid L. Comparison of the immuno- and neuro-pharmacological activities of MDP and Murabutide. In: Majde J A, editor. Immunopharmacology of infectious diseases: vaccine adjuvants and modulators of non-specific resistance. New York, N.Y: Alan R. Liss; 1987. pp. 213–222. [Google Scholar]
- 53.Rizza P, Santini S M, Logozzi M A, Lapenta C, Sestili P, Gherardi G, Lande R, Spada M, Parlato S, Belardelli F, Fais S. T-cell dysfunctions in hu-PBL-SCID mice infected with human immunodeficiency virus (HIV) shortly after reconstitution: in vivo effects of HIV on highly activated human immune cells. J Virol. 1996;70:7958–7964. doi: 10.1128/jvi.70.11.7958-7964.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenberg E S, Altfeld M, Poon S H, Phillips M N, Wilkes B M, Eldridge R L, Robbins G K, D'Aquila R T, Goulder P J, Walker B D. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 55.Sandhu J, Shpitz B, Gallinger S, Hozumi N. Human primary immune response in SCID mice engrafted with human peripheral blood lymphocytes. J Immunol. 1994;152:3806–3813. [PubMed] [Google Scholar]
- 56.Sandstrom E, Wahren B. Therapeutic immunisation with recombinant gp160 in HIV-1 infection: a randomised double-blind placebo-controlled trial. Nordic VAC-04 Study Group. Lancet. 1999;353:1735–1742. doi: 10.1016/s0140-6736(98)06493-9. [DOI] [PubMed] [Google Scholar]
- 57.Schwartz S, Felber B K, Benko D M, Fenyo E M, Pavlakis G N. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J Virol. 1990;64:2519–2529. doi: 10.1128/jvi.64.6.2519-2529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shearer G M. HIV-induced immunopathogenesis. Immunity. 1998;9:587–593. doi: 10.1016/s1074-7613(00)80656-1. [DOI] [PubMed] [Google Scholar]
- 59.Smithgall M D, Wong J G, Critchett K E, Haffar O K. IL-7 up-regulates HIV-1 replication in naturally infected peripheral blood mononuclear cells. J Immunol. 1996;156:2324–2330. [PubMed] [Google Scholar]
- 60.Starr S E. Immune reconstitution in HIV-infected individuals—what will it take? Immunologist. 1998;6:19–22. [Google Scholar]
- 61.Steger D J, Eberharter A, John S, Grant P A, Workman J L. Purified histone acetyltransferase complexes stimulate HIV-1 transcription from preassembled nucleosomal arrays. Proc Natl Acad Sci USA. 1998;95:12924–12929. doi: 10.1073/pnas.95.22.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stoddart C A, Moreno M E, Linquist-Stepps V D, Bare C, Bogan M R, Gobbi A, Buckheit R W, Bedard J, Rando R F, McCune J M. Antiviral activity of 2′-deoxy-3′-oxa-4′-thiocytidine (BCH-10652) against lamivudine-resistant human immunodeficiency virus type 1 in SCID-hu Thy/Liv mice. Antimicrob Agents Chemother. 2000;44:783–786. doi: 10.1128/aac.44.3.783-786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugimoto M, Germain R N, Chedid L, Benacerraf B. Enhancement of carrier-specific helper T cell function by the synthetic adjuvant, N-acetyl muramyl-L-alanyl-D-isoglutamine (MDP) J Immunol. 1978;120:980–982. [PubMed] [Google Scholar]
- 64.Sumaroka M V, Litvinov I S, Khaidukov S V, Golovina T N, Kamraz M V, Komal'eva R L, Andronova T M, Makarov E A, Nesmeyanov V A, Ivanov V T. Muramyl peptide-binding sites are located inside target cells. FEBS Lett. 1991;295:48–50. doi: 10.1016/0014-5793(91)81381-h. [DOI] [PubMed] [Google Scholar]
- 65.Sun Y, Clark E A. Expression of the c-myc proto-oncogene is essential for HIV-1 infection in activated T cells. J Exp Med. 1999;189:1391–1398. doi: 10.1084/jem.189.9.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun Y, Li L, Lau F, Beavo J A, Clark E A. Infection of CD4+ memory T cells by HIV-1 requires expression of phosphodiesterase 4. J Immunol. 2000;165:1755–1761. doi: 10.4049/jimmunol.165.4.1755. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki T, Hashimoto S, Toyoda N, Nagai S, Yamazaki N, Dong H Y, Sakai J, Yamashita T, Nukiwa T, Matsushima K. Comprehensive gene expression profile of LPS-stimulated human monocytes by SAGE. Blood. 2000;96:2584–2591. [PubMed] [Google Scholar]
- 68.Telzak E, Wolff S M, Dinarello C A, Conlon T, el Kholy A, Bahr G M, Choay J P, Morin A, Chedid L. Clinical evaluation of the immunoadjuvant murabutide, a derivative of MDP, administered with a tetanus toxoid vaccine. J Infect Dis. 1986;153:628–633. doi: 10.1093/infdis/153.3.628. [DOI] [PubMed] [Google Scholar]
- 69.ten Hagen T L, van Vianen W, Savelkoul H F, Heremans H, Buurman W A, Bakker-Woudenberg I A. Involvement of T cells in enhanced resistance to Klebsiella pneumoniae septicemia in mice treated with liposome-encapsulated muramyl tripeptide phosphatidylethanolamine or gamma interferon. Infect Immun. 1998;66:1962–1967. doi: 10.1128/iai.66.5.1962-1967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tenu J P, Adam A, Souvannavong V, Yapo A, Petit J F, Douglas K. Photoaffinity labelling of macrophages and B-lymphocytes using 125I-labelled aryl-azide derivatives of muramyldipeptide. Int J Immunopharmacol. 1989;11:653–661. doi: 10.1016/0192-0561(89)90151-3. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z M, Liu C, Dziarski R. Chemokines are the main proinflammatory mediators in human monocytes activated by Staphylococcus aureus, peptidoglycan, and endotoxin. J Biol Chem. 2000;275:20260–20267. doi: 10.1074/jbc.M909168199. [DOI] [PubMed] [Google Scholar]
- 72.Weidemann B, Schletter J, Dziarski R, Kusumoto S, Stelter F, Rietschel E T, Flad H D, Ulmer A J. Specific binding of soluble peptidoglycan and muramyldipeptide to CD14 on human monocytes. Infect Immun. 1997;65:858–864. doi: 10.1128/iai.65.3.858-864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Werner G H, Jolles P. Immunostimulating agents: what next? A review of their present and potential medical applications. Eur J Biochem. 1996;242:1–19. doi: 10.1111/j.1432-1033.1996.0001r.x. [DOI] [PubMed] [Google Scholar]
- 74.Wyde P R, Six H R, Ambrose M W, Throop B J. Muramyl peptides and polyinosinic-polycytidylic acid given to mice prior to influenza virus challenge reduces pulmonary disease and mortality. J Biol Response Modif. 1990;9:98–102. [PubMed] [Google Scholar]
- 75.Yoo J, Chen H, Kraus T, Hirsch D, Polyak S, George I, Sperber K. Altered cytokine production and accessory cell function after HIV-1 infection. J Immunol. 1996;157:1313–1320. [PubMed] [Google Scholar]
- 76.Zidek Z. Differences in proinflammatory activity of several immunomodulatory derivatives of muramyl dipeptide (MDP) with special reference to the mechanism of the MDP effects. Agents Actions. 1992;36:136–145. doi: 10.1007/BF01991241. [DOI] [PubMed] [Google Scholar]
- 77.Zunic M, Kricek F, Dukor P, Bahr G M. Oral administration of muramyl dipeptide into mice modulates cell proliferation, immunoglobulin synthesis and cytokine mRNA levels in gut associated lymphoid tissues. Int J Immunopharmacol. 1996;18:155–162. doi: 10.1016/0192-0561(95)00114-x. [DOI] [PubMed] [Google Scholar]