Abstract
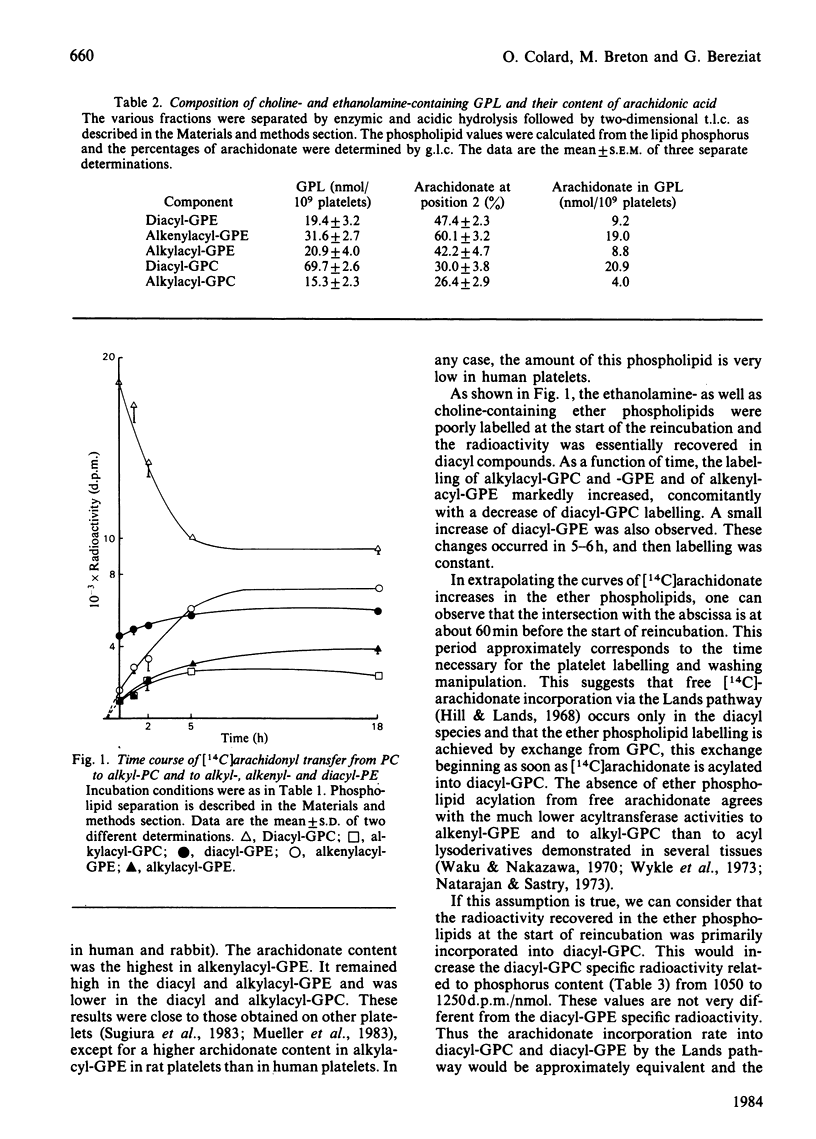
High levels of ether phospholipids were found in rat platelets. Alkylacyl compounds constituted 18 and 29% of glycerophosphocholine (GPC) and glycerophosphoethanolamine (GPE). Alkenylacyl compounds, not detected in GPC, represented 40% of GPE. Arachidonate comprised 60%, 42% and 26% of the acyl residues in the sn-2 position of alkenylacyl-GPE, alkylacyl-GPE and alkylacyl-GPC respectively. Based on all arachidonate being linked to the sn-2 position of the diacyl species, the arachidonate level was 47% in diacyl-GPE and 30% in diacyl-GPC. The incorporation and metabolic fate of arachidonate in various phospholipid classes of resting platelets was examined. Arachidonate was essentially recovered in the diacyl phospholipids and very poorly in alkylacyl- and alkenylacyl-GPE and GPC after 30 min incubation in the presence of [14C]arachidonic acid. Upon reincubation of the platelets after removal of free arachidonate, the radioactivity was gradually lost from diacyl-GPC. Concomitantly, the radioactivities in alkylacyl-GPC, alkylacyl-GPE, alkenylacyl-GPE and to a lower extent in diacyl-GPE were increased. Labelling of glycerophosphoinositol was not changed. This labelling transfer was linear up to 5-6 h, except for alkylacyl-GPC; then labelling remained constant. These data strongly suggest that free arachidonate incorporation through the Lands pathway occurs only for diacyl species and that arachidonate incorporation into the ether phospholipids is achieved by exchange from diacyl-GPC. Based on specific activities related to phosphorus content, the arachidonate incorporation rates into diacyl-GPE and diacyl-GPC were approximately equivalent. The very large differences between specific radioactivities related to arachidonate observed at the starting reincubation time were strongly attenuated when labelling equilibrium was reached. The turnover rate by this exchange pathway was higher in alkylacyl-GPC than in alkyl- and alkenylacyl-GPE. This finding agrees with the selectivity for arachidonate observed in the acylation of PAF-acether in human neutrophils [Chilton, O'Flaherty, Ellis, Swendsen & Wykle (1983) J. Biol. Chem. 258, 7268-7271].
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli V. M. Platelet lipids (3). In vitro incorporation of 1-14C linoleic acid into lipid fractions of rabbit platelets. Eur J Pharmacol. 1969 Sep;7(3):314–318. doi: 10.1016/0014-2999(69)90098-3. [DOI] [PubMed] [Google Scholar]
- Ardlie N. G., Packham M. A., Mustard J. F. Adenosine diphosphate-induced platelet aggregation in suspensions of washed rabbit platelets. Br J Haematol. 1970 Jul;19(1):7–17. doi: 10.1111/j.1365-2141.1970.tb01596.x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Benveniste J., Tencé M., Varenne P., Bidault J., Boullet C., Polonsky J. Semi-synthèse et structure proposée du facteur activant les plaquettes (P.A.F.): PAF-acether, un alkyl ether analogue de la lysophosphatidylcholine. C R Seances Acad Sci D. 1979 Nov 26;289(14):1037–1040. [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Selective release of archidonic acid from the phospholipids of human platelets in response to thrombin. J Clin Invest. 1977 Jul;60(1):1–6. doi: 10.1172/JCI108745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M. L., Wykle R. L., Snyder F. The retention of arachidonic acid in ethanolamine plasmalogens of rat testes during essential fatty acid deficiency. Biochim Biophys Acta. 1973 Jul 19;316(1):28–34. doi: 10.1016/0005-2760(73)90163-x. [DOI] [PubMed] [Google Scholar]
- Béréziat G., Chambaz J., Trugnan G., Pépin D., Polonovski J. Turnover of phospholipid linoleic and arachidonic acids in human platelets from plasma lecithins. J Lipid Res. 1978 May;19(4):495–500. [PubMed] [Google Scholar]
- Chilton F. H., O'Flaherty J. T., Ellis J. M., Swendsen C. L., Wykle R. L. Metabolic fate of platelet-activating factor in neutrophils. J Biol Chem. 1983 May 25;258(10):6357–6361. [PubMed] [Google Scholar]
- Chilton F. H., O'Flaherty J. T., Ellis J. M., Swendsen C. L., Wykle R. L. Selective acylation of lyso platelet activating factor by arachidonate in human neutrophils. J Biol Chem. 1983 Jun 25;258(12):7268–7271. [PubMed] [Google Scholar]
- Cohen P., Derksen A. Comparison of phospholipid and fatty acid composition of human erythrocytes and platelets. Br J Haematol. 1969 Oct;17(4):359–371. doi: 10.1111/j.1365-2141.1969.tb01382.x. [DOI] [PubMed] [Google Scholar]
- Colard O., Breton M., Bereziat G. Induction by lysophospholipids of CoA-dependent arachidonyl transfer between phospholipids in rat platelet homogenates. Biochim Biophys Acta. 1984 Mar 27;793(1):42–48. doi: 10.1016/0005-2760(84)90051-1. [DOI] [PubMed] [Google Scholar]
- Demopoulos C. A., Pinckard R. N., Hanahan D. J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979 Oct 10;254(19):9355–9358. [PubMed] [Google Scholar]
- Diagne A., Fauvel J., Record M., Chap H., Douste-Blazy L. Studies on ether phospholipids. II. Comparative composition of various tissues from human, rat and guinea pig. Biochim Biophys Acta. 1984 Apr 18;793(2):221–231. doi: 10.1016/0005-2760(84)90324-2. [DOI] [PubMed] [Google Scholar]
- El Tamer A., Record M., Fauvel J., Chap H., Douste-Blazy L. Studies on ether phospholipids. I. A new method of determination using phospholipase A1 from guinea pig pancreas: application to Krebs II ascites cells. Biochim Biophys Acta. 1984 Apr 18;793(2):213–220. doi: 10.1016/0005-2760(84)90323-0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fauvel J., Bonnefis M. J., Sarda L., Chap H., Thouvenot J. P., Douste-Blazy L. Purification of two lipases with high phospholipase A1 activity from guinea-pig pancreas. Biochim Biophys Acta. 1981 Feb 23;663(2):446–456. doi: 10.1016/0005-2760(81)90173-9. [DOI] [PubMed] [Google Scholar]
- Hill E. E., Lands W. E. Incorporation of long-chain and polyunsaturated acids into phosphatidate and phosphatidylcholine. Biochim Biophys Acta. 1968 May 1;152(3):645–648. doi: 10.1016/0005-2760(68)90109-4. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Deykin D. Arachidonoyl transacylase in human platelets. Coenzyme A-independent transfer of arachidonate from phosphatidylcholine to lysoplasmenylethanolamine. J Biol Chem. 1983 Nov 25;258(22):13806–13811. [PubMed] [Google Scholar]
- Lagarde M., Guichardant M., Menashi S., Crawford N. The phospholipid and fatty acid composition of human platelet surface and intracellular membranes isolated by high voltage free flow electrophoresis. J Biol Chem. 1982 Mar 25;257(6):3100–3104. [PubMed] [Google Scholar]
- Mueller H. W., O'Flaherty J. T., Wykle R. L. Biosynthesis of platelet activating factor in rabbit polymorphonuclear neutrophils. J Biol Chem. 1983 May 25;258(10):6213–6218. [PubMed] [Google Scholar]
- Mueller H. W., Purdon A. D., Smith J. B., Wykle R. L. 1-O-alkyl-linked phosphoglycerides of human platelets: distribution of arachidonate and other acyl residues in the ether-linked and diacyl species. Lipids. 1983 Nov;18(11):814–819. doi: 10.1007/BF02534641. [DOI] [PubMed] [Google Scholar]
- Natarajan V., Sastry P. S. In vitro studies on the acylation of 1-O-alkenyl glycero-3-phosphorylethanolamine by rat brain preparations. FEBS Lett. 1973 May 15;32(1):9–12. doi: 10.1016/0014-5793(73)80722-7. [DOI] [PubMed] [Google Scholar]
- Ninio E., Mencia-Huerta J. M., Heymans F., Benveniste J. Biosynthesis of platelet-activating factor. I. Evidence for an acetyl-transferase activity in murine macrophages. Biochim Biophys Acta. 1982 Jan 15;710(1):23–31. doi: 10.1016/0005-2760(82)90185-0. [DOI] [PubMed] [Google Scholar]
- Pieroni G., Hanahan D. J. Metabolic behavior of acetyl glyceryl ether phosphorylcholine on interaction with rabbit platelets. Arch Biochem Biophys. 1983 Jul 15;224(2):485–493. doi: 10.1016/0003-9861(83)90236-9. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Russell F. A., Deykin D. Mobilization of arachidonic acid in human platelets. Kinetics and Ca2+ dependency. Biochim Biophys Acta. 1977 Sep 28;488(3):370–380. doi: 10.1016/0005-2760(77)90196-5. [DOI] [PubMed] [Google Scholar]
- Sugiura T., Katayama O., Fukui J., Nakagawa Y., Waku K. Mobilization of arachidonic acid between diacyl and ether phospholipids in rabbit alveolar macrophages. FEBS Lett. 1984 Jan 9;165(2):273–276. doi: 10.1016/0014-5793(84)80184-2. [DOI] [PubMed] [Google Scholar]
- Sugiura T., Soga N., Nitta H., Waku K. Occurrence of alkyl ether phospholipids in rabbit platelets: compositions and fatty chain profiles. J Biochem. 1983 Nov;94(5):1719–1722. [PubMed] [Google Scholar]
- Touqui L., Jacquemin C., Vargaftig B. B. Conversion of 3H-PAF acether by rabbit platelets is independent from aggregation: evidences for a novel metabolite. Biochem Biophys Res Commun. 1983 Feb 10;110(3):890–893. doi: 10.1016/0006-291x(83)91045-8. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Fouque F., Chignard M. Interference of bromophenacyl bromide with platelet phospholipase A2 activity induced by thrombin and by the ionophore A23187. Thromb Res. 1980 Jan 1;17(1-2):91–102. doi: 10.1016/0049-3848(80)90297-2. [DOI] [PubMed] [Google Scholar]
- Waku K., Nakazawa Y. Acyltransferase activity to 1-O-alkyl-glycero-3-phosphorylcholine in sarcoplasmic reticulum. J Biochem. 1970 Oct;68(4):459–466. doi: 10.1093/oxfordjournals.jbchem.a129376. [DOI] [PubMed] [Google Scholar]
- Wykle R. L., Blank M. L., Snyder F. The enzymic incorporation of arachidonic acid into ether-containing choline and ethanolamie phosphoglycerides by deacylation-acylation reactions. Biochim Biophys Acta. 1973 Oct 17;326(1):26–33. doi: 10.1016/0005-2760(73)90024-6. [DOI] [PubMed] [Google Scholar]
- Wykle R. L., Malone B., Snyder F. Enzymatic synthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, a hypotensive and platelet-aggregating lipid. J Biol Chem. 1980 Nov 10;255(21):10256–10260. [PubMed] [Google Scholar]


