Abstract
Valve-in-mitral annular calcification presents a great challenge with a risk of left ventricular outflow tract obstruction (LVOTO). We demonstrate the first-in-human experience of performing percutaneous electrosurgery-guided perforation and balloon dilation of the anterior mitral valve leaflet followed by transcatheter valve implantation to prevent LVOTO.
Key Words: anterior mitral valve leaflet modification, electrosurgery, left ventricular outflow obstruction, valve-in-MAC
Graphical Abstract
Although transcatheter therapies for mitral valve disease have been quickly evolving, therapies for native mitral stenosis do present challenges. Neo–left ventricular outflow tract (neo-LVOT) obstruction in patients undergoing mitral valve replacement can be hemodynamically devastating, with immediate periprocedural complications. Different leaflet modification techniques have been used to prevent or minimize the risk of left ventricular outflow tract obstruction (LVOTO), with laceration of the anterior mitral leaflet to prevent outflow obstruction (LAMPOON) being the most accepted technique. We report the first-in-human experience of performing percutaneous electrosurgery-guided perforation and balloon dilation of the native anterior mitral valve leaflet to prevent LVOTO.
Take-Home Messages
-
•
LVOTO with TMVR can be a devastating complication, and appropriate preprocedural planning should be done in all TMVR cases with multimodality imaging and 3D model printing.
-
•
Leaflet modification of the native anterior mitral valve leaflet with subsequent balloon dilation and deployment of the valve can be a viable option to treat ViMAC.
History of Presentation
An 89-year-old man presented to our tertiary care center (Heart and Vascular Institute, Cleveland Clinic, Cleveland, Ohio, USA) for evaluation of progressively worsening shortness of breath.
Past Medical History
His past medical history was significant for a previous bioprosthetic aortic valve replacement and myectomy.
Investigations and Management
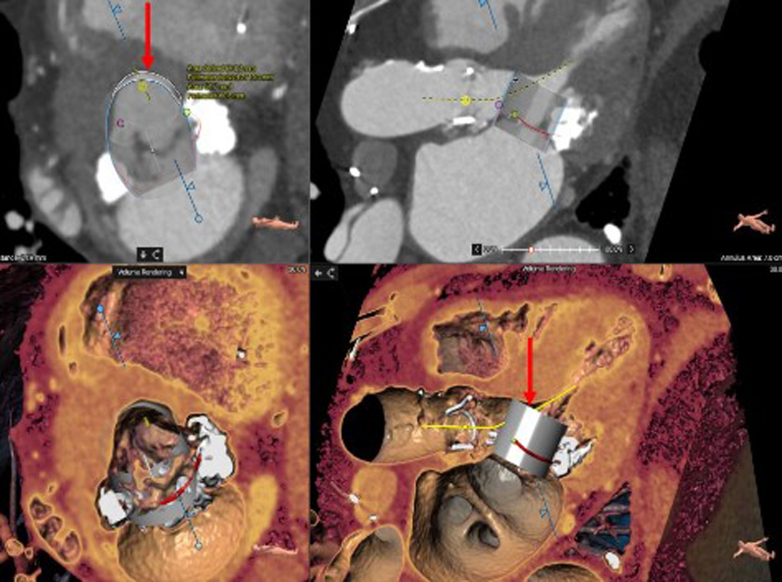
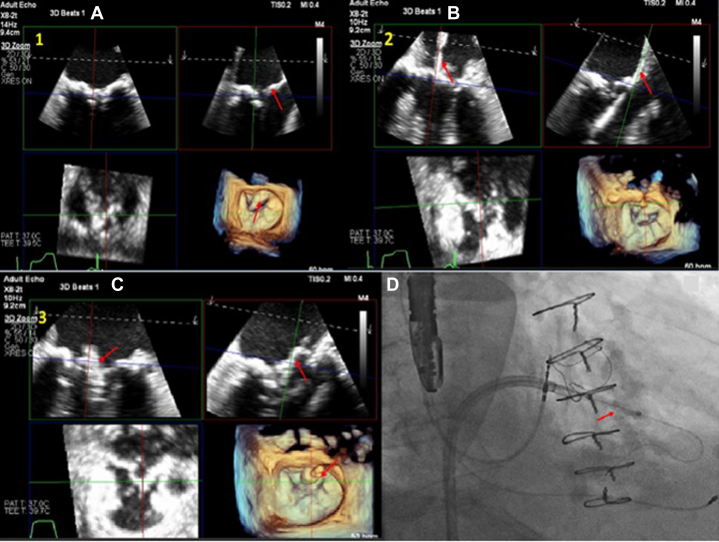
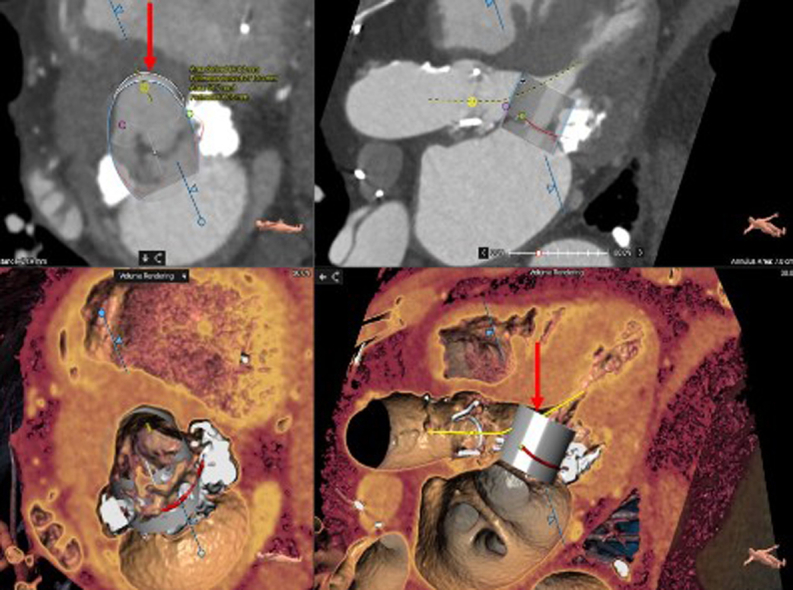
Transthoracic echocardiography (TTE) was performed. It showed that he had severe mitral annular calcification (MAC) with elevated gradients (peak/mean of 19/11 at a heart rate of 76 beats/min) across the mitral valve, with moderately severe mitral regurgitation as well. Initial management included optimization of medical therapy; however, the patient continued to remain symptomatic. He was deemed to be a very high risk for redo surgery, thus prompting evaluation for percutaneous options. Cardiac-gated contrast-enhanced computed tomography (CT) of the chest raised the concern for neo-LVOT obstruction with transcatheter mitral valve replacement (TMVR) (Figure 1), considering the left ventricular outflow tract (LVOT) size and the length of the anterior mitral leaflet.
Figure 1.
Computed Tomography Reconstruction
The images show the neo-left ventricular outflow tract measurement with an embedded 29-mm SAPIEN 3 Ultra valve (Edwards Lifesciences) (arrows).
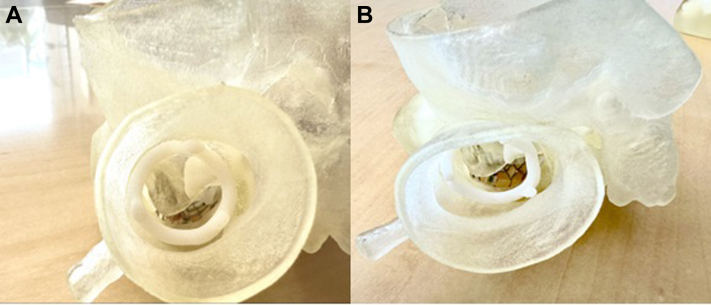
A 3-dimensional (3D) model was printed (Figure 2A) to review treatment options that could be pursued to mitigate the risk of LVOTO. TTE demonstrated an upper septal thickness of 1.6 cm. To reduce the risk of neo-LVOT obstruction, we performed alcohol septal ablation of the first medium-sized septal perforator (Figures 3A and 3B, pre- and post-ablation). Two months later, another gated contrast-enhanced CT scan of the chest was performed and showed a slight improvement in the neo-LVOT, although it was still not large enough to prevent obstruction (Figure 4). A 3D model of the heart was again printed, and it did show some decrease in septum thickness (Figure 2B). The patient was evaluated for enrollment in TMVR trials but did not meet inclusion criteria because of his annular and LVOT anatomy. The plan was to perform a valve-in-mitral annular calcification (ViMAC) procedure using a SAPIEN 3 Ultra valve (Edwards Lifesciences) with adjunctive leaflet modification to prevent LVOTO.
Figure 2.
Neo–Left Ventricular Outflow Tract Before and After Ablation
(A) A 3-dimensional printed model of the heart showing the neo–left ventricular outflow tract before alcohol septal ablation. (B) A 3-dimensional printed model of the heart showing the neo–left ventricular outflow tract post alcohol septal ablation.
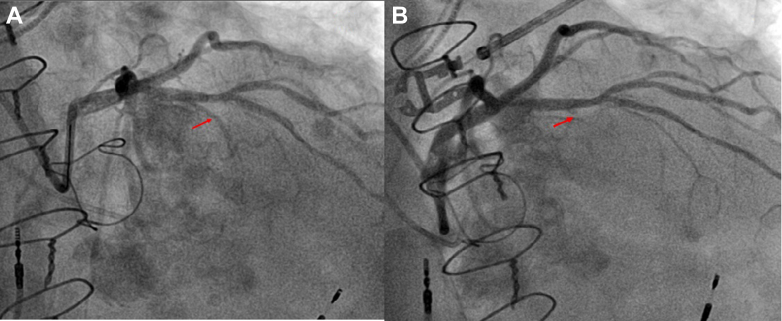
Figure 3.
Angiograms Before and After Ablation
(A) The left anterior descending artery and the first septal perforator before alcohol septal ablation (arrow). (B) The first septal perforator post alcohol septal ablation (arrow).
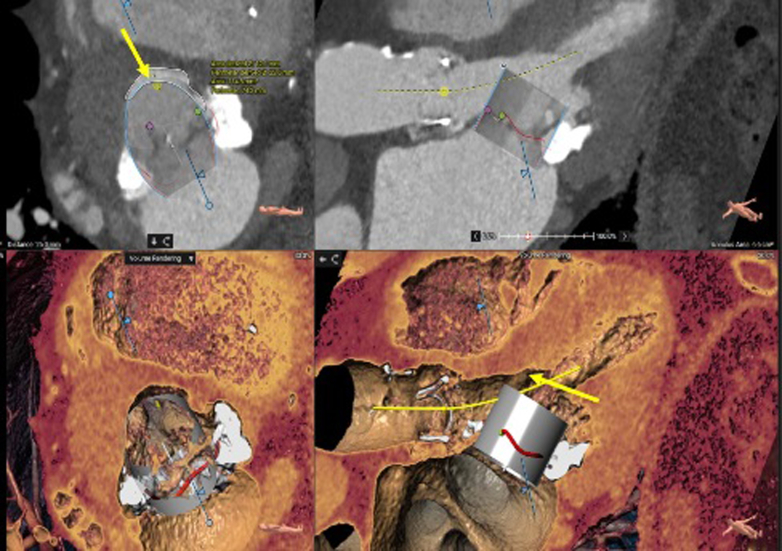
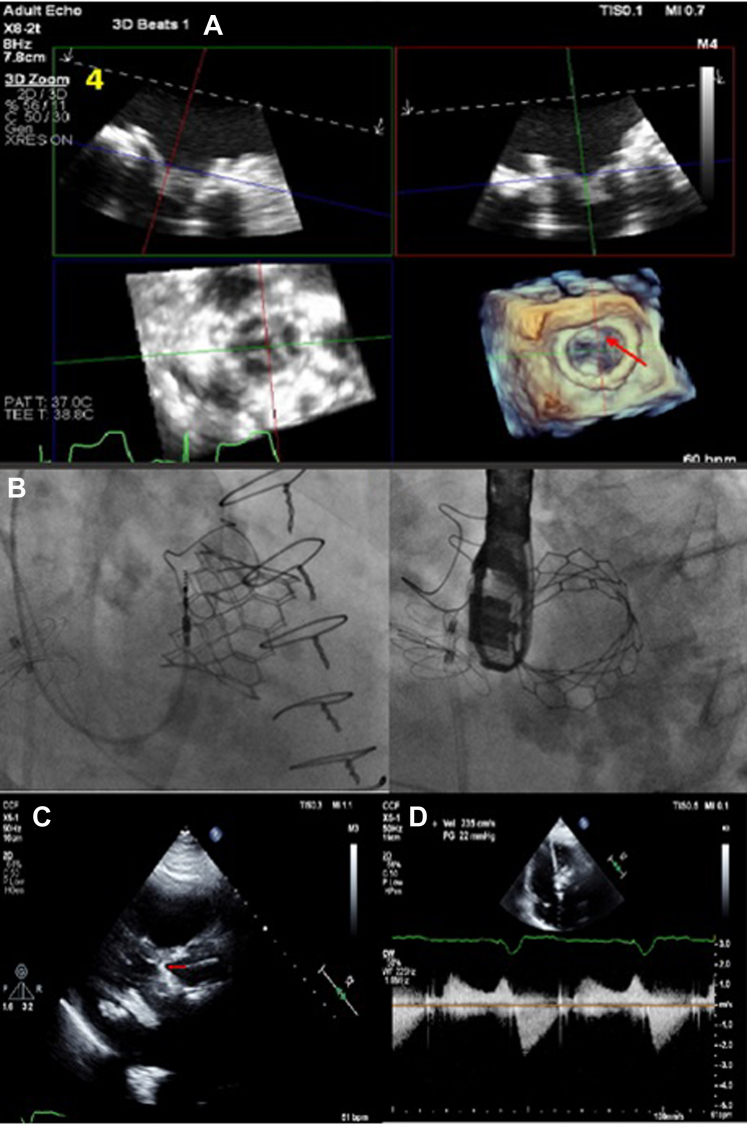
Figure 4.
Neo-Left Ventricular Outflow Tract Measurement
Measurement with an embedded 29-mm SAPIEN 3 Ultra valve (Edwards Lifesciences) (arrows) after alcohol septal ablation.
Leaflet modification technique
A novel percutaneous leaflet modification technique (previously used in a mitral valve-in-valve replacement)1 was planned in which the anterior mitral leaflet was perforated using an electrosurgical technique and was subsequently dilated using a balloon to facilitate valve deployment and mitigate the risk of neo-LVOT obstruction. A similar leaflet modification technique was performed surgically through a transapical approach.2 A similar technique of leaflet modification has also been subsequently described for a valve-in-ring procedure.3
Procedural details
The procedure was performed using general anesthesia. A cerebral embolic protection device was placed. A 16-F eSheath (Edwards Lifesciences) was then inserted in the right femoral vein, and an 8-F sheath was also placed in the right femoral artery for placement of an intra-aortic balloon pump (IABP) if needed for hemodynamic support. Following transseptal puncture, a medium-curl Agilis steerable sheath (Abbott) was directed toward the anterior mitral leaflet. Under transesophageal echocardiography (TEE) guidance, the center base of the anterior mitral leaflet was identified. Using the back end (straight) of an 0.035-inch wire through a 4 mm × 40 mm peripheral Charger balloon (Boston Scientific), the native anterior mitral leaflet was perforated (“cut” setting at 70 W) (Figures 5A and 5B, Video 1). The wire was then advanced to the left ventricle. We then dilated through the perforated anterior mitral leaflet with the 4.0 mm × 40 mm Charger balloon over a Wholey wire. The Wholey wire was exchanged for an 0.035” Confida wire (Medtronic) through an angled glide catheter, and the Agilis catheter was subsequently removed. Next the leaflet was further dilated with a 12 mm × 40 mm Charger balloon (Figures 5C and 5D). A 40-cc IABP was placed for hemodynamic support if needed, given the short duration of wide-open mitral regurgitation from perforation of the leaflet. The 29-mm SAPIEN 3 Ultra valve was deployed through the modified leaflet (20% atrial and 80% ventricular) with rapid ventricular pacing at 180 beats/min (Video 2). Figures 6A and 6B show the corresponding fluoroscopic and TEE images with the valve implanted in the mitral position.
Figure 5.
Multiplanar Reconstruction Images of the Procedure
(A and B) Multiplanar reconstruction images of the wire at the center base of the anterior leaflet (arrows) followed by perforation of the anterior mitral leaflet. (C) Multiplanar reconstruction images and (D) fluoroscopy showing balloon dilation of the perforated anterior mitral leaflet (arrows).
Figure 6.
Postprocedural Imaging
(A) Multiplanar reconstruction images and (B) fluoroscopy showing the 29-mm SAPIEN 3 Ultra valve (Edwards Lifesciences) placed in the mitral position (arrow in A). (C) Transthoracic echocardiography showing the SAPIEN 3 Ultra valve in the mitral position with the frame of the valve touching the septum (arrow). (D) Gradient across the neo–left ventricular outflow tract showing no significant dynamic obstruction.
TEE immediately post valve deployment showed a well seated SAPIEN 3 Ultra valve in the mitral position with no evidence of mitral regurgitation or paravalvular leak.
Outcome and Follow-Up
The patient was monitored in the hospital overnight and was discharged the next day. Echocardiography performed on postprocedure day 1 showed a mean gradient of 5 mm Hg across the mitral valve with no flow acceleration across the LVOT suggestive of obstruction (Figure 6C). The echocardiogram did demonstrate that there would have been definite obstruction if steps had not been taken to modify the mitral valve leaflet (Figure 6D). Clinical follow-up at 1 week and 6 months continued to show symptomatic improvement.
Discussion
LVOTO, if it occurs, can lead to very rapid deterioration and a poor outcome.4 Careful assessment is required to determine risk of obstruction with multimodality imaging.5,6 In this case, we additionally decided to print a 3D model to assess the risk of obstruction before and after performing alcohol septal ablation. Different techniques have been described to decrease the risk of LVOTO, one of which is the LAMPOON procedure, which has been shown to be quite effective.7
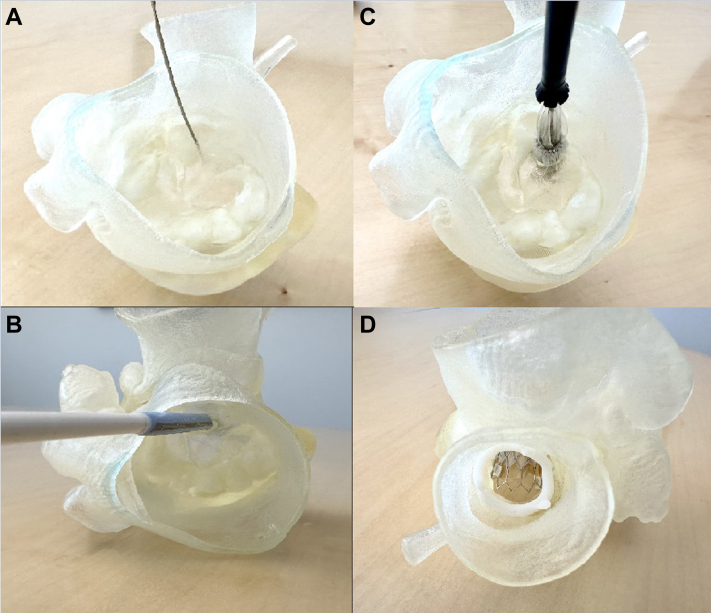
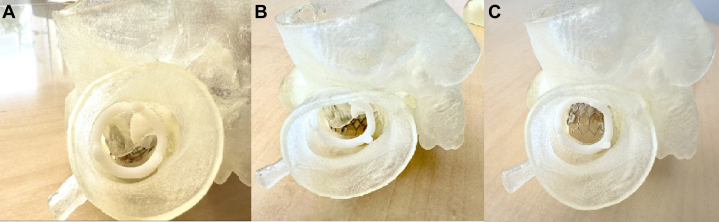
We propose a new and novel technique providing safe and controlled perforation of the anterior mitral leaflet at the center base of the leaflet with a wire and balloon dilation (Figures 7A to 7D). Figures 8A to 8C show a side-by-side comparison of an implanted SAPIEN 3 Ultra valve in the mitral position before alcohol septal ablation, after ablation, and after leaflet modification. In LAMPOON, cutting of the leaflet is dependent on the direction of the pull, which would not have been an issue in this case.7,8 In cases where the leaflets are extremely calcified, the cut may not be linear, and it can be off-axis from the LVOT. In our procedure, modifying the leaflet from the center base mitigates the risk of outflow obstruction by displacing the leaflets away from the LVOT. Additionally, the number of steps and wire exchanges is less, making it a simpler workflow procedure. There is, however, a risk of hemodynamic compromise from severe mitral regurgitation after leaflet modification, although this risk was mitigated in our case by the use of an IABP and rapid deployment of the transcatheter heart valve.
Figure 7.
Procedural Steps on the 3-Dimensional-Printed Model
(A) Perforating the anterior mitral leaflet from the atrial side. (B) The eSheath (Edwards Lifesciences) from the ventricular view through the anterior leaflet after balloon dilation of the leaflet. (C) Positioning of the valve from the atrial view with the device advanced through the anterior leaflet. (D) The 29-mm SAPIEN 3 Ultra valve (Edwards Lifesciences) implanted in the mitral position with no leaflet obstructing the left ventricular outflow tract through the aorta.
Figure 8.
Side-by-Side Comparison of Valve Deployment Through the Left Ventricular Outflow Tract View
(A) The valve deployed before alcohol septal ablation. (B) The valve deployed after alcohol septal ablation. (C) The valve deployed after leaflet modification and deployment of the valve after perforation through the anterior mitral leaflet.
Conclusions
ViMAC presents a great challenge with a risk of LVOTO. TMVR using electrosurgery-guided perforation of the anterior mitral leaflet followed by balloon dilation and valve deployment may be a feasible option. Further studies need to be performed to show safety and reproducibility of this leaflet modification technique.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Perforating the AML
Fluoroscopy of the back end of the J wire perforating the anterior mitral leaflet.
S3 Inflation
Deployment of the 29-mm SAPIEN 3 Ultra valve (Edwards Lifesciences) (S3) through the perforated anterior mitral valve leaflet.
References
- 1.Spilias N., Miyasaka R., Kapadia S.R., Krishnaswamy A. A novel method of prosthetic leaflet modification to enable transcatheter mitral valve-in-valve replacement. Catheter Cardiovasc Interv. 2023;102(6):1149–1153. doi: 10.1002/ccd.30872. [DOI] [PubMed] [Google Scholar]
- 2.Helmy T., Hui D.S., Smart S., Lim M.J., Lee R. Balloon assisted translocation of the mitral anterior leaflet to prevent left ventricular outflow obstruction (BATMAN): a novel technique for patients undergoing transcatheter mitral valve replacement. Catheter Cardiovasc Interv. 2020;95(4):840–848. doi: 10.1002/ccd.28496. [DOI] [PubMed] [Google Scholar]
- 3.Oliva A., Mangieri A., Cozzi O., et al. Transseptal balloon-assisted translocation of the mitral anterior leaflet (BATMAN) in mitral valve-in-ring implantation. JACC Cardiovasc Interv. 2024;17(4):568–570. doi: 10.1016/j.jcin.2023.10.040. [DOI] [PubMed] [Google Scholar]
- 4.Yoon S.H., Bleiziffer S., Latib A., et al. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. JACC Cardiovasc Interv. 2019;12(2):182–193. doi: 10.1016/j.jcin.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Meduri C.U., Reardon M.J., Lim D.S., et al. Novel multiphase assessment for predicting left ventricular outflow tract obstruction before transcatheter mitral valve replacement. JACC Cardiovasc Interv. 2019;12(23):2402–2412. doi: 10.1016/j.jcin.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Eleid M.F., Collins J.D., Mahoney P., et al. Emerging approaches to management of left ventricular outflow obstruction risk in transcatheter mitral valve replacement. JACC Cardiovasc Interv. 2023;16(8):885–895. doi: 10.1016/j.jcin.2023.01.357. [DOI] [PubMed] [Google Scholar]
- 7.Khan J.M., Babaliaros V.C., Greenbaum A.B., et al. Anterior leaflet laceration to prevent ventricular outflow tract obstruction during transcatheter mitral valve replacement. J Am Coll Cardiol. 2019;73(20):2521–2534. doi: 10.1016/j.jacc.2019.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Case B.C., Lisko J.C., Babaliaros V.C., et al. LAMPOON techniques to prevent or manage left ventricular outflow tract obstruction in transcatheter mitral valve replacement. Ann Cardiothorac Surg. 2021;10(1) doi: 10.21037/acs-2020-mv-25. 17279-17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Perforating the AML
Fluoroscopy of the back end of the J wire perforating the anterior mitral leaflet.
S3 Inflation
Deployment of the 29-mm SAPIEN 3 Ultra valve (Edwards Lifesciences) (S3) through the perforated anterior mitral valve leaflet.