Abstract
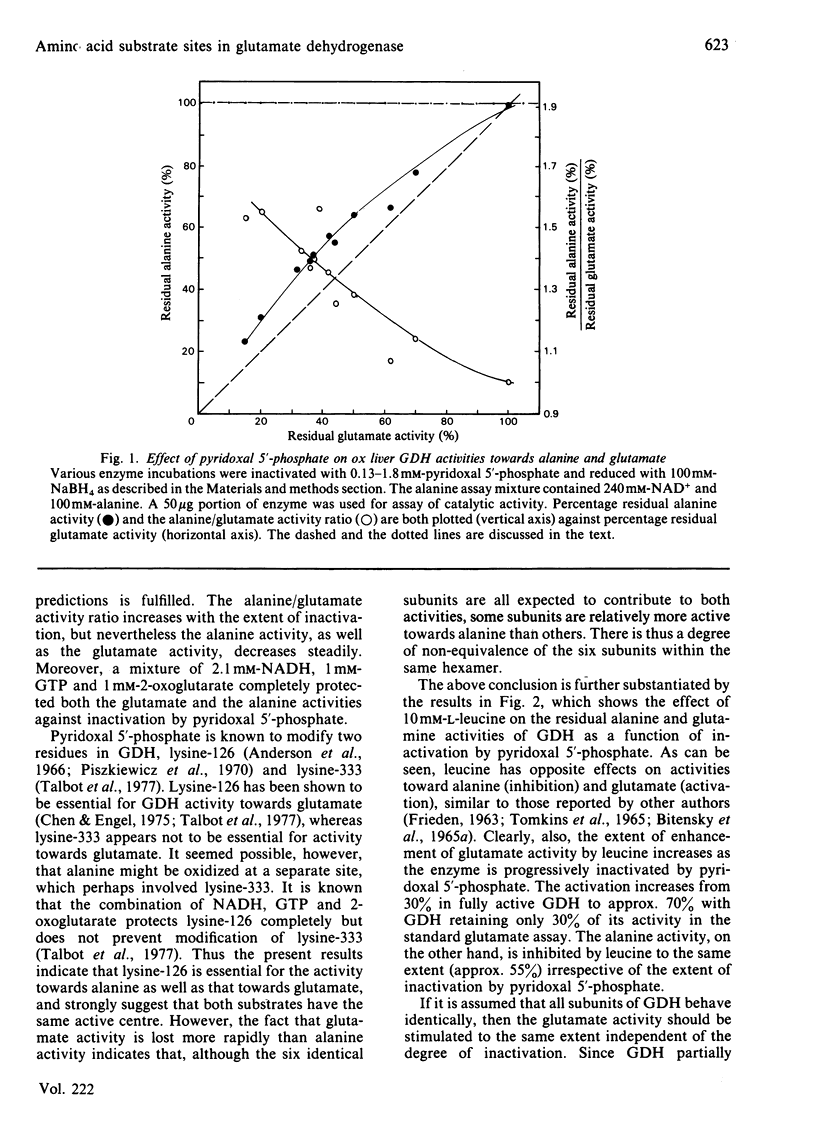
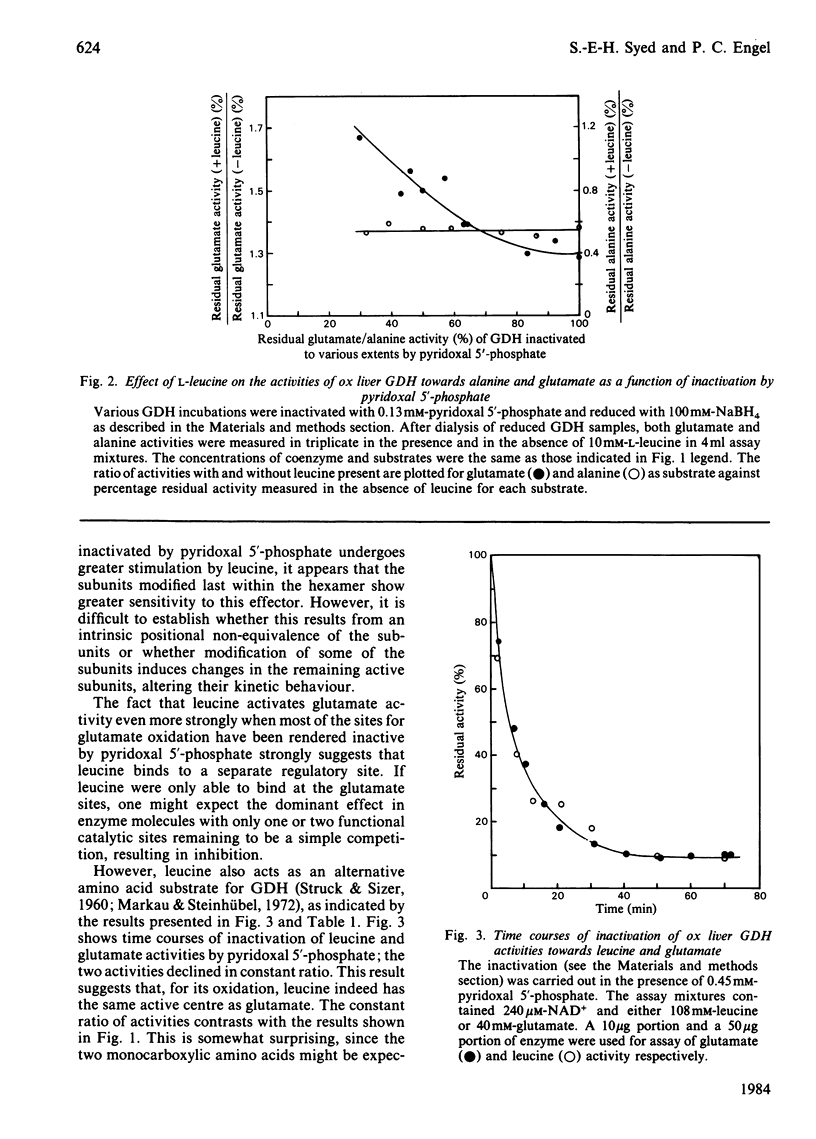
The effect of pyridoxal 5'-phosphate on the activity of ox liver glutamate dehydrogenase towards different amino acid substrates was investigated. Both alanine and glutamate activities decreased steadily in the presence of pyridoxal 5'-phosphate. The alanine/glutamate activity ratio increased as a function of inactivation by pyridoxal 5'-phosphate, indicating that glutamate activity is lost more rapidly than alanine activity. A mixture of NADH, GTP and 2-oxoglutarate completely protected the alanine and glutamate activities against inactivation by pyridoxal 5'-phosphate. The activity of glutamate dehydrogenase towards glutamate and leucine decreased steadily in a constant ratio in the presence of pyridoxal 5'-phosphate. The effect of leucine on the alanine and glutamate activities as a function of inactivation by pyridoxal 5'-phosphate was studied. The results are interpreted to suggest that the subunits of glutamate dehydrogenase hexamer are kinetically non-equivalent with regard to activity towards the two monocarboxylic amino acids as well as glutamate, and that all three substrates share the same active centre. However, leucine is also able to bind at a separate regulatory site.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. M., Anderson C. D., Churchich J. E. Inhibition of glutamic dehydrogenase by pyridoxal 5'-phosphate. Biochemistry. 1966 Sep;5(9):2893–2900. doi: 10.1021/bi00873a017. [DOI] [PubMed] [Google Scholar]
- BITENSKY M. W., YIELDING K. L., TOMKINS G. M. RECIPROCAL CHANGES IN ALANINE AND GLUTAMATE DEHYDROGENASE ACTIVITIES AFTER EXPOSURE OF CRYSTALLINE BOVINE L-GLUTAMATE DEHYDROGENASE TO ORGANIC MERCURY. J Biol Chem. 1965 Feb;240:663–667. [PubMed] [Google Scholar]
- BITENSKY M. W., YIELDING K. L., TOMKINS G. M. THE REVERSAL BY ORGANIC MERCURIALS OF "ALLOSTERIC" CHANGES IN GLUTAMATE DEHYDROGENASE. J Biol Chem. 1965 Feb;240:668–673. [PubMed] [Google Scholar]
- Bell J. E., Dalziel K. A conformational transition of the oligomer of glutamate dehydrogenase induced by half-saturation with NAD + or NADP + . Biochim Biophys Acta. 1973 May 5;309(1):237–242. doi: 10.1016/0005-2744(73)90336-7. [DOI] [PubMed] [Google Scholar]
- Brown A., Culver J. M., Fisher H. F. Mechanism of inactivation of L-glutamate dehydrogenase by pyridoxal and pyridoxal phosphate. Biochemistry. 1973 Oct 23;12(22):4367–4373. doi: 10.1021/bi00746a011. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Engel P. C. Ox liver glutamate dehydrogenase. The role of lysine-126 reappraised in the light of studies of inhibition and inactivation by pyridoxal 5'-phosphate. Biochem J. 1975 Sep;149(3):619–626. doi: 10.1042/bj1490619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee C. J., Bradshaw R. A., Goldin B. R., Frieden C. Identification of the sites of modification of bovine liver glutamate dehydrogenase reacted with trinitrobenzenesulfonate. Biochemistry. 1971 Sep 14;10(19):3516–3526. doi: 10.1021/bi00795a005. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M., Engel P. C. The preparation of pure salt-free nicotinamide coenzymes. Anal Biochem. 1977 Oct;82(2):523–531. doi: 10.1016/0003-2697(77)90191-9. [DOI] [PubMed] [Google Scholar]
- Egan R. R., Dalziel K. Active centre equivalent weight of glutamate dehydrogenase from dry weight determinations and spectrophotometric titrations of abortive complexes. Biochim Biophys Acta. 1971 Oct;250(1):47–50. doi: 10.1016/0005-2744(71)90118-5. [DOI] [PubMed] [Google Scholar]
- Engel P. C., Dalziel K. Kinetic studies of glutamate dehydrogenase with glutamate and norvaline as substrates. Coenzyme activation and negative homotropic interactions in allosteric enzymes. Biochem J. 1969 Dec;115(4):621–631. doi: 10.1042/bj1150621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER H. F., CROSS D. G., McGREGOR L. L. Catalytic activity of sub-units of glutamic dehydrogenase. Nature. 1962 Dec 1;196:895–896. doi: 10.1038/196895b0. [DOI] [PubMed] [Google Scholar]
- Freedman R. B., Radda G. K. Chemical modification of glutamate dehydrogenase by 2,4,6-trinitrobenzenesulphonic acid. Biochem J. 1969 Sep;114(3):611–619. doi: 10.1042/bj1140611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin B. R., Frieden C. Effect of trinitrophenylation of specific lysyl residues on the catalytic, regulatory, and molecular properties of bovine liver glutamate dehydrogenase. Biochemistry. 1971 Sep 14;10(19):3527–3534. doi: 10.1021/bi00795a006. [DOI] [PubMed] [Google Scholar]
- Hershko A., Kindler S. H. Mode of interaction of purine nucleotides and amino acids with glutamate dehydrogenase. Biochem J. 1966 Dec;101(3):661–664. doi: 10.1042/bj1010661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo M., Pantaloni D. Régulation de l'activité de la glutamate déshydrogènase par les effecteurs GTP et ADP: étude par "stopped flow". Bull Soc Chim Biol (Paris) 1967 Dec 18;49(11):1563–1572. [PubMed] [Google Scholar]
- Markau K., Steinhübel I. Kinetic measurements with monocarboxylic acids as substrates and effectors of glutamate dehydrogenase. FEBS Lett. 1972 Nov 15;28(1):115–120. doi: 10.1016/0014-5793(72)80690-2. [DOI] [PubMed] [Google Scholar]
- Pal P. K., Wechter W. J., Colman R. F. Affinity labeling of the inhibitory DPNH site of bovine liver glutamate dehydrogenase by 5'-fluorosulfonylbenzoyl adenosine. J Biol Chem. 1975 Oct 25;250(20):8140–8147. [PubMed] [Google Scholar]
- Piszkiewicz D., Landon M., Smith E. L. Bovine liver flutamate dehydrogenase. Sequence of a hexadecapeptide containing a lysyl residue reactive with pyridoxal 5'-phosphate. J Biol Chem. 1970 May 25;245(10):2622–2626. [PubMed] [Google Scholar]
- Piszkiewicz D., Smith E. L. Bovine liver glutamate dehydrogenase. Equilibria and kinetics of imine formation by lysine-97 with pyridoxal 5'-phosphate. Biochemistry. 1971 Nov 23;10(24):4544–4552. doi: 10.1021/bi00800a031. [DOI] [PubMed] [Google Scholar]
- Prough R. A., Culver J. M., Fisher H. F. Spectrophotometric evidence for a glutamate dehydrogenase. I. Leucine complex. Arch Biochem Biophys. 1972 Apr;149(2):414–418. doi: 10.1016/0003-9861(72)90339-6. [DOI] [PubMed] [Google Scholar]
- Rasool C. G., Nicolaidis S., Akhtar M. The asymmetric distribution of enzymic activity between the six subunits of bovine liver glutamate dehydrogenase. Use of D- and L-glutamyl alpha-chloromethyl ketones (4-amino-6-chloro-5-oxohexanoic acid. Biochem J. 1976 Sep 1;157(3):675–686. doi: 10.1042/bj1570675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisler E., Eisenberg H. Solubility of toluene in bovine liver glutamate dehydrogenase solutions and enhancement of enzyme association. Biochim Biophys Acta. 1972 Feb 28;258(2):351–357. doi: 10.1016/0005-2744(72)90226-4. [DOI] [PubMed] [Google Scholar]
- STRUCK J., Jr, SIZER I. W. The substrate specificity of glutamic acid dehydrogenase. Arch Biochem Biophys. 1960 Feb;86:260–266. doi: 10.1016/0003-9861(60)90415-x. [DOI] [PubMed] [Google Scholar]
- Smith T., Bell J. E. Mechanism of hysteresis in bovine glutamate dehydrogenase: role of subunit interactions. Biochemistry. 1982 Feb 16;21(4):733–737. doi: 10.1021/bi00533a023. [DOI] [PubMed] [Google Scholar]
- TOMKINS G. M., YIELDING K. L., CURRAN J. Steroid hormone activation of L-alanine oxidation catalyzed by a subunit of crystalline glutamic dehydrogenase. Proc Natl Acad Sci U S A. 1961 Mar 15;47:270–278. doi: 10.1073/pnas.47.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot J. C., Gros C., Cosson M. P., Pantaloni D. Physicochemical evidence for the existence of two pyridoxal 5'-phosphate binding sites on glutamate dehydrogenase and characterization of their functional role. Biochim Biophys Acta. 1977 Sep 27;494(1):19–32. doi: 10.1016/0005-2795(77)90131-3. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Yielding K. L., Curran J. F., Summers M. R., Bitensky M. W. The dependence of the substrate specificity on the conformation of crystalline glutamate dehydrogenase. J Biol Chem. 1965 Oct;240(10):3793–3798. [PubMed] [Google Scholar]
- YIELDING K. L., TOMKINS G. M. An effect of L-leucine and other essential amino acids on the structure and activity of glutamic dehydrogenase. Proc Natl Acad Sci U S A. 1961 Jul 15;47:983–989. doi: 10.1073/pnas.47.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T. Regulation of glutamate dehydrogenase by histidine. Biochim Biophys Acta. 1971 Feb 10;227(2):241–247. doi: 10.1016/0005-2744(71)90057-x. [DOI] [PubMed] [Google Scholar]


