Abstract
The partial control of viremia during acute human immunodeficiency virus type 1 (HIV-1) infection is accompanied by an HIV-1-specific cytotoxic T-lymphocyte (CTL) response and an absent or infrequent neutralizing antibody response. The control of HIV-1 viremia has thus been attributed primarily, if not exclusively, to CTL activity. In this study, the role of antibody in controlling viremia was investigated by measuring the ability of plasma or immunoglobulin G from acutely infected patients to inhibit primary strains of HIV-1 in the presence of natural-killer (NK) effector cells. Antibody that inhibits virus when combined with effector cells was present in the majority of patients within days or weeks after onset of symptoms of acute infection. Furthermore, the magnitude of this effector cell-mediated antiviral antibody response was inversely associated with plasma viremia level, and both autologous and heterologous HIV-1 strains were inhibited. Finally, antibody from acutely infected patients likely reduced HIV-1 yield in vitro both by mediating effector cell lysis of target cells expressing HIV-1 glycoproteins and by augmenting the release of β-chemokines from NK cells. HIV-1-specific antibody may be an important contributor to the early control of HIV viremia.
During acute human immunodeficiency virus type 1 (HIV-1) infection, the plasma viremia level rises to a peak and then drops coincident with the development of a specific immune response (11, 12). The level of viremia eventually attained represents a set point that correlates well with subsequent immunological and clinical events and is an important factor in the decision to institute antiretroviral therapy (22, 35).
The initial control of viremia has been attributed to an HIV-1-specific cytotoxic T-lymphocyte (CTL) response (6, 17, 27, 31, 39). CTLs targeting several epitopes can be detected early in infection, and depletion of CD8+ cells from monkeys infected with simian immunodeficiency virus (SIV) abrogates the fall in viremia normally seen during acute infection (6, 17, 31, 36). The apparent importance of CTLs in controlling viremia has had a large impact on vaccine development, where many recent efforts have centered on eliciting strong cellular immune responses (1, 2, 19, 20).
Unlike CTL activity, antibodies which neutralize HIV infectivity are often undetectable during acute infection (18, 23, 24, 30). Although a recent study demonstrated consistent neutralizing activity in sera from patients with early infection when macrophages were used as target cells, many of the sera were obtained several months after infection and possibly after the viremia set point was reached (34). The low frequency of neutralizing activity during the period of falling viremia has led to the notion that antibodies do not play a major role in controlling viremia.
Although neutralizing antibodies may be undetectable or at low titer during acute HIV-1 infection, antibodies with other functions could play a role in controlling viremia. Antibody-dependent cellular cytotoxicity (ADCC) occurs when antibody forms a bridge between a target cell bearing foreign antigens on its surface and an effector cell expressing Fc receptors; this interaction results in the lysis or apoptosis of the target cell. Like CTL activity, ADCC could eliminate infected cells and thereby reduce viral burden. In a small number of acutely infected patients, Connick et al. found antibodies which mediated ADCC to be present at about the same time as CTLs became detectable (11). We recently demonstrated that ADCC, measured by 51Cr release assay using target cells transfected with HIV env and an autologous combination of patient serum and peripheral blood mononuclear cells (PBMCs), correlated inversely with viral load in chronically infected patients not receiving antiretroviral therapy (14). Thus, ADCC antibodies may be present during acute infection, and it is biologically plausible that ADCC plays a role in determining the virological set point.
ADCC is typically assessed in 51Cr release assays, which provide a measure of target cell death. However, in elucidating the role of ADCC in viral infections, it may be more biologically relevant to directly measure the ability of antibody and effector cells to inhibit virus; this is particularly true if mechanisms other than cytotoxicity contribute to the antiviral effect. In this study, we examined the ability of antibody from acutely infected patients, in combination with effector cells from healthy individuals, to directly inhibit heterologous and autologous clinical strains of HIV-1.
MATERIALS AND METHODS
Patients.
Plasma from 15 patients with acute HIV infection, none of whom had ever received antiretroviral therapy, was collected as part of an ongoing prospective study of acute and early HIV infection at the University of California, San Diego School of Medicine, and at Cedars-Sinai Medical Center. Criteria for inclusion of patients included the following: (i) negative HIV-specific antibody measured by enzyme-linked immunosorbent assay (ELISA) and either a positive plasma HIV-1 RNA by PCR or a positive p24 antigen, (ii) positive ELISA with an indeterminate HIV-specific Western blot and a positive plasma HIV RNA or p24 antigen, or (iii) positive Western blot within 30 days of a negative or indeterminate Western blot. Plasma was collected between 3 and 56 days (median = 18 days) following onset of symptoms of acute HIV infection (13 patients) or at 34 and 38 days following a known exposure to HIV from two patients (Table 1). From all but three patients, plasma was collected during the period of declining viremia. Samples obtained later in the course of acute infection were also available from 11 of the 15 patients. All plasma was collected in EDTA or acid citrate dextrose and frozen at −70°C prior to use in the assays described below. Due to a limited supply, plasma samples from different patients were pooled for some experiments. This research was approved by Institutional Review Boards at the University of California, Irvine, the University of California, San Diego, and Cedars Sinai Medical Center.
TABLE 1.
Antiviral activity and HIV RNA level in plasma from 15 patients with acute HIV infection
| Patient | Inhibition by plasma (%)a
|
HIV RNA (log10 copies/ml) | Days from symptom onset | |
|---|---|---|---|---|
| With NK cells | Without NK cells | |||
| 1 | 48 | 18 | 6.96 | 3 |
| 2 | 84 | 5 | 5.87 | 6 |
| 3 | 0 | 0 | 6.98 | 9 |
| 4 | 0 | 0 | 6.91 | 10 |
| 5 | 98 | 0 | 5.68 | 34b |
| 6 | 4 | 1 | 7.68 | 14 |
| 7 | 55 | 16 | 6.14 | 14 |
| 8 | 94 | 12 | 6.26 | 38b |
| 9 | 99 | 0 | 5.28 | 18 |
| 10 | 99 | 46 | 5.36 | 22 |
| 11 | 5 | 0 | 4.61 | 22 |
| 12 | 71 | 0 | 4.12 | 24 |
| 13 | 44 | 30 | 3.68 | 37 |
| 14 | 22 | 6 | 6.00 | 39 |
| 15 | 97 | 15 | 3.73 | 56 |
Calculated as {1 − ([p24i]/[p24u])} × 100, where [p24i] and [p24u] are the concentrations of supernatant fluid p24 produced by HIV92US657-infected CD4+ lymphocytes 7 or 8 days after the addition of 10% plasma from acutely infected patients or from uninfected controls, respectively, with or without NK effector cells from uninfected donors. For each patient, p24 determinations were repeated using supernatant fluid obtained at one or two additional times; percent inhibition was generally higher when obtained at earlier times and lower when collected later.
Days following a known HIV exposure.
Virus.
HIV92US657 is an R5 primary isolate obtained from the NIH AIDS Reagents Program through the Multicenter AIDS Cohort Study and the UNAIDS Network for HIV Isolation and Characterization and from the Division of AIDS, National Institute of Allergy and Infectious Diseases. Autologous patient isolates were obtained by cocultivating patient PBMCs with phytohemagglutinin (PHA)-stimulated PBMCs from a healthy donor. Virus was passaged an additional time on PHA-stimulated PBMCs and stocks were stored at −80°C until used.
Separation of IgG from plasma of acutely infected patients and generation of Fab fragments.
Immunoglobulin G (IgG) was separated from plasma samples by affinity chromatography using protein G-coated Sepharose beads. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to analyze the purity of the IgG fraction and to adjust the concentration of IgG used in subsequent assays. Fab fragments were generated by incubating IgG with 5% (vol/vol) papain and 1 mM EDTA for 16 h at 37°C; Fc fragments were captured by protein G-coated Sepharose beads, and eluate containing Fab fragments was analyzed by SDS-PAGE and stored at 4°C until use.
Virus inhibition assay.
PBMCs obtained by Ficoll-Hypaque centrifugation of buffy coats from healthy donors were allowed to adhere to polystyrene flasks for 1 h. Nonadherent cells were collected and stimulated for 24 h with PHA in RPMI 1640 medium supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal bovine serum (medium). CD4+ lymphocytes were then magnetically separated from the PBMCs with anti-CD4 monoclonal antibody (Miltenyi Biotech, Auburn, Calif.) and infected with HIV-1 at a multiplicity of infection of approximately 0.05 for various time periods. Next, 2 × 106 infected cells were added to 12.5-cm3 flasks to which effector cells and either 10% heat-inactivated patient plasma, patient IgG, plasma from uninfected controls, or IgG from uninfected controls (Cytogam; a gift from Nami Park, MedImmune, Gaithersburg, Md.) was added (total volume = 2 ml). Effector cells were prepared from PBMCs obtained from healthy, HIV-seronegative donors different from the target cell donors. Natural killer (NK) effector cells were magnetically separated from the PBMCs using anti-CD56 monoclonal antibody (Miltenyi Biotec) and added to the infected cells at an effector/target cell (E:T) ratio of 10:1 immediately after addition of plasma. Supernatant fluid was sampled for p24 by ELISA (ZeptoMetrix; Buffalo, N.Y.) between 3 and 10 days after the addition of plasma and effector cells. Virus inhibition was calculated as follows: % inhibition = {1 − ([p24i]/[p24u])} × 100, where [p24i] is the concentration of p24 in the supernatant of flasks containing plasma or IgG from acutely infected patients, and [p24u] is the concentration of p24 from flasks containing plasma or IgG from uninfected controls. This formula was applied to the calculation of virus inhibition due to antibody alone and due to antibody combined with effector cells (in which case [p24i] and [p24u] were determined in flasks containing effector cells).
Immunoadsorption with envelope glycoprotein-expressing cells.
A total of 8 × 107 CEM213 cells, which are stably transfected with env from a laboratory strain of HIV (provided by Myles Cloyd, University of Texas, Galveston), or nontransfected CEM cells were incubated with 0.4 ml of plasma for 60 min at room temperature. The cells were then pelleted, and the supernatant fluid was collected.
51Cr release assay.
To evaluate the ability of plasma to lyse cells expressing HIV-1 envelope glycoproteins, serial dilutions of plasma were incubated in triplicate with 51Cr-labeled CEM213 cells. PBMC effector cells, from healthy donors, were then added at an E:T ratio of 100:1, and following a 4-h incubation, supernatant fluid was assayed by scintillography. Cytotoxicity was determined as follows: % cytotoxicity = cpmplasma + effector cells − cpmspontaneous/cpmmaximum − cpmspontaneous.
Generation of soluble antiviral substances.
CEM213 cells were incubated with IgG from acutely infected patients or from uninfected controls for 30 min and washed carefully to remove unbound antibody. NK effector cells were then added at an E:T ratio of 10:1. After 18 h, the cells were pelleted and the supernatant fluid (conditioned medium) was collected. Conditioned medium was added neat to CD4+ lymphocytes 54 h after infection with HIV92US657; virus yield was determined 5 days later by measuring p24 in the supernatant fluid bathing the CD4+ lymphocytes.
Chemokines and chemokine neutralization.
To determine if conditioned medium generated from a target cell-antibody-effector cell interaction contained β-chemokines which might explain the antiviral effect of the conditioned medium, goat antibodies against macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and anti-RANTES (regulated upon activation, normal T-cell expressed, presumed secreted; NIH AIDS Reagents Program) were used for immunoadsorption; normal goat serum was used as a control. Briefly, the anti-β-chemokine antibodies were used to coat six-well culture plates; conditioned medium was then added to the plates and, after a 60-min incubation at room temperature, collected for subsequent determinations of antiviral activity. The quantity of β-chemokines in conditioned medium was measured by ELISA (R & D Systems, Minneapolis, Minn.) according to the manufacturer's instructions.
Statistics.
Correlations between continuous variables were analyzed using Pearson's correlation on ranked data. The Kruskal-Wallis test was used to evaluate continuous variables divided into two different groups.
RESULTS
Plasma from acutely infected patients inhibits HIV-1 in the presence of NK effector cells.
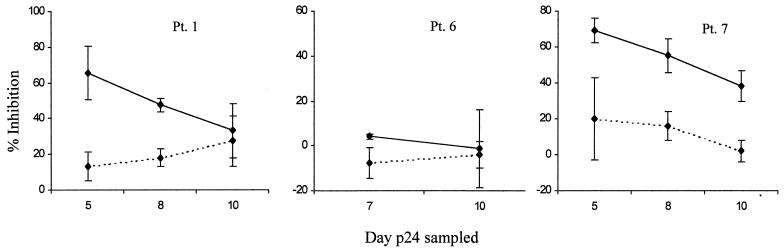
To determine if plasma from acutely infected patients could inhibit HIV-1 and to define the variability of the virus inhibition assay, plasma from three acutely infected patients was assayed two to five times each in six separate experiments; plasma was collected 3 (patient 1) or 14 (patients 6 and 7) days after onset of symptoms of acute HIV infection. CD4+ lymphocytes were infected with HIV92US657 for 48 h before addition of 10% plasma and effector cells, and virus yield was determined at various times thereafter. Plasma was left in solution during the entire assay, thus allowing neutralization of cell-free virus to take place. Nevertheless, plasma alone had little or no effect on virus yield relative to plasma from uninfected controls (Fig. 1; Table 1). NK cells combined with HIV-seronegative plasma also did not reduce viral yield (data not shown); this lack of inhibition by NK cells and seronegative plasma was confirmed in nine separate assays, each with different donors. In contrast to patient plasma alone or control plasma plus NK cells, when plasma from acutely infected patients was combined with NK cells from heterologous, healthy donors, supernatant p24 was decreased in two of the three patient samples assayed (Fig. 1).
FIG. 1.
Inhibition of HIV-1 due to 10% plasma from acutely infected patients (Pt.) with (solid lines) or without (broken lines) NK effector cells (E:T = 10:1). CD4+ lymphocytes were infected for 48 h prior to the addition of plasma and effector cells, and p24 antigen was determined subsequently in supernatant fluids on the days indicated. Percent inhibition = {1 − ([p24i]/[p24u])} × 100, where [p24i] and [p24u] are the concentrations of supernatant fluid p24 produced by HIV-1-infected CD4+ lymphocytes in the presence of plasma from acutely infected patients or from uninfected controls, respectively. This formula was applied to the calculation of virus inhibition due to antibody alone and due to antibody combined with effector cells. Each error bars represents the standard error of two to five experiments.
Different effector cell and/or target cell donors were used in each assay, and peak p24 yield with HIV-seronegative control plasma ranged from about 200 to 1,700 pg/ml. Thus, some interassay variability was expected. However, individual plasma samples generally yielded similar results in repeated assays, especially when p24 was sampled on day 7 or 8 after the addition of plasma and NK cells (Fig. 1). These results demonstrate that the direct effect of plasma and effector cells on HIV yield can be reproducibly measured and that plasma from acutely infected patients has antiviral activity in the presence of NK effector cells.
Whole antibody directed against envelope glycoproteins mediates the antiviral effect.
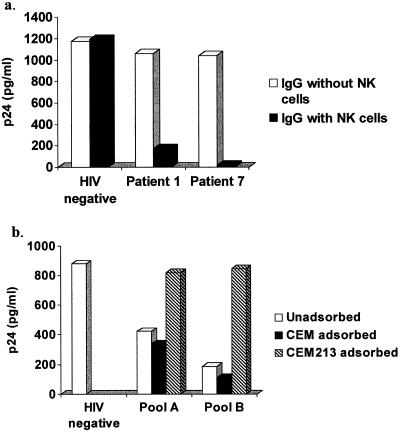
To establish that antibody, rather than some other component of plasma, was responsible for reducing viral yield in the presence of effector cells, IgG was purified from plasma of two acutely infected patients. Samples eluted from the column gave the same pattern on SDS-PAGE as a commercially available IgG with high anticytomegalovirus antibody titer (Cytogam; MedImmune) and contained approximately the same amount of IgG as the original 10% plasma from which the IgG was extracted. IgG from both patients resulted in substantial reductions in viral yield when combined with NK effector cells (Fig. 2a). Fab fragments generated from the patient IgG did not inhibit virus (data not shown).
FIG. 2.
(a) IgG from acutely infected patients inhibits HIV-1 in the presence of NK effector cells. IgG from two acutely infected patients or HIV-seronegative controls without or with NK effector cells was added to CD4+ lymphocytes infected with HIV-1 for 48 h. Supernatant fluid p24 was sampled 7 days later; similar results were obtained when p24 was sampled at 5 days. Data shown are representative of two separate experiments. (b) The antiviral activity of antibody was removed by adsorbing with cells expressing HIV-1 surface glycoproteins (CEM213 cells). Prior to measurement of antiviral activity in the presence of NK effector cells, plasma pooled from HIV-seronegative controls or from acutely infected patients (pool A, consisting of plasma from patients 5 and 9; pool B, consisting of plasma from patients 6 and 10) was left unadsorbed or (for patient plasma) was adsorbed with either untransfected CEM cells or CEM213 cells. p24 was sampled from supernatant fluid 10 days after the addition of plasma and effector cells; similar results were obtained when p24 was sampled at 6 days. Data shown are representative of two separate experiments.
Using whole plasma, we next performed immunoadsorption with CEM213 cells, which are transfected with env and express HIV gp41 and gp120 on their surface. Two separate pools of plasma, each made of samples from two patients (patients 5 and 9 in pool A and patients 6 and 10 in pool B), were used for immunoadsorption. Adsorption with CEM213 cells, but not with untransfected CEM cells, reduced antiviral activity to the same level as plasma from uninfected controls (Fig. 2b). Similar results were obtained when this experiment was repeated using an individual plasma from an acutely infected patient, as well as an additional pool of plasma from four acutely infected patients (data not shown). Thus, the antiviral activity of plasma from acutely infected patients is contained within the IgG fraction, requires intact antibody, and, as expected, targets HIV glycoproteins expressed on the surface of infected cells.
Prevalence of antiviral antibody during acute HIV infection.
To determine the prevalence of antiviral antibody, plasma from 15 acutely infected patients was assayed for antiviral activity against HIV92US657 with and without NK effector cells. Antiviral activity was measured using 10% plasma, NK effector cells from healthy donors, and PHA-stimulated CD4+ lymphocytes infected with HIV92US657 for 48 h as target cells. Supernatant fluid was assayed for p24 activity between 3 and 10 days after the addition of plasma and effector cells. Only day 7 or day 8 results are reported (Table 1); in general, earlier sampling resulted in greater inhibition of viral yield, whereas later sampling gave less inhibition (Fig. 1a). Plasma from each patient was assayed in one of five separate assays, each using different effector and target cell donors. With the exception of plasma samples from patients 1, 6, and 7 (which were assayed multiple times), each patient's plasma was assayed once. Compared with plasma from HIV-negative controls combined with NK effector cells, plasma samples from eight patients, collected at the earliest time points, inhibited virus by >50% in the presence of NK effector cells, and five samples inhibited by >90% (Table 1). In most cases, there was little or no inhibition due to patient plasma alone (compared to HIV-negative plasma alone), consistent with other studies showing poor neutralizing activity early in HIV infection (Table 1). There was no statistically significant correlation between the antiviral activity of plasma in the presence of NK effector cells and the antiviral activity of plasma in the absence of effector cells (R = 0.31, P = 0.27 [Table 1]). Thus, for the majority of patients, an antiviral antibody response occurs very early in acute infection at the time of declining viremia; again, this response requires effector cells.
The magnitude of the antiviral antibody response during acute HIV infection increases as plasma viremia level decreases.
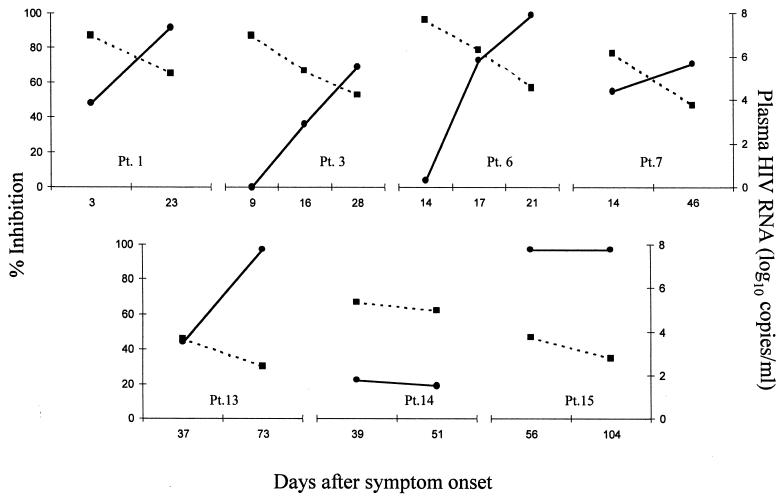
To evaluate the relationship between the antiviral antibody response and plasma viremia, we assayed plasma obtained at multiple time points from seven patients. Plasma HIV RNA was measured by reverse transcription-PCR (Roche Molecular Systems, Branchburg, N.J.). Antiviral activity was measured as described above for determining the prevalence of antibody. To eliminate the variability caused by different target or effector cells, multiple samples from an individual patient were assayed in parallel using a single effector cell donor and a single target cell donor (effector cells and target cells were from different healthy donors). In five patients, the antiviral antibody activity increased while viral load decreased (without antiviral therapy) as much as 3 log10 copies/ml (Fig. 3, patients 1, 3, 6, 7, and 13). Patient 15's viral load decreased while antiviral antibody activity remained constant at 97% (Fig. 3). Patient 14 had very little antiviral activity at either of two time points; this patient's level of viremia stayed constant at about 6 log10 copies/ml during this period (Fig. 3).
FIG. 3.
Evolution of antiviral antibody response and plasma HIV RNA during acute infection. Solid lines, percent inhibition (calculated as described for Fig. 1 and in the text) for 10% plasma in the presence of NK effector cells; broken lines, HIV RNA level. Multiple samples from individual patients were assayed for virus inhibition in parallel (in three separate assays), using single effector cell and target cell donors in each assay (effector cells and target cells were from different donors, and the E:T ratio was 10:1). Parallel assays were run once for each sample; however, the first time points from patients (Pt.) 1, 6, and 7 represent the mean values from an additional one to four assays.
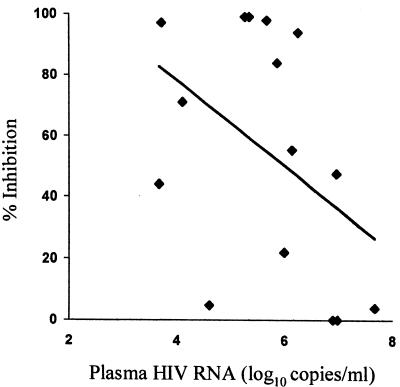
We also compared the antiviral activity from all 15 patients with their HIV RNA levels obtained at the same time. Among patients with multiple samples, only the earliest sample was included in this analysis (Table 1), in order to eliminate the potential bias of using nonindependent measurements. Despite the fact that five different effector cell-target cell donor pairs were used to measure the antiviral activity of plasma (which would normally increase the variability of the measurement), there was an inverse relationship between antiviral activity and viremia level (R = −0.48, P = 0.067 [Fig. 4]). Furthermore, patients with plasma viremia levels less than or equal to the median value (5.87 copies/ml) had significantly greater antiviral antibody activity than patients with plasma viremia levels of >5.87 copies/ml (medians = 91 and 22% inhibition, respectively; P = 0.028). Although the antiviral antibody activity increased over time for most patients with multiple samples, overall there was a poor correlation between antibody activity and the number of days after onset of symptoms that the specimen was obtained (R = 0.18, P = 0.52). Since almost all patients were studied during the period of declining viremia, there was a strong inverse correlation between the day the specimen was obtained and the level of viremia (R = 0.66, P = 0.007).
FIG. 4.
Antiviral antibody activity in plasma from acutely infected patients is inversely associated with plasma HIV-1 RNA level. Percent inhibition of viral yield by 10% patient plasma (from the earliest time point tested) and NK effector cells from uninfected donors is shown as a continuous variable with linear regression line.
Antibody present during acute infection inhibits autologous and heterologous strains of HIV.
The HIV-specific neutralizing antibody response has been reported to be strain specific, particularly early in infection (23). To determine the breadth of the effector cell-mediated antiviral antibody response during acute infection, we used a panel of autologous and heterologous isolates in the virus inhibition assay. Two separate assays were performed, and p24 was measured in the supernatant fluid of HIV-1-infected CD4+ lymphocytes 3, 7, and 10 days after addition of plasma and NK effector cells; the growth kinetics for all isolates were similar. Three of five plasma samples with activity against the reference strain (HIV92US657) had nearly identical antiviral activity against autologous strains or against heterologous strains from other acutely infected patients; a plasma sample which lacked activity against HIV92US657 also did not inhibit the autologous isolate (Table 2). Plasma from patient 3, obtained 16 days after onset of symptoms of acute HIV infection, had about half the activity against the reference strain as against the autologous strain; plasma from the same patient obtained 12 days later was equally active against both strains. Plasma from patient 6 markedly inhibited HIV92US657 but had little if any effect on the isolate from patient 11. On the other hand, the isolate from patient 11 was strongly inhibited by plasma from patient 9, implying that there was no general difficulty inhibiting the patient 11 isolate. These results suggest that the early antiviral antibody response is often broadly reactive.
TABLE 2.
Inhibition of different primary strains of HIV-1 by antibody from acutely infected patients and NK effector cellsa
| Plasma source (days from symptom onset) | % Inhibition of virus fromb
|
||||
|---|---|---|---|---|---|
| HIV92US657 | Pt. 3 | Pt. 9 | Pt. 11 | Pt. 12 | |
| Pt. 3 (16) | 36 | 72 | — | — | — |
| Pt. 3 (28) | 69 | 70 | — | — | — |
| Pt. 6 (21) | 99 | — | — | 13 | — |
| Pt. 9 (18) | 99 | — | 98 | 98 | — |
| Pt. 11 (22) | 5 | — | 0 | 0 | — |
| Pt. 12 (24) | 71 | — | — | — | 62 |
Percent inhibition is calculated as described for Table 1. p24 was sampled from supernatant fluid 7 days after the addition of plasma and NK effector cells to infected CD4+ lymphocytes; p24 determinations were repeated using supernatant fluid sampled at 3 and 10 days, and the relative inhibition of individual strains of virus remained nearly identical; Pt., patient; —, not done.
Virus strains were isolated from patient plasma on day 9 (patients 3 and 12), day 18 (patient 9), and day 10 (patient 11) after onset of symptoms of acute HIV infection.
Plasma from acutely infected patients mediates ADCC and triggers the release of β-chemokines from effector cells.
ADCC causes target cell death and is therefore a likely mechanism by which antibody in the presence of NK effector cells might lead to virus inhibition. To investigate the possible role of ADCC in controlling viremia during acute infection, we measured the ADCC antibody activity of plasma obtained at multiple time points from four acutely infected patients. ADCC was measured in a 51Cr release assay using CEM213 target cells and PBMC effector cells from a healthy donor at an E:T of 100:1. All four patients developed ADCC antibody during the period of declining viremia (Fig. 5). These results indicate that lysis of target cells by ADCC is likely to be responsible, at least in part, for the antiviral antibody activity during acute infection.
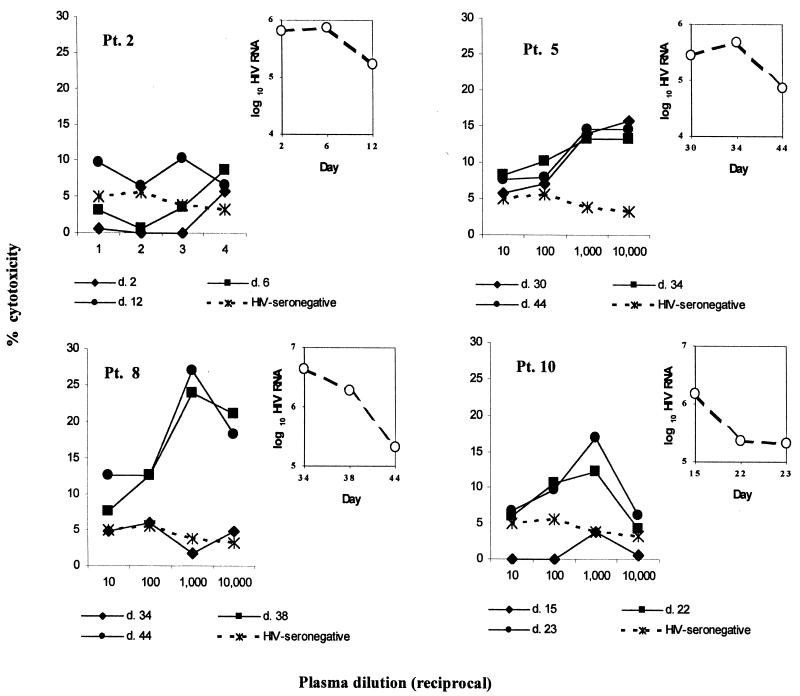
FIG. 5.
Plasma samples from acutely infected patients mediate cytotoxicity of HIV-1 env-transfected CEM cells in the presence of PBMC effector cells. 51Cr-labeled CEM213 target cells were incubated in triplicate with serial dilutions of patient plasma and with PBMCs from a healthy donor in a 4-h 51Cr release assay; mean counts were used to calculate percent cytotoxicity as described in the text. Plasma samples were obtained at different days (d. 2, d. 12, etc.) following the onset of symptoms of acute HIV infection or, in the case of patients (Pt.) 5 and 8, following a known exposure to HIV. Also shown (insets) are the HIV RNA levels from plasma obtained on each day.
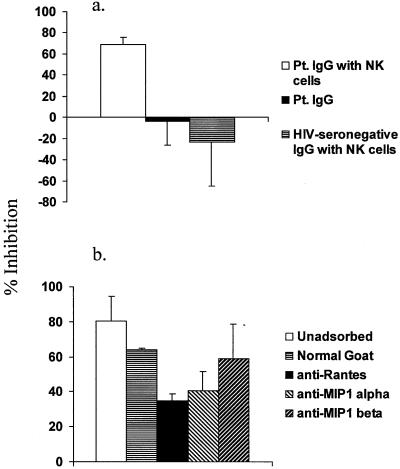
We also explored noncytolytic antiviral mechanisms by determining the ability of conditioned medium, generated from the combination of CEM213 target cells, pooled patient (or control) IgG, and effector cells, to inhibit HIV-1. Conditioned medium from flasks containing patient IgG combined with NK cells reduced virus yield from CD4+ lymphocytes by a mean of 69% compared with infected CD4+ lymphocytes that did not receive conditioned medium (Fig. 6a). Conditioned medium from flasks containing either patient IgG alone or HIV-seronegative IgG combined with NK effector cells had little or no effect on virus yield (Fig. 6a). In a 51Cr release assay, none of these conditioned media were cytolytic (data not shown). Finally, conditioned medium containing a freeze-thaw lysate of the CEM213 target cells did not inhibit virus (data not shown).
FIG. 6.
(a) Medium conditioned by an interaction between antibody, NK cells, and HIV envelope-expressing target cells inhibits a primary, R5 strain of HIV-1. Conditioned medium was obtained from flasks containing CEM213 target cells and either NK effector cells from a healthy donor plus IgG from a pool of acutely infected patients (Pt.), IgG from acutely infected patients without NK effector cells, or NK effector cells plus IgG from HIV-seronegative donors. Percent inhibition was calculated by the formula 100 × {1 − ([p24cm]/[p24vc])}, where [p24cm] is the concentration of p24 obtained from supernatant fluid of freshly infected CD4+ lymphocytes after 5 days incubation with conditioned medium, and [p24vc] is the concentration of p24 obtained with supernatant fluid of freshly infected CD4+ lymphocytes after 5 days incubation with medium alone (i.e., virus control). Data represent means ± standard errors of 10 experiments in the case of effector cells and patient IgG; effector cells plus HIV-seronegative IgG and patient IgG without effector cells were included in four and five of these experiments, respectively. (b) Antibodies against β-chemokines inhibit the antiviral effect of conditioned medium. Medium conditioned with CEM213 target cells, IgG from acutely infected patients, and NK effector cells was incubated with HIV-infected CD4+ lymphocytes. The conditioned medium was used without adsorption or after adsorption with normal goat serum, goat anti-RANTES antibody, goat anti-MIP-1α antibody, or goat anti-MIP-1β antibody. Data represent means ± standard errors of three experiments.
In view of a study indicating that β-chemokines are secreted from NK cells after cross-linking of Fc receptors by anti-CD16 antibody (28), we determined if β-chemokines were responsible for the antiviral effect of the conditioned medium. First, combining IgG from acutely infected patients with CEM213 target cells and NK effector cells resulted in increased amounts of MIP-1α and RANTES compared with target cells combined with HIV-seronegative IgG plus NK effector cells; note that NK cells in the absence of HIV-1-specific IgG produced all three β-chemokines when combined with target cells (Table 3). We also found that adsorption of conditioned medium with goat anti-MIP-1α and anti-RANTES antibodies reduced the antiviral activity by 36 and 46%, respectively, compared with adsorption with normal goat serum; adsorption with anti-MIP-1β had little effect (Fig. 6b). Thus, MIP-1α and RANTES production is augmented by the interaction between HIV-1-specific antibody, env-expressing target cells, and NK effector cells and results in reduced virus yield.
TABLE 3.
Chemokine production due to the interaction between HIV-1-specific antibody, target cells expressing HIV envelope glycoproteins, and NK effector cells
| Antibody-effector cell combinationsa | β-Chemokine (pg/ml)b
|
||
|---|---|---|---|
| MIP-1α | MIP-1β | RANTES | |
| Patient IgG + NK cells | 4,454 ± 1,935 | 992 ± 637 | 8,260 ± 1,725 |
| HIV-negative IgG + NK cells | 1,507 ± 752 | 1,032 ± 489 | 6,021 ± 2,006 |
| Patient Fab + NK cells | 1,740 ± 940 | 964 ± 680 | 6,084 ± 1,916 |
| Patient IgG (no NK cells) | <10 ± 0 | 70 ± 56 | <10 ± 0 |
Antibody-effector cell combinations were added to CEM213 target cells for 18 h, and the supernatant fluid was assayed for β-chemokine levels by ELISA.
Mean ± standard error of three separate experiments, except in the case of the Fab-NK combination, where values represent the mean ± standard error of two separate experiments. Significant differences (P < 0.05) were noted by paired t test for RANTES production by patient IgG + NK cells versus HIV-negative IgG + NK cells or patient IgG (no NK cells).
DISCUSSION
The marked decline in plasma HIV-1 level that occurs during acute infection strongly suggests that immune responses are capable of partially controlling HIV viremia. Since CTL activity is detectable early in infection, whereas neutralizing antibody is generally absent, immunological control of HIV-1 has been attributed primarily to the CTL response (6, 17, 18, 23, 24, 27, 30, 31, 39). We have found, however, that in marked contrast to neutralizing activity, antibody directed against infected cells and capable of inhibiting HIV-1 in the presence of NK effector cells is detectable in the majority of patients when viremia is declining and as early as a few days after the onset of symptoms of acute infection. Furthermore, we found that the magnitude of this effector cell-mediated antiviral antibody response is inversely associated with plasma viremia level and that autologous and heterologous primary HIV strains are inhibited. These findings indicate that HIV-1-specific antibody could play an important role in the control of viremia during acute infection.
Our data strongly support ADCC as a mechanism responsible for the antiviral activity in plasma from acutely infected patients. ADCC occurs when antibody forms a bridge between target cells expressing antigens with which the antibody binds and effector cells bearing Fc receptors. We have shown that the antiviral activity is contained within the IgG fraction of antibody and requires NK effector cells (which express Fc receptors [32]). Furthermore, the antiviral antibody binds to envelope glycoproteins, and activity requires intact antibody rather than Fab fragments. Finally, in cytotoxicity assays directly measuring ADCC, plasma from acutely infected patients, in the presence of effector cells, lysed target cells expressing HIV glycoproteins.
ADCC, like CTL activity, results in the death of infected cells (21). It is therefore biologically plausible that ADCC plays a role in controlling viremia during HIV-1 infection. Baum et al. have shown that in patients with chronic infection, higher ADCC antibody titer is associated with less rapid progression of disease (4). We have previously found that ADCC, measured by 51Cr release assay using target cells transfected with HIV-1 env and an autologous combination of patient serum and PBMC effector cells, correlates inversely with viral load in chronically infected patients not receiving antiretroviral therapy (14). Importantly, the effector cells of ADCC—NK cells as well as macrophages—can be found in key sites of HIV replication, such as lymph nodes (M. Lu, N. Kouttab, N. Raja, D. L. Zheng, and G. Skowron, Abstr. 7th Conf. Retroviruses Opportunistic Infect., abstr. 368, 2000; 26). In SIV-infected rhesus macaques with rapidly progressive disease, the passive infusion of plasma from animals with more slowly progressive disease reduces plasma viremia in a manner most consistent with ADCC (5). Furthermore, during acute SIV infection, a rapid increase in NK activity (measured in a 51Cr release assay) and in the number of activated NK cells precedes the decline in viremia (15); since NK cells are a major effector cell for ADCC (38), it is possible that these activated NK cells are mediating ADCC. In a recent study describing synergism between antibody and immune T cells in protecting mice from herpes simplex virus type 2 genital infection, the authors suggested that T cells responding to the virus produce cytokines that activate NK cells, which in turn mediate ADCC in the presence of antibody (25). Such a three-way interaction between innate, humoral, and T-cell immunity could also explain the strong correlation between HIV-1-specific CD4+ lymphocyte activity and control of viremia during HIV-1 infection (33). Finally, the recent demonstration that infusion of an anti-CD8 monoclonal antibody results in a transient increase in plasma SIV viremia is also consistent with a role for ADCC in the control of lentivirus infection, since macaque NK cells generally express CD8 and would likely be depleted along with CTLs (8, 36). From our results together with those of Connick et al. showing a temporal relationship between antiviral antibodies and the fall in viremia during acute infection (11), there is mounting evidence supporting an important role for ADCC in controlling viremia during HIV infection.
By definition, ADCC results in the lysis of target cells. Most of our experiments used reduction in viral yield, rather than cell lysis, as an indicator of the biological activity of antibody. It is likely that much of the antiviral activity that we measured in plasma from acutely infected patients was due to the death and removal of virus-producing cells. On the other hand, noncytolytic mechanisms, due to soluble substances secreted as a result of the interaction between target cells, antibody, and effector cells, also reduced viral yield. Some of the noncytolytic antiviral effect was neutralized by antibodies against MIP-1α and RANTES. Furthermore, HIV-specific antibody, in the presence of envelope-expressing target cells, augmented MIP-1α and RANTES release from NK cells. Thus, it is likely that these β-chemokines, released from NK cells via Fc receptor stimulation, were responsible for some or all of the soluble antiviral effect. Their role relative to that of cytotoxicity in inhibiting virus was not ascertained.
Presumably, the β-chemokines act by inhibiting the entry of newly released virus into uninfected cells (13). β-Chemokine release from NK cells through cross-linking of Fc receptors has been previously documented (28). However, in that study, Fc receptor cross-linking was accomplished by a specific antibody-antigen interaction directed against the receptor. Our study shows, for the first time, that a physiological interaction between an HIV-1-specific antibody, HIV-1 glycoprotein-expressing cells, and effector cells bearing Fc receptors results in the release of β-chemokines and that a consequent antiviral effect occurs. Thus, we define a new biological activity of antibody, related to ADCC through its component parts but acting through an entirely different mechanism.
In general, antibody alone had little effect on viral yield. Our experimental conditions allowed antibody added to HIV-infected cells to inhibit cell-to-cell spread of virus, either by neutralizing cell-free virus or by interacting with budding virions. In any case, the limited activity of antibody alone is consistent with other studies showing poor neutralizing antibody activity during acute infection (18, 23, 24, 30). Nonetheless, a recent study reported consistent neutralizing activity early in infection when macrophages, rather than lymphocytes, were used as target cells; however, many of the sera tested were obtained several months after infection and likely well after the steepest declines in viremia (34). We did not find a strong correlation between the antiviral effect of antibody alone and that of antibody combined with effector cells. Thus, it is unclear whether antibodies that mediate an antiviral effect toward infected cells in the presence of effector cells are the same as those that neutralize cell-free virus. Addressing this question will require further studies focused on determining the antigenic specificity and the antibody-antigen affinity of the early antiviral response.
Plasma antiviral activities were generally similar when measured against a reference strain, other heterologous strains, or autologous strains. Although we did not determine the degree of genetic diversity in the isolates used, these results suggest that the antiviral response during acute infection, when measured in the presence of effector cells, is broadly reactive. Again, this differs from the neutralizing antibody response, which tends to be strain specific, particularly early in infection (23). If further studies verify the breadth and importance of the effector cell-mediated antiviral response in controlling viremia in vivo, the antigenic determinants of this response may prove to be key components of a protective or therapeutic HIV vaccine.
An unexpected finding of our investigation was the lack of antiviral activity of NK cells in the absence of HIV-specific antibody. NK activity is thought to require a positive signal generated through a specific ligand-receptor interaction or the absence of inhibitory signals mediated by major-histocompatibility complex molecules on target cells and inhibitory receptors on NK cells (3, 7, 10). HIV infection down regulates the expression of some major histocompatibility complex class I molecules, which should render infected cells targets for NK activity (37). On the other hand, HLA C and HLA E molecules may not be down regulated and thus remain available to interact with inhibitory receptors (9). It should be noted that we did not activate NK cells before using them in virus inhibition assays. However, the use of NK effector cells and CD4+ lymphocyte target cells from different donors likely resulted in some activation over the 7-day assay period. Furthermore, it is possible that NK cells could have been effective in reducing viral yield in cells infected for a shorter time. In any case, our results lead us to question any significant role for NK cells—in the absence of Fc receptor cross-linking by antibody—in controlling viremia. If the activation of NK cells, which occurs just prior to the decline in viremia during acute SIV infection (15), is important in controlling viremia, it may be due to the capacity of NK cells to act as effector cells for an early antiviral antibody response.
In summary, antibody capable of inhibiting autologous and heterologous primary strains of HIV-1, in the presence of NK effector cells, is present early in acute HIV infection, and the magnitude of this antibody response correlates inversely with plasma HIV-1 viremia level. HIV-1-specific antibody may thus be an important contributor to the early control of HIV-1 viremia, and antigens that elicit an effector cell-mediated, antiviral antibody response may be important components of an HIV vaccine.
ACKNOWLEDGMENTS
Financial support was provided by National Institutes of Health grant A144610 (D.N.F.), Universitywide AIDS Research Program Individual Investigator Award R97-I-068 (D.N.F.), Universitywide AIDS Research Program grant PH97-CS-202 (E.S.D.), National Center for Research Resources grant M01-RR00425 (E.S.D.), the Japan Foundation for Health (E.S.D.), the Women's Guild (E.S.D.), and the California Collaborative Treatment Group of the Universitywide AIDS Research Program (D.N.F.).
We acknowledge the contributions of Jacqui Pitt and Susan Little.
REFERENCES
- 1.Allen T M, Vogel T U, Fuller D H, Mothé B R, Steffen S, Boyson J E, Shipley T, Fuller J, Hanke T, Sette A, Altman J D, Moss B, McMichael A J, Watkins D I. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J Immunol. 2000;164:4968–4978. doi: 10.4049/jimmunol.164.9.4968. [DOI] [PubMed] [Google Scholar]
- 2.Barouch D H, Santra S, Schmitz J E, Kuroda M J, Fu T-M, Wagner W, Bilska M, Craiu A, Zheng X X, Krivulka G R, Beaudry K, Lifton M A, Nickerson C E, Trigona W L, Punt K, Freed D C, Guan L, Dubey S, Casimiro D, Simon A, Davies M-E, Chastain M, Strom T B, Gelman R S, Montefiori D C, Lewis M G, Emini E A, Shiver J W, Letvin N L. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 3.Bauer S, Groh V, Wu J, Steinle A, Phillips J H, Lanier L L, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 4.Baum L L, Cassutt K J, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger C A, Nishanian P, Henrard D R, Phair J. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2168–2173. [PubMed] [Google Scholar]
- 5.Binley J M, Clas B, Gettie A, Vesanen M, Montefiori D C, Sawyer L, Booth J, Lewis M, Marx P A, Bonhoeffer S, Moore J P. Passive infusion of immune serum into simian immunodeficiency virus-infected rhesus macaques undergoing a rapid disease course has minimal effect on plasma viremia. Virology. 2000;270:237–249. doi: 10.1006/viro.2000.0254. [DOI] [PubMed] [Google Scholar]
- 6.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braud V M, Allan D S, O'Callaghan C A, Söderström K, D'Andrea A, Ogg G S, Lazetie S, Young N T, Bell J I, Phillips J H, Lanier L L, McMichael A J. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 8.Carter D L, Shieh T M, Blosser R L, Chadwick K R, Margolick J B, Hildreth J E K, Clements J E, Zink M C. CD56 identifies monocytes and not natural killer cells in rhesus macaques. Cytometry. 1999;37:41–50. [PubMed] [Google Scholar]
- 9.Cohen G B, Gandhi R T, Davis D M, Mandelboim O, Chen B K, Strominger J L, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 10.Colonna M, Borsellino G, Falco M, Ferrara G B, Strominger J L. HLA-C is the inhibitory ligand that determines dominant resistance to lysis by NK1- and NK2-specific natural killer cells. Proc Natl Acad Sci USA. 1993;90:12000–12004. doi: 10.1073/pnas.90.24.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connick E, Marr D G, Zhang X Q, Clark S J, Saag M S, Schooley R T, Curiel T J. HIV-specific cellular and humoral immune responses in primary HIV infection. AIDS Res Human Retrovir. 1996;12:1129–1140. doi: 10.1089/aid.1996.12.1129. [DOI] [PubMed] [Google Scholar]
- 12.Daar E S, Moudgil T, Meyer R D, Ho D D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991;324:961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- 13.Dragie T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 14.Forthal D N, Landucci G, Keenan B. The relationship between antibody-dependent cellular cytotoxicity, plasma HIV-1 RNA, and CD4+ lymphocyte count. AIDS Res Hum Retrovir. 2001;17:553–561. doi: 10.1089/08892220151126661. [DOI] [PubMed] [Google Scholar]
- 15.Giavedoni L D, Velasquillo M C, Parodi L M, Hubbard G B, Hodara V L. Cytokine expression, natural killer cell activation, and phenotypic changes in lymphoid cells from rhesus macaques during acute infection with pathogenic simian immunodeficiency virus. J Virol. 2000;74:1648–1657. doi: 10.1128/jvi.74.4.1648-1657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jewett A, Giorgi J V, Bonavida B. Antibody-dependent cellular cytotoxicity against HIV-coated target cells by peripheral blood monocytes from HIV seropositive asymptomatic patients. J Immunol. 1990;145:4065–4071. [PubMed] [Google Scholar]
- 17.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legrand E, Pellegrin I, Neau D, Pellegrin J L, Ragnaud J M, Dupon M, Guillemain B, Fleury H J. Course of specific T lymphocyte cytotoxicity, plasma and cellular viral loads, and neutralizing antibody titers in 17 recently seroconverted HIV type 1-infected patients. AIDS Res Hum Retrovir. 1997;13:1383–1394. doi: 10.1089/aid.1997.13.1383. [DOI] [PubMed] [Google Scholar]
- 19.Letvin N L, Schmitz J E, Jordan H L, Seth A, Hirsch V M, Reimann K A, Kuroda M J. Cytotoxic T lymphocytes specific for the simian immunodeficiency virus. Immunol Rev. 1999;170:127–134. doi: 10.1111/j.1600-065x.1999.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 20.Leung N J, Aldovini A, Young R, Jarvis M A, Smith J M, Meyer D, Anderson D E, Carlos M P, Gardner M B, Torres J V. The kinetics of specific immune responses in rhesus monkeys inoculated with live recombinant BCG expressing SIV Gag. Pol, Env, and Nef proteins. Virology. 2000;268:94–103. doi: 10.1006/viro.1999.0131. [DOI] [PubMed] [Google Scholar]
- 21.Lyerly H K, Matthews T J, Langlois A J, Bolognesi D P, Weinhold K J. Human T-cell lymphotropic virus IIIB glycoprotein (gp120) bound to CD4 determinants on normal lymphocytes and expressed by infected cells serves as target for immune attack. Proc Natl Acad Sci USA. 1987;84:4601–4605. doi: 10.1073/pnas.84.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellors J W, Rinaldo C R, Jr, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 23.Moog C, Fleury H J, Pellegrin I, Kirn A, Aubertin A M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore J P, Cao Y, Ho D D, Koup R A. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J Virol. 1994;68:5142–5155. doi: 10.1128/jvi.68.8.5142-5155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison L A, Zhu L, Thebeau L G. Vaccine-induced serum immunoglobin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J Virol. 2001;75:1195–1204. doi: 10.1128/JVI.75.3.1195-1204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller H, Falk S, Schmidts H L, Stutte H J. In situ immunophenotyping of lymphocytes/macrophages: grading of lymphadenopathy, staging and pathophysiology of HIV infection. Res Virol. 1990;141:171–184. doi: 10.1016/0923-2516(90)90019-f. [DOI] [PubMed] [Google Scholar]
- 27.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 28.Oliva A, Kinter A L, Vaccarezza M, Rubbert A, Catanzaro A, Moir S, Monaco J, Ehler L, Mizell S, Jackson R, Li Y, Romano J W, Fauci A S. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Investig. 1998;102:223–231. doi: 10.1172/JCI2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orenstein J M, Feinberg M, Yoder C, Schrager L, Mican J M, Schwartzentruber D J, Davey R T, Jr, Walker R E, Falloon J, Kovacs J A, Miller K D, Fox C, Metcalf J A, Masur H, Polis M A. Lymph node architecture preceding and following 6 months of potent antiviral therapy: follicular hyperplasia persists in parallel with p24 antigen restoration after involution and CD4 cell depletion in an AIDS patient. AIDS. 1999;13:2219–2229. doi: 10.1097/00002030-199911120-00004. [DOI] [PubMed] [Google Scholar]
- 30.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 31.Price D A, Goulder P J, Klenerman P, Sewell A K, Easterbrook P J, Troop M, Bangham C R, Phillips R E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravetch J V, Perussia B. Alternative membrane forms of Fc gamma RIII (CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170:481–497. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 34.Ruppach H, Nara P, Raudonat I, Elanjikal Z, Rübsamen-Waigmann H, Dietrich U. Human immunodeficiency virus (HIV)-positive sera obtained shortly after seroconversion neutralize autologous HIV type 1 isolates on primary macrophages but not on lymphocytes. J Virol. 2000;74:5403–5411. doi: 10.1128/jvi.74.12.5403-5411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schacker T W, Hughes J P, Shea T, Coombs R W, Corey L. Biological and virologic characteristics of primary HIV infection. Ann Intern Med. 1998;128:613–620. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz O, Maréchal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 38.Tyler D S, Nastala C L, Stanley S D, Matthews T J, Lyerly H K, Bolognesi D P, Weinhold K J. GP120 specific cellular cytotoxicity in HIV-1 seropositive individuals. Evidence for circulating CD16+ effector cells armed in vivo with cytophilic antibody. J Immunol. 1989;142:1177–1182. [PubMed] [Google Scholar]
- 39.Wilson J D, Ogg G S, Allen R L, Davis C, Shaunak S, Downie J, Dyer W, Workman C, Sullivan S, McMichael A J, Rowland-Jones S L. Direct visualization of HIV-1-specific cytotoxic T lymphocytes during primary infection. AIDS. 2000;14:225–233. doi: 10.1097/00002030-200002180-00003. [DOI] [PubMed] [Google Scholar]