Abstract
Ischemic electrocardiographic changes in the setting of pulmonary embolism are typically the result of dilatation of the right cavities and/or right ventricular ischaemia, without coronary occlusion. We present a patient with pulmonary embolism and concomitant myocardial infarction, with the aim of exploring the possible links between these 2 distinct entities.
Key Words: pulmonary embolism, posterior myocardial infarction, ST-segment depression, ST-segment elevation
Graphical Abstract
History of Presentation
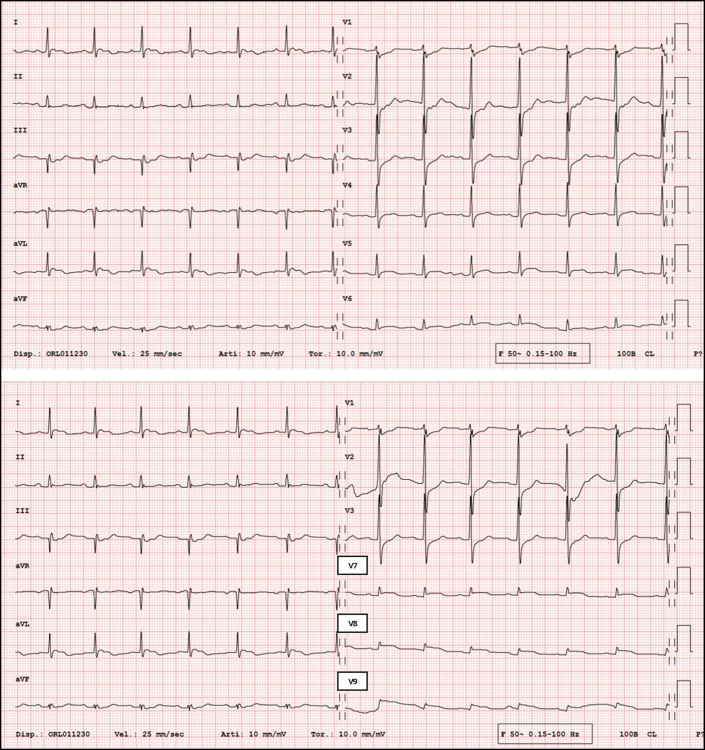
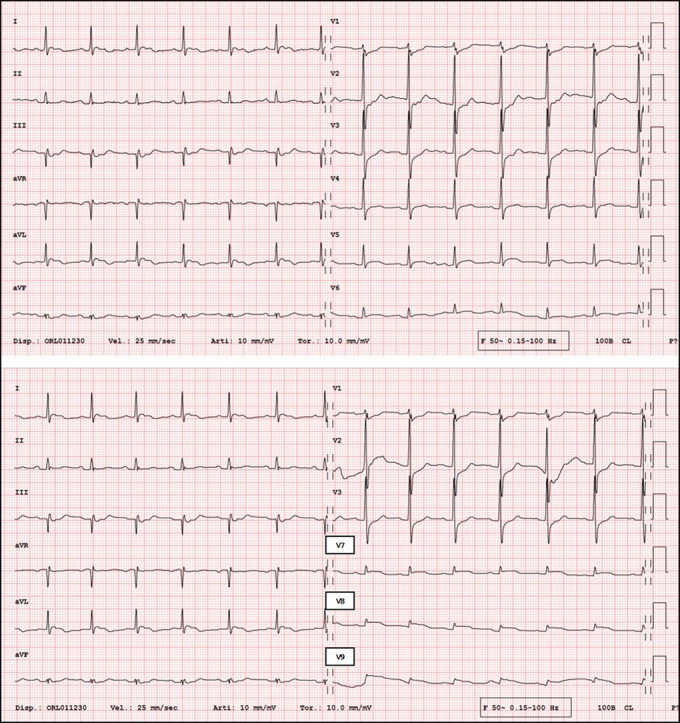
An 88-year-old woman was transported by ambulance to the emergency department of the Regional Hospital Civico of Lugano, Switzerland, with a report of dyspnea, retrosternal oppressive pain, diaphoresis, and generalized malaise. On arrival at the hospital, she was hemodynamically stable, her blood pressure was 120/70 mm Hg, her heart rate was 75 beats/min, and her oxygen saturation was 90% with 2.5 L/min oxygen by nasal cannula. Her right lower limb was swollen and painful on palpation. Blood tests showed elevations of D-dimer at 30.9 mg/L (normal value <0.50 mg/L) and of high-sensitivity troponin T at 256 ng/L (normal value <14 ng/L). Arterial blood gas measurement showed low-normal pH (7.34) with elevated lactate (4.8 mmol/L), hypoxemia (Po2, 8.7 kPa), and normal Pco2 (4.32 kPa). The electrocardiogram (ECG) showed sinus rhythm at 85 beats/min, ST-segment depression in leads V1, to V3, and ST-segment elevation in the posterior leads V7, to V9 (Figure 1).
Learning Objectives
-
•
To recognize MI when it occurs together with PE.
-
•
To identify the possible pathophysiologic links between simultaneously occurring PE and MI.
-
•
To suggest the consideration of systemic thrombolytic therapy in patients with intermediate- to high-risk PE and evidence of ST-segment elevation MI.
Figure 1.
Electrocardiogram
(Top) Standard 12-lead electrocardiogram showing ST-segment depression in leads V1, V2, and V3. (Bottom) Electrocardiogram with posterior leads showing ST-segment elevation in leads V7, V8, and V9.
Past Medical History
The patient was known to have previous breast cancer in remission and chronic kidney disease (Kidney Disease: Improving Global Outcomes stage G3b). Her cardiovascular risk factors were hypertension and insulin-dependent type 2 diabetes.
Differential Diagnosis
The initial differential diagnosis was between pulmonary embolism (PE) and posterior ST-segment elevation myocardial infarction (MI).
Investigations
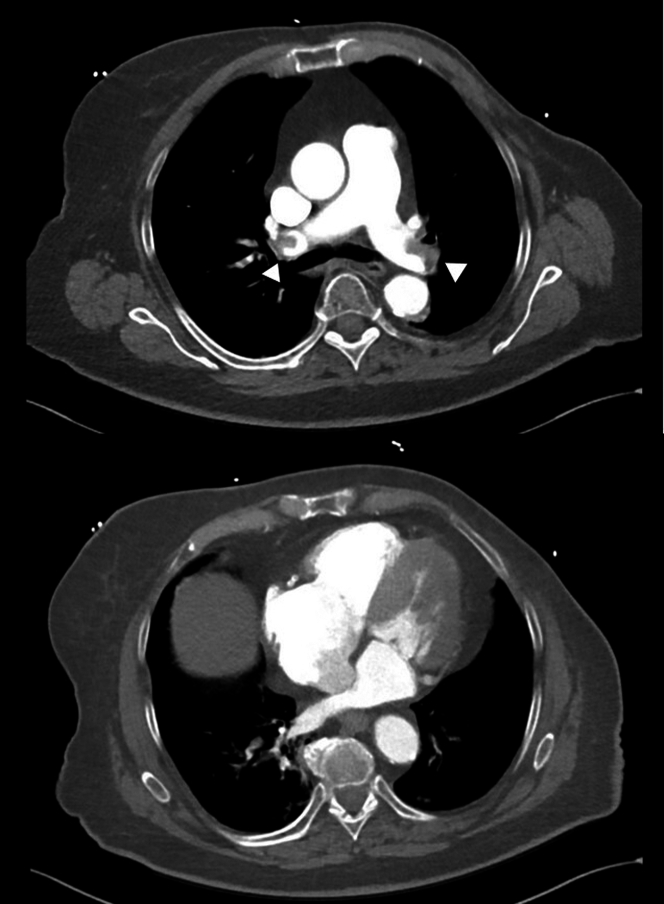
Pulmonary computed tomography angiography was performed and showed a massive saddle PE (Figure 2). Bedside transthoracic echocardiography documented right ventricular free wall hypokinesia with sparing of the apex (McConnell sign). There was no left ventricular wall motion abnormality. Compression ultrasound of the limbs showed right femoral thrombosis.
Figure 2.
Chest Computed Tomography Angiography
(Top) Bilateral emboli can be noted in the main pulmonary arteries (arrowheads), as well as right ventricular dilatation with a 1:1 ratio compared with (bottom) left ventricular basal diameter.
Management
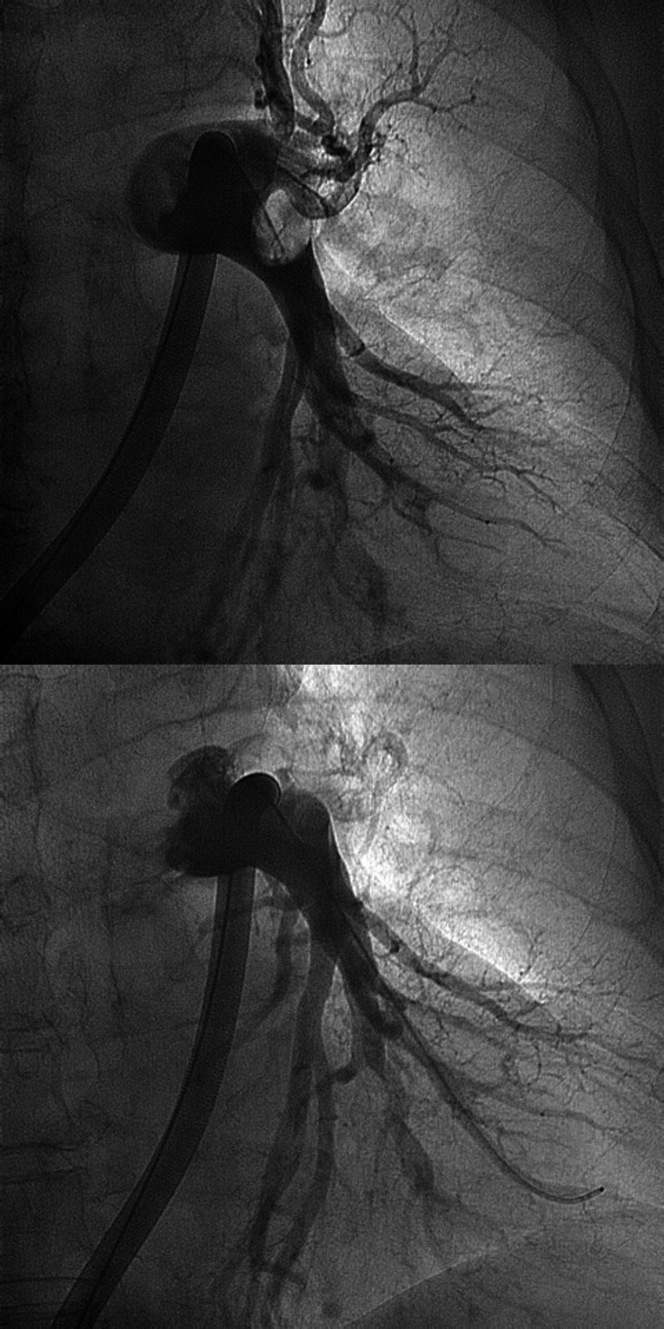
The case was discussed within the PE response team. Under a working diagnosis of intermediate- to high-risk PE (no hemodynamic instability, a PE severity index [PESI] of 118 determining risk Class IV, a simplified PESI of 2, elevation of troponins, and right ventricular dysfunction), we performed percutaneous treatment with catheter-based pulmonary thrombectomy with the use of the Inari FlowTriever System (Inari Medical)1 (Figure 3). We did not perform coronary angiography concomitantly because we interpreted the ECG changes as secondary to PE and given the absence of left ventricular wall motion abnormality. The patient remained hemodynamically stable throughout the procedure and was then transferred to the intensive care unit for monitoring.
Figure 3.
Pulmonary Artery Angiography
Left pulmonary artery angiography (top) before and (bottom) after multiple cycles of thromboaspiration with the Inari FlowTriever system (Inari Medical).
In the following hours, the patient’s chest pain gradually improved, and the right ventricular hypokinesia resolved (Video 1). Nevertheless, a repeat ECG showed the persistence of ST-segment elevation in the posterior leads without new Q waves, repeat transthoracic echocardiography showed new inferolateral midbasal hypokinesia of the left ventricle (Video 2, Video 3, Video 4), and blood test results showed a progressive increase in high-sensitivity troponin T up to 8,665 ng/L. In parallel, however, the patient developed severe anemia (hemoglobin dropped from 112 g/L to 74 g/L) and bilateral pneumonia with persisting dyspnea, hypoxia, fever, and bilateral infiltrates on chest radiography. Because of this clinical deterioration, even although the ECG and echocardiography suggested ischemia, coronary angiography was performed only several days later (day 12 after admission) because the patient’s clinical status was deemed too unstable for her to undergo an earlier invasive procedure. Table 1 summarizes the main events, diagnostic examinations, and symptom evolution during the hospitalization.
Table 1.
Days of Hospitalization With Diagnostic Examinations, Evolution of Symptoms, and Laboratory Analysis
| Days of Hospitalizationa |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16-17 | 18 | 19 | 20 | |
| CT angiography | X | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Lower limb duplex | — | — | — | — | X | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| TTE controls | X | — | — | — | — | — | — | — | — | — | — | — | — | — | X | — | — | — | — |
| Inari protocol | X | — | —— | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Coronary angiography | — | — | — | — | — | — | — | — | — | — | — | X | — | — | — | — | — | — | — |
| HS-troponin (<14 ng/L) | 5,175 | 8,665 | 4,586 | 5,031 | 4,649 | 4,137 | — | — | 2,901 | — | 1,932 | 1,305 | 1,013 | — | — | — | — | — | — |
| Hb (120-160 g/L) | 112 | 74 | 93 | 86 | 82 | 83 | — | 84 | 80 | 80 | 88 | 82 | 88 | 86 | 91 | — | 96 | — | — |
| Creatinine (44-80 μmol/L) | 139 | 113 | 100 | 103 | — | 113 | — | 98 | 119 | 109 | 128 | 129 | 123 | 127 | 121 | — | 132 | — | 101 |
| GFR (>60 mL/min/1.73 m2) | 35 | 43 | 47 | 45 | — | 40 | — | 48 | 38 | 42 | 35 | 34 | 36 | 35 | 37 | — | 33 | — | — |
| CRP (<5 mg/L) | 3 | — | 82 | 108 | 88 | 92 | — | 59 | 36 | 23 | — | 14 | 10 | 7 | 6 | — | — | — | <1 |
| NT-pro-BNP (<623 ng/L) | 68 | — | 3,435 | 3,691 | — | — | — | 2,768 | — | — | 1,849 | 2,081 | 1,458 | — | — | — | — | — | |
| Symptoms and signs | Chest pain | Dyspnea NYHA functional class II | — | — | — | — | — | — | — | — | — | ||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |||
| Hypoxemia | — | — | — | — | — | — | — | — | — | ||||||||||
CRP = C-reactive protein; CT = computed tomography; GFR =glomerular filtration rate; Hb = hemoglobin; HS = high-sensitivity; NT-proBNP = N-terminal pro–B-type natriuretic peptide; TTE = transthoracic echocardiography.
“X” indicates that a procedure was performed; values shown in parentheses are normal values.
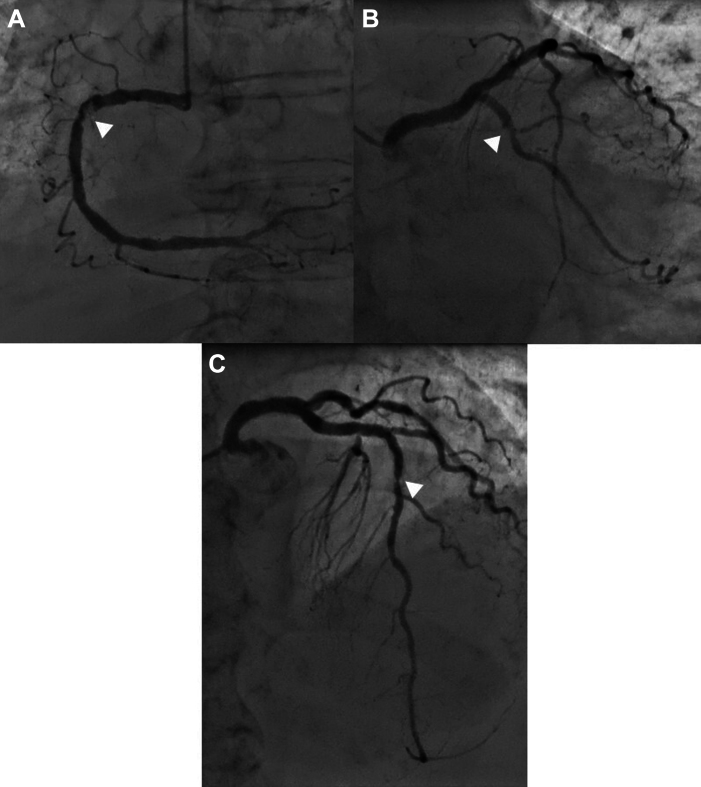
Invasive coronary angiography showed 3-vessel coronary artery disease with a markedly calcified and apparently thrombotic critical stenosis of the proximal right coronary artery (RCA), a critical focal stenosis of the mid-left anterior descending artery, and a critical stenosis of the mid-left circumflex artery (Figures 4A to 4C), with TIMI flow grade 3 in all vessels. After multidisciplinary discussion, conservative treatment was pursued, taking into account the patient’s age and the inherent risks of revascularization.
Figure 4.
Coronary Angiography
Critical focal stenoses (white arrowheads) can be noted in (A) the proximal right coronary artery, (B) the proximal left circumflex artery, and (C) the mid-left anterior descending artery.
At discharge, transthoracic echocardiography showed preserved global left ventricular function with midbasal inferolateral akinesia. The right ventricle was normal in size, with preserved longitudinal and radial function indices. Cardiac magnetic resonance to confirm the diagnosis was not performed because the available clinical and echocardiographic data were deemed sufficient to diagnose MI and given the lack of long-term clinical implications.
Discussion
We present the case of an older adult patient who presented with acute PE and an ECG consistent with a posterior MI.
The most common ECG changes during PE are tachycardia, right bundle branch block, right-axis deviation, a S1Q3T3 pattern, the presence of “P pulmonale,” ST-segment depression, and T-wave inversion in leads V1 to V3.2,3 Ischemic ECG patterns have been often described in the setting of PE, and the interpretation of such a findings as independent risk factors for worse outcome has been proposed.4 Fewer cases have been reported of ST-segment elevation during PE, but in most of them a coronary obstructive disease was not found.2
We initially interpreted the ECG changes as secondary to the acute PE. When invasive coronary angiography was performed, however, severe 3-vessel disease with subtotal stenosis in the proximal RCA was observed. This finding was consistent with the ECG and echocardiographic changes observed after the thrombectomy procedure, and it prompted us to reinterpret the situation and conclude that, concomitantly with the PE, the patient had also experienced an inferolateral MI. In retrospect, a noninvasive approach with systemic thrombolytic therapy could have also been considered to address both events simultaneously.
Concomitant MI and PE is a rare occurrence.2,5 The most likely interpretation for the MI in this setting is the presence of a mismatch between myocardial oxygen demand and supply with severe 3-vessel disease (type 2 MI), aggravated by hemodynamic overload of the PE on the right ventricle directly and the left ventricle indirectly.3 Alternative explanations cannot be ruled out. An embolic MI could have occurred secondary to a right-to-left shunt (ie, patent foramen ovale [PFO]), facilitated by the elevated right-sided heart pressures resulting from the PE,3 and the thrombus could have already been resolved by the time coronary angiography was performed. Echocardiography did not show indirect signs of a PFO or other intracardiac shunt. A formal test with microbubbles was not performed, however, given the lack of clinical implication for an older adult patient who would not qualify for a PFO closure and who would need long-term anticoagulant therapy regardless in the setting of PE with intermediate recurrence risk. Finally, a temporal association between primary coronary thrombosis and PE cannot be excluded because coronary angiography was performed well after the acute event.
Because patient data were anonymized, ethical approval for describing the case was not requested, but the patient signed an informed consent for the use of her clinical data for research purposes.
Follow-Up
At the 3-month follow-up, the patient was stable, reporting only mild dyspnea (NYHA functional class II). Echocardiography showed a preserved left ventricular ejection fraction with midbasal inferolateral wall akinesia.
Conclusions
Albeit rare, the coexistence of PE and MI should be suspected in patients whose symptoms do not promptly resolve on treatment of 1 of these 2 diseases, as well as when the ECG presentation cannot be entirely explained by secondary alterations caused by PE. In our case, both conditions were met, yet the perceived low a priori likelihood of 2 concomitant diseases led us to a delayed diagnosis and potentially suboptimal treatment.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Drs Alessandro Caretta, Carlo Alberto Caruzzo, Gianluca Guarnieri, and Andrea Milzi of the Cardiocentro Ticino Institute for their helpful contribution.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Echocardiography After Percutaneous Treatment of Acute PE, With Normal Right Ventricular Size and Function in the Apical 4-Chamber View
Apical 3-Chamber View With New Akinesia of the Basal and Midventricular Inferolateral Wall
Short-Axis View of the Basal LV With Inferolateral Akinesia
Short-Axis View of the Midventricular LV With Inferolateral Akinesia
References
- 1.Capanegro J., Quinn E., Arndt M., Sherard D. Successful removal of a Life-threatening PE using the INARI flow triever device. Radiol Case Rep. 2021;16(7):1878–1881. doi: 10.1016/j.radcr.2021.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khurana K.V., Ranjan A. ST-segment elevation in conditions of non-cardiovascular origin mimicking an acute myocardial infarction: a narrative review. Cureus. 2022;14(10) doi: 10.7759/cureus.30868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yue X.L., Shi X.Y., Jiang M., Li R.J. Acute pulmonary embolism presenting with electrocardiographic signs and serum biomarkers of ST-segment elevation myocardial infarction: a case report. J Int Med Res. 2023;51(9) doi: 10.1177/03000605231197063. doi:10.1177/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kukla P., McIntyre W.F., Fijorek K., et al. Use of ischemic ECG patterns for risk stratification in intermediate-risk patients with acute PE. Am J Emerg Med. 2014;32(10):1248–1252. doi: 10.1016/j.ajem.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqa A., Haider A., Job A., Yue B., Krim N.R. Pulmonary embolism presenting as ST-elevation myocardial infarction: a diagnostic trap. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.927923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiography After Percutaneous Treatment of Acute PE, With Normal Right Ventricular Size and Function in the Apical 4-Chamber View
Apical 3-Chamber View With New Akinesia of the Basal and Midventricular Inferolateral Wall
Short-Axis View of the Basal LV With Inferolateral Akinesia
Short-Axis View of the Midventricular LV With Inferolateral Akinesia