Abstract
There is growing appreciation for inherited structural heart diseases and their genetic causes. One causal gene for congenital cardiac and vascular lesions is FLNA which encodes a critical protein for cytoskeletal and extracellular matrix development. A newborn infant male, with prenatally diagnosed polyvalvular dysfunction, presented with low cardiac output and postnatally detected aortic arch hypoplasia and coarctation. Attempted palliative coarctation intervention resulted in vascular complications that ultimately contributed to his demise. This case report highlights polyvalvular dysplasia, vascular abnormalities, and a likely causal de novo missense variant in the FLNA gene (c.5180 C>T p.P1727L) not previously described.
Key Words: cardiac catheterization filamin A, variant effect prediction, vasculopathy
Graphical Abstract
History of Presentation
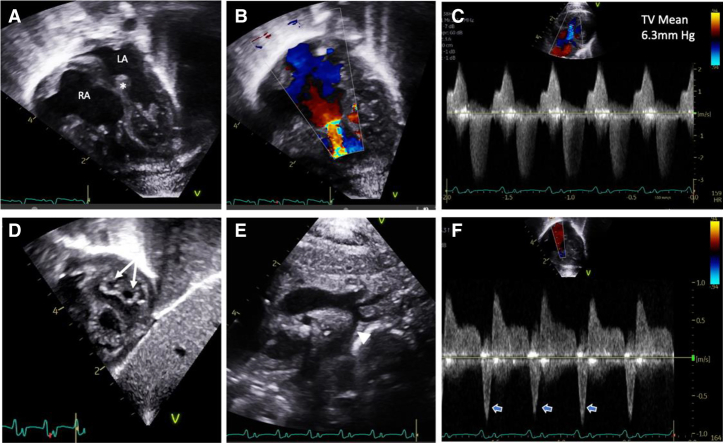
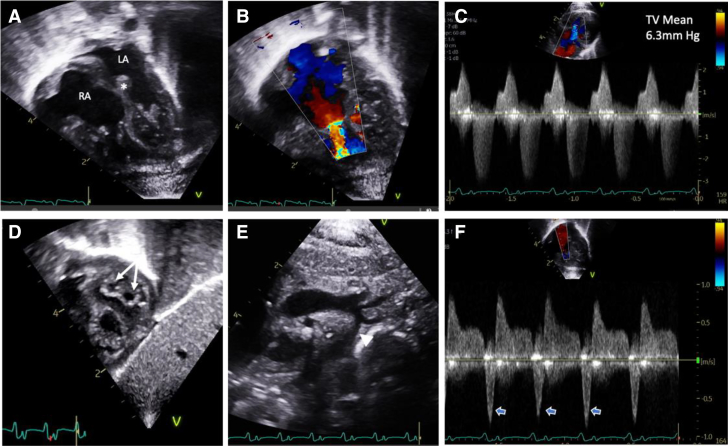
A 2.5-kg baby boy was born at 36 weeks’ gestation to a 23-year-old G1P1 mother. Fetal echocardiography was concerning for polyvalvar dysplasia with biatrial enlargement and abnormal myocardial architecture. The initial postnatal echocardiogram clarified extensive structural defects, including polyvalvular dysplasia, biventricular hypertrophy, and aortic isthmus hypoplasia without coarctation. Valvular abnormalities included tricuspid stenosis (mean gradient, 6.3 mm Hg) with moderate regurgitation, a double-orifice mitral valve with moderate stenosis (mean gradient, 6.0 mm Hg), a bicuspid aortic valve with mild stenosis, and a mildly thickened, well-functioning pulmonary valve (Figures 1A to 1F).
Take-Home Messages
-
•
We advocate for whole exome sequencing in congenital polyvalvular disease, and this case report is the first to describe a presumed causative FLNA gene (c.5180 C>T p.P1727L) sequence variant.
-
•
Clinicians need to consider the vascular integrity of patients with known or suspected FLNA variants before performing any invasive procedure.
Figure 1.
Newborn Echocardiogram
(A) The 4-chamber view demonstrating biatrial enlargement and thickened atrioventricular valves and atrial septum (asterisk). Color Doppler imaging of the tricuspid valve (TV) showing (B) narrow inflow secondary to restricted opening with (C) evidence for tricuspid stenosis and regurgitation on continuous-wave Doppler imaging. (D) The subcostal short-axis view demonstrates a double-orifice mitral valve (arrows). (E) Diffuse aortic arch hypoplasia (arrowhead) is noted. (F) Pulmonary vein Doppler imaging with deep flow reversal with atrial systole (open arrows) suggests elevated filling pressures. LA = left atrium; RA = right atrium.
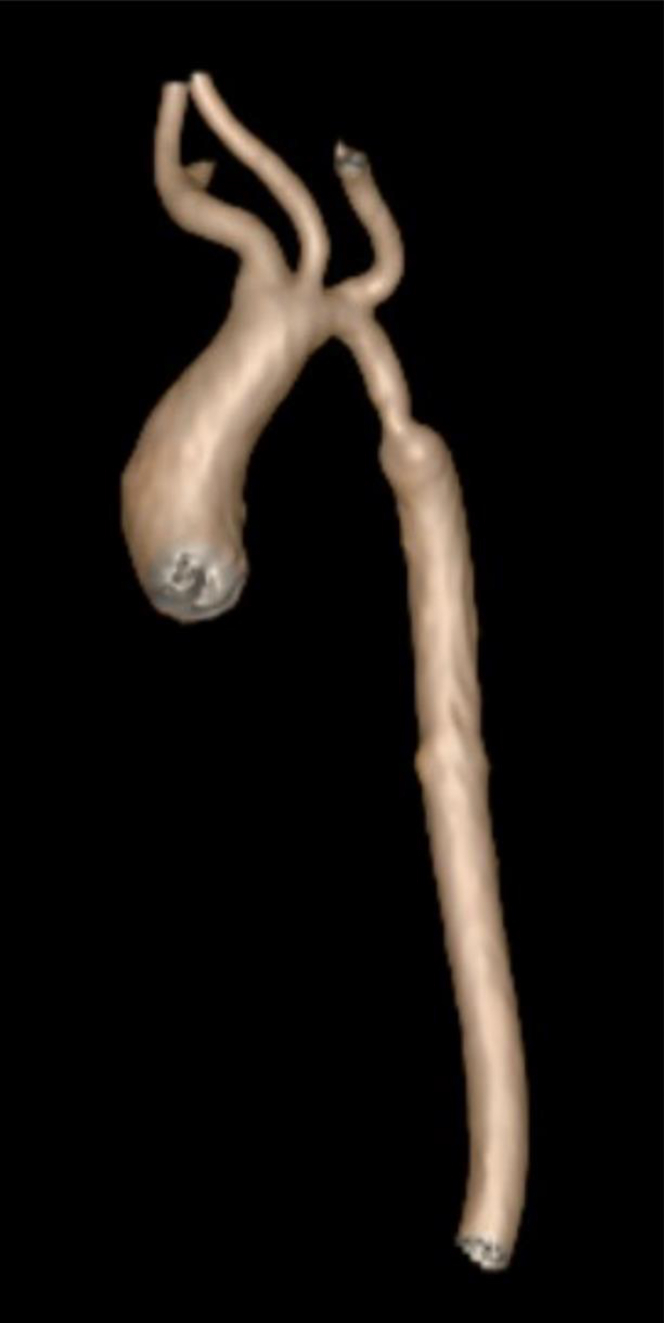
The baby was admitted to the neonatal intensive care unit (NICU) for management of tachypnea, hypoglycemia, and complex cardiac defects. Physical examination was notable for no dysmorphic features, mild acrocyanosis, and a 2-3/6 systolic murmur. By day of life (DOL) 2, the baby developed worsening respiratory status and poor perfusion. A repeat echocardiogram showed evolving coarctation with long-segment distal arch hypoplasia, which was confirmed by computed tomography angiography (Figure 2). Prostaglandin (PGE) infusion was initiated without ductal (patent ductus arteriosus [PDA]) opening or relief of coarctation. Given his complex cardiac defects, whole exome sequencing was performed, which revealed a missense sequence variant in FLNA (NM_001456.3 c.5180 C>T, p.P1727L) on the X chromosome that was not present in the mother. No other coding sequence variants were found.
Figure 2.
3-Dimensional Computed Tomography Reconstruction of Aorta
Distal transverse aortic arch and proximal descending aorta hypoplasia with discrete coarctation, with a minimum diameter of 2 mm.
Management and Outcome
Given the patient’s extensive polyvalvular disease and diffusely hypoplastic aortic arch, he was deemed a poor candidate for surgery. On DOL 8, following a multidisciplinary discussion, he was referred for cardiac catheterization and possible temporizing coarctation intervention.
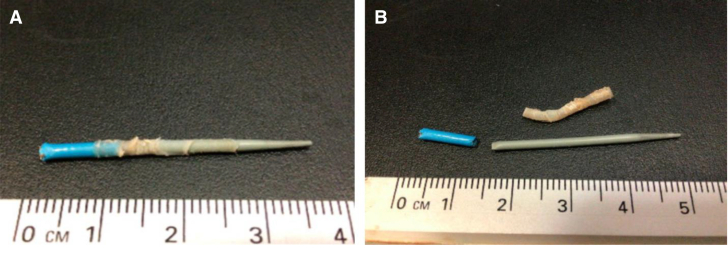
Several vascular anomalies were encountered during cardiac catheterization. Wire access to the right femoral vein under ultrasound guidance was easily accomplished and confirmed radiographically but was followed by difficulty advancing a standard 6-F venous sheath over the 0.018-inch access wire. Gradual dilation of the access site allowed accommodation of a 6-F sheath, but with wire and dilator removal, the sheath would not draw, and on removal, approximately 1.5 cm of venous intimal lining was removed with the sheath. The left femoral artery and vein were then accessed, and 4-F sheaths were placed. A 15 to 20 mm Hg aortic arch gradient was recorded, and venous saturations were 25% to 30%, consistent with severely depressed cardiac output. A 3-F pigtail catheter was advanced in retrograde fashion through the aortic valve to measure mitral valve gradients over an 0.025-inch angle glide. Aortic angiography following hemodynamic measures confirmed the coarctation anatomy without PDA, but it also documented new moderate to severe aortic valve insufficiency. A similar vascular issue was encountered with attempts at upsizing the femoral artery sheath to 5-F for aortic stenting; nearly 2 cm of arterial intima was removed with the sheath (Figures 3A and 3B). As a result, no intervention was performed, PGE infusion was increased, and the patient was returned to the NICU.
Figure 3.
Femoral Artery Intimal Degeneration and Adhesion to Cardiac Catheter Sheath
(A) Approximately 1.5 cm of left femoral artery intimal lining adhered to a 5-F Ansel sheath. (B) The intimal lining was removed from the 5-F sheath.
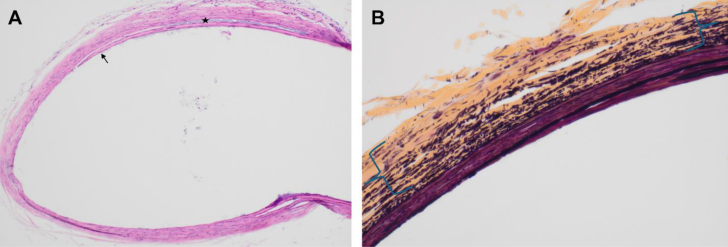
On DOL 12, the baby was compassionately extubated in the setting of low cardiac output and multiorgan failure. Arterial tissue sent for surgical pathologic examination showed disruption of the normal elastic lamellar units (Figures 4A and 4B). Elastic fibers were noted to be short, fragmented, and loosely arranged within a background of collagen fibrosis. Smooth muscle fibers were also small and focally disorganized. This constellation of histologic changes is compatible with medial degeneration.
Figure 4.
Femoral Artery Histology Demonstrating Intrinsic Vascular Disorganization
(A) Absent adventitia with disruption of superficial media (arrow) by mucoid material (star). (hematoxylin and eosin, 100×). (B) Loose, fragmented, and disorganized arraignments of elastic fibers in media (between brackets) that are without normal lamellar arrangement. There is also collagen deposition (yellow) (Movat pentachrome stain, 400×).
Discussion
Although acquired forms of valvular disease have been long established, there is growing appreciation for inherited structural heart diseases and their genetic causes. One causal gene is FLNA, which encodes the actin-binding protein filamin A, a critical protein for cytoskeletal and extracellular matrix development.1 Loss of function sequence variants in the FLNA gene are pleiotropic and are associated with a spectrum of clinical features, including seizure disorders, cardiovascular disease, pulmonary hypertension, gastrointestinal disorders, and joint hypermobility.2,3 Several cardiac features are associated with FLNA deficiency, including ventricular septal defect, PDA, and valvular dystrophy, along with peripheral vascular anomalies, including thoracic aortic dilation and aneurysm, which have been observed in up to 20% of cases.4
Although loss of function sequence variants in FLNA are associated with a spectrum of clinical features, this patient’s presentation is similar to that in individuals with isolated X-linked cardiac valvular dystrophy (XCVD; OMIM 314400).5 Male patients with XCVD often show severe polyvalvular disease characterized by stenosis, regurgitation, and prolapse, particularly of the mitral and aortic valves. Genome sequencing of patients with XCVD has identified 3 missense sequence variants (P637Q, H743P, and G288R) in FLNA at highly conserved residues.5 Although this patient’s sequence variant (P1727L) has not been implicated in XCVD, his phenotypic similarity to those with known pathogenic variants suggests P1727L to be causal.
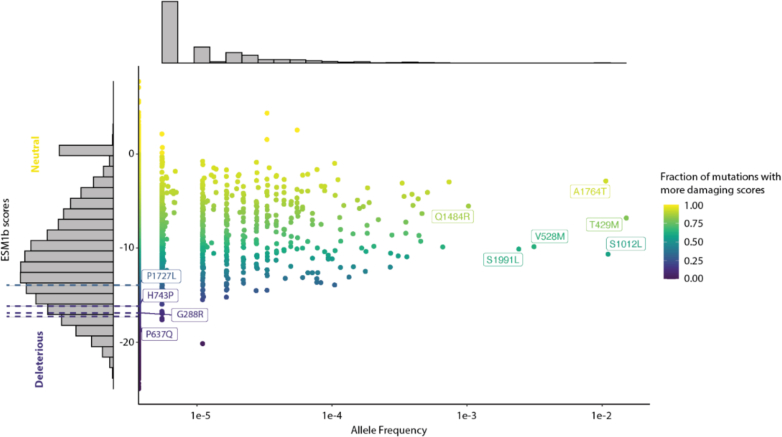
To evaluate the functional consequence of this patient’s variant, we used several computational tools to predict its pathogenicity. P1727L and several known pathogenic variants are predicted to be damaging by the evolutionary model ESM1b (Figure 5).6 This was corroborated by a structured-based variant effect predictor, DDMut,7 using AlphaFold’s structural prediction of FLNA. Again, P1727L and several pathogenic variants were strongly destabilizing (−0.94, −0.33, −2.08, −1.89, and −2.69 kcal/mol for P1727L, G288R, P637Q, H743P, and V711D, respectively). Importantly, almost all high-frequency benign variants from the Genome Aggregation Database (gnomAD) have DDMut scores near 0 kcal/mol, including A1764T (0.06), T429M (0.1), S1012L (0.08), V528M (−0.68), S1991L (0.02), and Q1484R (−0.16), findings suggesting that these predictions are well calibrated. Taken together, P1727L is likely causal given that computational methods predict its pathogenicity and this patient’s clinical phenotype replicates that of known pathogenic FLNA variants.
Figure 5.
P1727L: A Rare, Likely Damaging Sequence Variation When Compared With the Landscape of Missense Sequence Variations in FLNA
Variant effect predictions for all 50,293 possible single missense variants on the basis of ESM1b are plotted vs allele frequency in the Genome Aggregation Database (gnomAD) 2.1.1. Sequence variations are colored on the basis of the fraction of missense variants with more damaging scores. P1727L, the 3 known rare pathogenic variants with allele frequencies of 0 in gnomAD, and 6 known benign or likely benign variants with large allele frequencies (and neutral scores) are labeled.
Although iatrogenic vascular injuries from percutaneous femoral access have been commonly reported in small children undergoing cardiac catheterization,8,9 intimal avulsions of both arterial and venous access are exceedingly uncommon, thus suggesting an underlying abnormal tissue consistency and loss of vascular structural integrity in this patient. Notably, other cases of missense sequence variants in FLNA are associated with vascular fragility reminiscent of that observed here. Surgical pathologic examination in this case showed findings consistent with medial degeneration, which is similar to the loss of elastic lamellae and the loss of elastic fiber organization seen in the inner media of the aortic wall and brachiocephalic truncus of a previously reported patient with an FLNA indel sequence variant.3 The similarity in histopathologic findings and the clinical presentation in our patient suggests that FLNA P1727L confers structural cardiac and vascular defects similar to those seen in known XCVD.
Conclusions
This case emphasizes the importance of whole exome sequencing in congenital polyvalvular disease. It also highlights the potential causal role of FLNA in congenital valvular disease and vascular abnormalities. Notably, this is the first reported case of FLNA 5180 C>T P1727L in a neonate, and we suggest that this gene sequence variant could be pathogenic. Given the profound complications encountered here, it is crucial to consider this FLNA 5180 C>T P1727L gene sequence variant as pathologic before consideration of any invasive procedures.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
All data are publicly accessible. ESM1b scores were downloaded from https://huggingface.co/spaces/ntranoslab/esm_variants, and DDMut scores were downloaded from https://biosig.lab.uq.edu.au/ddmut/.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Feng Y., Chen M.H., Moskowitz I.P., et al. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci U S A. 2006;103(52):19836–19841. doi: 10.1073/pnas.0609628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Wit M.C., de Coo I., Lequin M., Halley D., Roos-Hesselink J., Mancini G. Combined cardiological and neurological abnormalities due to filamin A gene mutation. Clin Res Cardiol. 2011;100(1):45–50. doi: 10.1007/s00392-010-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinstein E., Frentz S., Morgan T., et al. Vascular and connective tissue anomalies associated with X-linked periventricular heterotopia due to mutations in Filamin A. Eur J Hum Genet. 2013;21(5):494–502. doi: 10.1038/ejhg.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M., Choudhury S., Hirata M., Khalsa S., Chang B., Walsh C. Thoracic aortic aneurysm in patients with loss of function Filamin A mutations: clinical characterization, genetics, and recommendations. Am J Med Genet. 2018;176(2):337–350. doi: 10.1002/ajmg.a.38580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Tourneau T., Le Scouarnec S., Cueff C., et al. New insights into mitral valve dystrophy: a Filamin-A genotype-phenotype and outcome study. Eur Heart J. 2018;39(15):1269–1277. doi: 10.1093/eurheartj/ehx505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandes N., Goldman G., Wang C., Ye C., Ntranos V. Genome-wide prediction of disease variant effects with a deep protein language model. Nat Genet. 2023;55:1512–1522. doi: 10.1038/s41588-023-01465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y., Pan Q., Pires D., Rodrigues C., Ascher D. DDMut: predicting effects of mutations on protein stability using deep learning. Nucleic Acids Res. 2023;51(W1):W122–W128. doi: 10.1093/nar/gkad472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge B., Copelan A., Scola D., Watts M. Iatrogenic percutaneous vascular injuries: clinical presentation, imaging, and management. Semin Interv Radiol. 2015;32(2):108–122. doi: 10.1055/s-0035-1549375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akkus N., Bahadur F., Varma J. Fracture of a guiding catheter in a tortuous iliac artery and its retrieval by a larger sheath. Rev Port Cardiol Engl Ed. 2013;32(4):341–344. doi: 10.1016/j.repc.2012.07.010. [DOI] [PubMed] [Google Scholar]