Highlights
-
•
Study on the implementation of National Action Plan on Antimicrobial Resistance.
-
•
The largest sample size to study the epidemiology and antimicrobial resistance.
-
•
No significant change in the prevalence of urinary tract infections.
-
•
Change in bacteria species causing urinary tract infections between two periods.
-
•
The trend of antimicrobial resistance is increasing significantly.
Keywords: Antimicrobial resistance (AMR) surveillance, National action plan on AMR, Urinary tract infection, Hospital levels
Abstract
Objectives
To delineate the epidemiology and antimicrobial resistance (AMR) trends of pathogens causing urinary tract infections (UTIs) during (June 2019-June 2020) and after (March-July 2023) the implementation of the National Action Plan on AMR 2017-2022 in Mwanza, Tanzania.
Methods
This cross-sectional study was conducted among 2097 patients with clinical symptoms of UTIs during (n = 1144) and after (n = 953) the National Action Plan on AMR 2017-2022. Quantitative urine culture was done to isolate significant bacteria causing UTI, which were then identified to the species level and tested for antimicrobial susceptibility. Tabulations, descriptive, and logistic regression analyses were used to analyze categorical and continuous variables, as well as the association between outcome and independent variables. Statistical significance was defined as P ≤0.05 at a 95% confidence interval (CI).
Results
The overall prevalence of culture-positive UTIs was 22.8% (479 of 2097; 95% CI: 21.1-24.7%), with no significant difference between the study periods (21.8% [249 of 1144; 95% CI: 19.5-24.3%]) vs 24.1% (230 of 953; 95% CI: 21.5-26.9%), P = 0.274). We observed a significant increase in resistance to ciprofloxacin (32.0% vs 45.8%, P = 0.0481) and third-generation cephalosporins (marked by extended-spectrum β-lactamase–producing Enterobacterales [ESBL-PE], 38.7% vs 56.9%, P = 0.0307). Additionally, UTIs caused by ESBL-PE is significantly common among patients in higher-tier hospitals (58.4% vs 34.0%; OR [95% CI]: 2.51 [1.41-4.48], P = 0.002).
Conclusions
There was a significant increase in bacterial resistance to ciprofloxacin and third-generation cephalosporins, as well as ESBL-PE. These results emphasize the critical need to enhance AMR surveillance, improve infection prevention and control measures, and strengthen antimicrobial stewardship programs.
Introduction
Antimicrobial resistance (AMR) is currently a highly significant global concern in the realm of public health [1]. Recently, the excessive and inappropriate use of antibiotics has resulted in the emergence and spread of bacteria resistant to multiple antimicrobials [1]. Consequently, managing infections, including urinary tract infections (UTIs), is now facing a substantial threat because existing antibiotic treatments have become less effective [1]. To address this issue, numerous countries, including Tanzania, are implementing their National Action Plans on AMR (NAP-AMR) adapted from the World Health Organization Global Action Plans on AMR [2,3].
UTIs are one of the most prevalent bacterial infections worldwide, for example, in 2019, more than 404.6 million people had UTIs [4]. The emergence of AMR has complicated the management of UTIs. The World Health Organization has identified 12 priority pathogens based on their AMR potentials [5]. Of these, critical priority pathogens include carbapenemase-producing Acinetobacter baumannii and Pseudomonas aeruginosa and carbapenemase and extended-spectrum β-lactamase (ESBL)–producing Enterobacterales, as well as the high priority pathogen, methicillin-resistant Staphylococcus aureus [5,6]. All these pathogens can potentially cause UTIs, underscoring an urgent need to institute AMR testing and surveillance to guide specific management. Tanzania rolled-out its NAP-AMR 2017-2022, which, among others, focuses on four objectives, i.e. creating awareness through information, education, and communication; fostering AMR research and surveillance; mitigating infection prevention and control; and promoting antimicrobial stewardship (AMS) [3]. However, AMS and AMR surveillance on UTIs and blood stream infections in Tanzania is exclusively focused on tertiary and secondary hospitals (higher-tier hospitals) and not on primary hospitals (lower-tier hospitals). Furthermore, the NAP-AMR program's roll-out was challenged by the COVID-19 pandemic, which was first declared on March 16, 2020 in Tanzania [7], resulting in a surge of antibiotics consumption used in health care facilities and the communities [8].
Therefore, this study was designed to address these critical research gaps by involving five hospitals spanning from primary (lower-tier) to secondary and tertiary (higher-tier) hospitals to delineate epidemiology and resistance trends of pathogens causing UTIs in Mwanza, Tanzania through a multisectoral and multicenter project, “Supporting the NAP-AMR” (SNAP-AMR), during the implementation of the Tanzanian NAP-AMR 2017-2022. The SNAP-AMR project collaborated with the Tanzanian Ministry of Health between June 2019 and June 2020 to support all four strategic objectives of the NAP-AMR and build laboratory capacity at lower-tier hospitals by establishing bacteriology laboratories to perform cultivation, phenotypic detection, and susceptibility testing and reporting of resistance phenotypes.
Materials and methods
Study design, duration, and setting
This cross-sectional hospital-based study was conducted between June 2019 and June 2020 (first cohort, during NAP-AMR) and between March and July 2023 (second cohort, after NAP-AMR) in five health care facilities in Mwanza, Tanzania. Bugando Medical Centre is a tertiary zonal referral hospital with a capacity of 1080 beds, serving a catchment of about 18.8 million people from the Lake Victoria Zone regions (Mwanza, Mara, Kagera, Kigoma, Simiyu, Shinyanga, and Geita). Sekou Toure Regional Referral Hospital, which has approximately 450 beds, is a regional referral hospital for the Mwanza Region, serving a population of 3.6 million people. Sumve Designated District Hospital (SDDH), with a bed capacity of 265, is a designated district hospital that serves the population of Kwimba district. Magu District Hospital (MDH) is a district hospital for Magu District, with an estimated bed capacity of 150. Misungwi District Hospital (MisDH) is a district hospital for Misungwi District, with a bed capacity of 128. SDDH, MDH, and MisDH serve a total population of 1.3 million people. In this study, higher-tier hospitals refer to Bugando Medical Centre and Sekou Toure Regional Referral Hospital, whereas lower-tier hospitals refer to MDH, MisDH, and SDDH.
Population, selection criteria, and data collection
In the current study, we enrolled patients exhibiting clinical symptoms of UTIs, such as pyuria, dysuria, lower abdominal pain, flank pain, or fever, according to Tanzania's standard treatment guidelines [9]. The clinical diagnosis of UTIs was done by clinicians in the respective health care facilities. Urine samples were then collected and sent to the microbiology laboratory of the corresponding health care facilities for culture and antibiotic susceptibility testing. Those patients whose urine samples were received in the laboratory were eligible for enrollment in this study. Each patient was enrolled once. The patients’ information such as age, sex, hospital number, and ward of admission or clinic of the visit were extracted from laboratory request forms or laboratory information management systems. The information captured from the patients’ request forms or laboratory information management systems was used to track patients to collect further related information; sociodemographic and clinical data were collected using a pretested structured questionnaire. A total of 51 (4.3%) patients enrolled during NAP-AMR were excluded from the final data set before data analysis due to incomplete information. However, no patient enrolled after NAP-AMR was excluded due to incomplete information.
Laboratory procedures
A 10-µl loop was used for quantitatively inoculation of urine sample on 5% sheep blood agar and MacConkey agar plates, followed by aerobic incubation at 35 ± 2°C for 18 to 24 hours. Bacterial growth of ≥105 CFU/mL was considered significant for UTIs. The in-house biochemical identification tests were used for identification of isolated bacteria as previously documented [10]. The Kirby-Bauer disc diffusion method was used for antimicrobial susceptibility testing (AST), and the zones of inhibition were interpreted based on Clinical and Laboratory Standards Institute guidelines [11]. All culture media and antibiotic discs were manufactured by HiMedia, Maharashtra, India. All laboratories followed the same standard operating procedures to ensure the reproducibility of laboratory results across hospitals. The isolates were initially analyzed in the laboratories of the hospitals to guide the patients’ management and then transported to the Microbiology Research Laboratory of the Catholic University of Health and Allied Sciences in Mwanza, Tanzania for confirmation and storage at −80°C until further analysis.
Bacterial species and AST were verified at the Institute for Hygiene and Microbiology of the Julius Maximilians University of Würzburg, Würzburg, Germany. Species identification was done by matrix-assisted laser desorption ionization-time of flight on VITEK MS (bioMérieux, Nürtingen, Germany) and 16S rRNA Sanger sequencing, whenever necessary. To determine bacterial susceptibility to antibiotic agents, minimum inhibitory concentrations were determined by VITEK 2 (bioMérieux, Nürtingen, Germany) using the following AST cards: AST-P654 (Staphylococcus spp. and other gram-positive bacteria [GPB]), AST-N214 (Enterobacterales and Acinetobacter spp.), AST-N371 (E. coli), AST-P655 (Enterococcus spp. and Streptococcus spp.), and AST-N248 (Pseudomonas spp.). To interpret the AST results, the European Committee on Antimicrobial Susceptibility Testing clinical break points (version 13.0, 2023) were applied [12].
In the current study, coagulase-negative Staphylococci (CoNS) and other rare bacterial pathogens were considered significant pathogens causing UTIs based on the following: (i) every patient enrolled in this study showed symptoms indicative of UTIs, (ii) pure (one type of microbial growth) and significant growth (at least 105 CFU/mL) on culture media, and (iii) previous studies reported that CoNS and rare bacterial pathogens possess important virulence genes/factors associated with infections, including UTIs [13,14].
Detection of multidrug-resistance phenotypes
S. aureus strains showing resistance toward cefoxitin were considered as methicillin-resistant S. aureus, whereas ESBL-producing strains of E. coli and K. pneumoniae were detected based on simultaneous assessment of the inhibitory effects of cefepime, cefotaxime, and ceftazidime alone and in the presence of clavulanic acid [12].
Quality control
E. coli ATCC 25922 and S. aureus ATCC 25923 were used as control organisms for the validation of laboratory protocols such as biochemical identification and antibiotic susceptibility testing.
Data management and analysis
Microsoft Excel was used for data cleaning and coding, whereas STATA 15.0 was used for data analysis. Continuous and categorical data are presented as median (interquartile range) and proportions or percentages, respectively. Univariate and multivariate logistic regression analyses were used to determine the association between independent variables (e.g. age, sex, exposure to antibiotics, etc.) and the outcome variable (i.e. UTIs by ESBL-producing Enterobacterales [ESBL-PE]). Independent variables showing significant levels of association (P <0.05) by univariate analysis were included in the multivariate analysis. In addition, the Wilcoxon rank-sum test (Mann–Whitney U test) was used to compare the median age of culture-positive and -negative groups. A P ≤0.05 at 95% confidence interval (CI) was considered statistically significant.
Results
Sociodemographic and clinical characteristics of patients with the clinical diagnosis of UTI during and after NAP-AMR
A total of 2097 patients were enrolled (1144 during NAP-AMR and 953 after NAP-AMR). The median (interquartile range) age of all patients was 26 (6-42) years and patients enrolled after NAP-AMR were slightly older than those enrolled during NAP-AMR (29 [8-44] vs 23 [5-40] years). Overall, female patients accounted for 59.3% (1244 of 2097), 56.9% (651 of 1144) during NAP-AMR and 62.2% (593 of 953) after NAP-AMR. Of all patients, 39.5% (828 of 2097) had primary education level and 44.0% (923 of 2097) were self-employed (Table 1).
Table 1.
Sociodemographic and clinical characteristics of patients with clinical diagnosis of urinary tract infections during and after the NAP-AMR.
| Characteristics | Categories | Overall (N = 2097) | During NAP-AMR (N = 1144) | After NAP-AMR (N = 953) | Chi-square test |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | P-value | ||
| Median (interquartile range) age in years | 26 (6-42) | 23 (5-40) | 29 (8-44) | <0.0001a | |
| Sex | Male | 853 (40.7) | 493 (43.1) | 360 (37.8) | 0.014 |
| Female | 1244 (59.3) | 651 (56.9) | 593 (62.2) | ||
| Residency | Rural | 1057 (50.4) | 595 (52.0) | 462 (48.5) | 0.107 |
| Urban | 1040 (49.6) | 549 (48.0) | 491 (51.5) | ||
| Education level | None | 700 (33.4) | 462 (40.4) | 238 (24.9) | <0.0001 |
| Primary | 828 (39.5) | 397 (34.7) | 431 (45.2) | ||
| Secondary | 340 (16.2) | 164 (14.3) | 176 (18.5) | ||
| Tertiary | 229 (10.9) | 121 (10.6) | 108 (11.3) | ||
| Occupation | None | 944 (45.0) | 565 (49.0) | 379 (39.8) | <0.0001 |
| Employed | 230 (11.0) | 140 (12.2) | 90 (9.4) | ||
| Self-employed | 923 (44.0) | 439 (38.4) | 484 (50.8) | ||
| Healthcare facility | BMC | 668 (31.8) | 273 (23.9) | 395 (41.4) | <0.0001 |
| SRRH | 312 (14.9) | 102 (8.9) | 210 (22.0) | ||
| MDH | 428 (20.4) | 280 (24.5) | 148 (15.5) | ||
| SDDH | 337 (16.1) | 212 (18.5) | 125 (13.1) | ||
| MisDH | 352 (16.8) | 277 (24.2) | 75 (7.9) | ||
| Level of healthcare facility | Higher-tier | 980 (46.7) | 375 (32.8) | 605 (63.5) | <0.0001 |
| Lower-tier | 1117 (53.2) | 769 (67.2) | 348 (36.5) | ||
| Patient category | Outpatient | 1411 (67.3) | 667 (58.3) | 744 (78.1) | <0.0001 |
| Inpatient | 686 (32.7) | 477 (41.7) | 209 (21.9) | ||
| Ward/clinic of enrollment | Medical | 1433 (68.3) | 618 (54.0) | 815 (82.8) | <0.0001 |
| Pediatric | 473 (22.5) | 389 (34.0) | 84 (8.8) | ||
| Surgical | 107 (5.1) | 88 (7.7) | 19 (2.0) | ||
| Othersb | 110 (5.2) | 49 (4.3) | 61 (6.4) | ||
| History of fever past 3 months | Yes | 868 (41.4) | 528 (46.1) | 340 (35.7) | <0.0001 |
| No | 1229 (58.6) | 616 (53.9) | 613 (64.3) | ||
| History of admission 3 months | Yes | 258 (12.3) | 171 (15.0) | 87 (9.1) | <0.0001 |
| No | 1839 (87.7) | 973 (85.0) | 866 (90.9) | ||
| History of antibiotic use 3 months | Yes | 606 (28.9) | 365 (31.9) | 241 (25.3) | 0.001 |
| No | 1491 (71.1) | 779 (68.1) | 712 (74.7) | ||
| Patient on antibiotic during sampling | Yes | 542 (25.8) | 539 (47.1) | 3 (0.3) | <0.0001 |
| No | 1555 (74.1) | 605 (52.9) | 950 (99.7) | ||
| Classes of antibiotics taken by patients during the study period | Penicillins | 310 (57.2) | 309 (57.3) | 1 (33.3) | <0.0001 |
| Cephalosporins | 130 (23.9) | 129 (23.9) | 1 (33.3) | ||
| Quinolones | 92 (16.9) | 92 (17.1) | 0 (0.0) | ||
| Aminoglycosides | 67 (12.4) | 65 (12.1) | 2 (66.7) | ||
| Macrolides | 23 (4.2) | 23 (4.3) | 0 (0.0) | ||
| Nitroimidazoles | 49 (9.0) | 47 (8.7) | 2 (66.7) | ||
| Carbapenems | 3 (0.5) | 3 (0.6) | 0 (0.0) | ||
| Chronic disease | Yes | 214 (10.2) | 122 (10.7) | 92 (9.6) | 0.447 |
| No | 1883 (89.8) | 1022 (89.3) | 861 (90.4) | ||
| Types of chronic diseases | Hypertension | 81 (37.8) | 45 (36.9) | 36 (39.1) | <0.0001 |
| Sickle cell disease | 38 (17.7) | 32 (26.2) | 6 (6.5) | ||
| Diabetes mellitus | 57 (26.6) | 31 (25.4) | 26 (28.3) | ||
| HIV | 31 (14.5) | 25 (20.6) | 6 (6.5) | ||
| Cancer | 19 (8.9) | 0 (0.0) | 19 (20.6) | ||
| Chronic kidney disease | 1 (0.5) | 1 (0.8) | 0 (0.0) | ||
AICU, adult intensive care unit; Gyn/Obs, gynecology/obstetrics; NICU, neonatal intensive care unit; NAP-AMR, National Action Plan on Antimicrobial Resistance.
Wilcoxon rank-sum (Mann–Whitney) test.
During NAP-AMR: others: neonatology unit (n = 21), AICU (n = 12), NICU (n = 10), and Gyn/Obs (n = 6). After NAP-AMR: others: Gyn/Obs (n = 28), oncology (n = 14), neonatology unit (n = 9), AICU (n = 8), and NICU (n = 2).
Of 2097 patients, 1117 (53.2%) were from lower-tier hospitals and 1141 (54.4%) were enrolled as outpatients. A higher proportion of patients was enrolled as outpatients after NAP-AMR (78.1%, 744 of 953) than during NAP-AMR (58.3%, 667 of 1144). Most patients (68.3%, 1433 of 2097) were enrolled from medical wards/clinics. The rates of admission within 3 months before enrollment in the current study during and after NAP-AMR were 15.0% and 9.1%, respectively. The rates of antibiotic use within 3 months before enrollment in the current study during and after NAP-AMR were 31.9% and 25.3%, respectively. Although, the current antibiotic use on admission or visit was 47.1% during NAP-AMR and 0.3% after NAP-AMR, with penicillins (57.2%) and cephalosporins (23.9%) predominating during NAP-AMR (Table 1).
Culture-positive UTIs in patients with the clinical diagnosis of UTIs
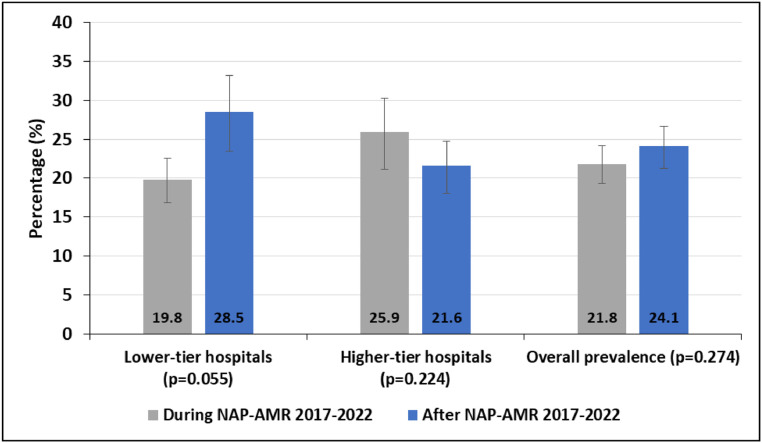
Of 2097 urine samples examined, 479 had significant microbial growth on culture, resulting in a cumulative prevalence of culture-positive UTIs of 22.8% (95% CI: 21.1-24.7%). The prevalence of UTIs increased slightly but not significantly, from 21.8% (249 of 1144; [95% CI: 19.5-24.3%]) during NAP-AMR to 24.1% (230 of 953; [95% CI: 21.5-26.9%]) after NAP-AMR (P = 0.274).
The decrease of UTIs in higher-tier hospitals from 25.9% (97 of 375; [95% CI: 21.5-30.6%]) during NAP-AMR to 21.6% (131 of 605; [95% CI: 18.4-25.2%]) after NAP-AMR (P = 0.224) and the increase of UTIs in lower-tier hospitals from 19.8% (152 of 769; [95% CI: 17.0-22.8%]) during NAP-AMR to 28.5% (99 of 348; [95% CI: 23.8-33.5%]) after NAP-AMR (P = 0.055) were not significant (Figure 1).
Figure 1.
Prevalence of laboratory-confirmed urinary tract infections in patients presenting with clinical symptoms of urinary tract infections. Error bars showing 95% confidence intervals.
NAP-AMR = national action plan on antimicrobial resistance.
Bacterial species causing UTIs
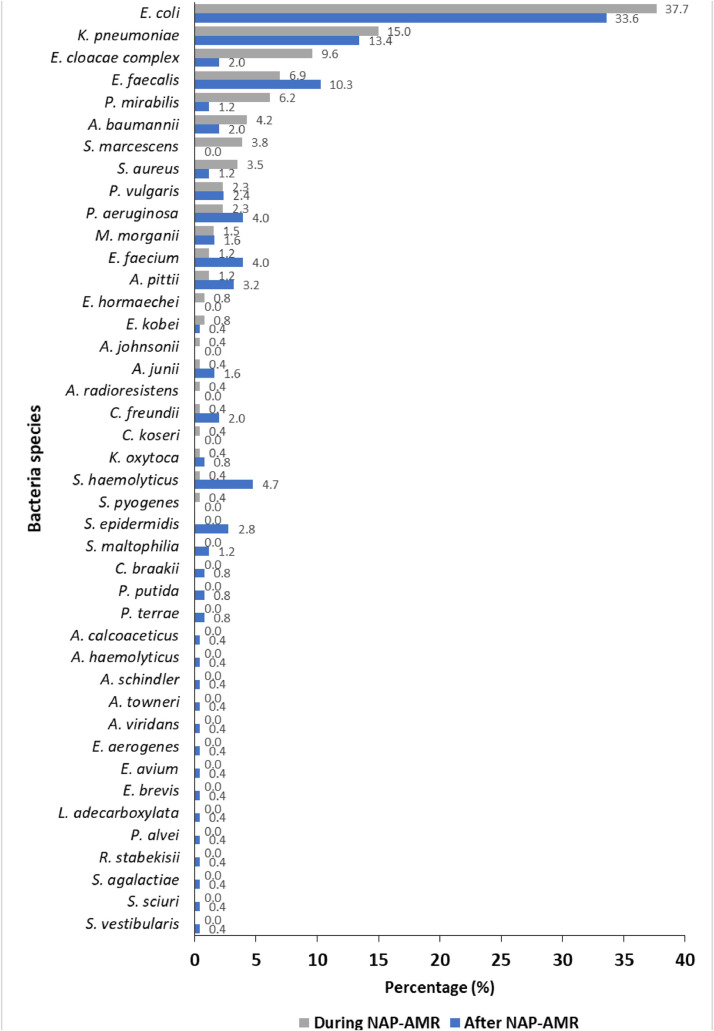
Of 479 positive urine culture, a total of 513 isolates were identified: 260 during NAP-AMR and 253 after NAP-AMR. Up to two different species were detected in one positive sample. Gram-negative bacteria (GNB) were most prevalent causative agents of UTIs in both periods; however, the proportion decreased significantly from 88.1% (229 of 260) during NAP-AMR to 74.3% (188 of 253) after NAP-AMR (P <0.0001). The species causing >50% of the cases during NAP-AMR were E. coli (37.7%, 98 of 260), K. pneumoniae (15.0%, 39 of 260), E. cloacae complex (9.6%, 25 of 260), E. faecalis (6.9%, 18 of 260), and P. mirabilis (6.1%, 16 of 260), whereas the species causing >50% of the cases after NAP-AMR were E. coli (33.6%, 85 of 253), K. pneumoniae (13.4%, 34 of 253), E. faecalis (10.3%, 26 of 253), S. haemolyticus (4.7%, 12 of 253), and E. faecium and P. aeruginosa (4.0%, 10 of 253 each). Further species identified during and after NAP-AMR are listed in Figure 2.
Figure 2.
Bacteria species causing urinary tract infections during and after NAP-AMR.
NAP-AMR = national action plan on antimicrobial resistance.
AMR trends of GNB causing UTIs
In the comparison of AMR during and after NAP-AMR, we observed an increased percentage resistance of GNB toward the antibiotic agents tested. A significant increase was observed toward cefpodoxime (42.2-57.4%, P = 0.0271), ceftazidime (23.2-41.3%, P = 0.0249), and ciprofloxacin (32.0-45.8%, P = 0.0481) (Table 2).
Table 2.
Antimicrobial resistance of gram-negative bacteria causing urinary tract infections during and after national action plan on antimicrobial resistance.
| Antibiotic agent tested | % Resistance in lower-tier hospitals |
% Resistance in higher-tier hospitals |
Overall % resistance |
||||||
|---|---|---|---|---|---|---|---|---|---|
| During (N = 55-133) | After (N = 35–77) | P-value | During (N = 43-96) | After (N = 50-111) | P-value | During (N = 98-229) | After (N = 85-188) | P-value | |
| %R [95% CI] | %R [95% CI] | %R [95% CI] | %R [95% CI] | %R [95% CI] | %R [95% CI] | ||||
| AMP | 83.2 [74.7-89.7] | 84.5 [72.5-92.6] | 0.4217 | 94.5 [86.5-98.5] | 92.5 [85.1-96.9] | 0.3092 | 87.8 [82.1-92.2] | 89.4 [83.4-93.8] | 0.3341 |
| AMC | 65.0 [48.3-79.4] | 72.4 [52.8-87.3] | 0.3087 | 79.4 [62.1-91.3] | 89.6 [77.3-96.5] | 0.1179 | 71.6 [59.9-81.5] | 83.1 [72.8-90.7] | 0.0678 |
| SXT | 61.9 [52.3-70.9] | 64.5 [51.3-76.3] | 0.3930 | 82.7 [72.7-90.2] | 78.1 [68.5-85.9] | 0.2458 | 70.6 [63.7-76.9] | 72.8 [65.1-79.5] | 0.3498 |
| CPD | 33.6 [24.8-43.4] | 46.4 [32.9-60.3] | 0.1539 | 54.8 [42.7-66.5] | 64.1 [53.5-73.9] | 0.1766 | 42.2 [34.9-49.8] | 57.4 [49.1-65.5] | 0.0271 |
| CTX | 25.2 [17.3-34.5] | 37.9 [22.5-51.6] | 0.1693 | 50.0 [37.9-62.0] | 55.8 [45.2-65.9] | 0.2952 | 35.2 [28.2-42.7] | 49.0 [40.8-57.2] | 0.0513 |
| CAZ | 17.6 [10.9-26.1] | 29.5 [18.5-42.6] | 0.1964 | 31.2 [21.1-42.7] | 48.1 [38.3-58.0] | 0.0838 | 23.2 [17.4-30.0] | 41.3 [33.8-49.2] | 0.0249 |
| GEN | 24.6 [16.9-33.5] | 16.1 [8.0-27.6] | 0.2899 | 46.5 [35.7-57.6] | 46.7 [36.9-56.7] | 0.4925 | 34.0 [27.5-41.0] | 35.3 [28.1-43.1] | 0.4390 |
| CIP | 22.8 [15.5-31.6] | 36.1 [24.2-49.4] | 0.1556 | 44.2 [33.5-55.3] | 51.4 [41.5-61.3] | 0.2481 | 32.0 [25.6-38.9] | 45.8 [38.0-53.7] | 0.0481 |
| TZP | 21.6 [14.4-30.4] | 25.0 [15.5-36.6] | 0.3979 | 44.0 [33.2-55.3] | 32.7 [23.9-42.4] | 0.1624 | 31.3 [24.8-38.3] | 29.6 [23.0-36.9] | 0.4221 |
| TGC | 7.5 [2.1-18.2] | 13.2 [4.4-28.1] | 0.3916 | 16.3 [7.3-29.6] | 9.1 [3.0-19.9] | 0.3560 | 11.7 [6.2-19.6] | 10.7 [5.3-18.9] | 0.4705 |
| NIT | 2.5 [0.1-13.2] | 0.0 [0.0-11.9] | - | 0.0 [0.0-10.3] | 2.1 [0.0-11.1] | - | 1.4 [0.0-7.3] | 1.3 [0.0-7.0] | - |
| IPM | 2.6 [0.5-7.5] | 0.0 [0.0-5.7] | - | 4.7 [1.3-11.5] | 10.5 [5.3-17.9] | 0.3639 | 3.5 [1.4-7.1] | 6.6 [3.3-11.5] | 0.3883 |
| MEM | 0.0 [0.0-3.2] | 0.0 [0.0-5.7] | - | 3.5 [0.7-11.5] | 9.5 [4.7-16.8] | 0.3693 | 1.5 [0.3-4.3] | 6.0 [2.9-10.7] | 0.3765 |
AMC, amoxicillin-clavulanic acid; AMP, ampicillin; CAZ, ceftazidime; CIP, ciprofloxacin; CPD, cefpodoxime; CTX, cefotaxime; GEN, gentamicin; IPM, imipenem; MEM, meropenem; NIT, nitrofurantoin; SXT, trimethoprim-sulfamethoxazole; TGC, tigecycline; TZP, piperacillin-tazobactam.
AMR trends of GPB causing UTIs
Overall, the percentage of resistance of GPB increased to some antibiotics (trimethoprim-sulfamethoxazole, erythromycin, tetracycline, levofloxacin, and fosfomycin) and decreased to others (penicillin and gentamicin). However, the differences were not significant. Interestingly, we observed no resistance of GPB toward linezolid, daptomycin, mupirocin, rifampicin, and vancomycin in both periods (Supplementary Table I). Due to the small numbers of GPB, the comparison between health care facilities was not reasonable.
The trend of extended-spectrum β-lactamase–producing Enterobacterales causing UTIs
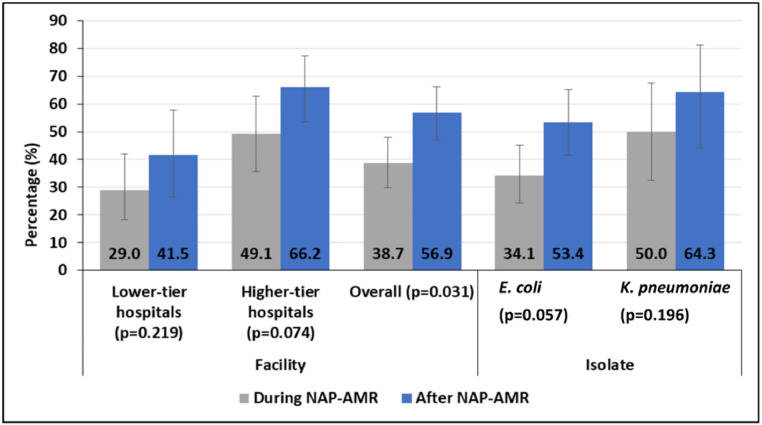
A total of 228 Enterobacterales isolates, consisting of E. coli (n = 162) and K. pneumoniae (n = 66), were tested for ESBL production. Of these, 108 (47.4%; 95% CI: 40.7-54.1%) were identified as ESBL-PE. The proportion of ESBL-PE increased significantly from 38.7% (46 of 119 [95% CI: 29.9-48.0%]) during NAP-AMR to 56.9% (62 of 109 [95% CI: 47.1-66.3%]) after NAP-AMR (P = 0.0307) (Figure 3).
Figure 3.
Proportions of ESBL-PE among E. coli and K. pneumoniae causing urinary tract infections.
NAP-AMR, national action plan on antimicrobial resistance.
Factors associated with culture-positive UTIs caused by ESBL-PE
The univariate logistic regression analysis revealed that UTIs caused by ESBL-PE were significantly common among inpatients (57.1% vs 42.4%; odds ratio [OR] [95% CI]: 1.81 [1.04-3.15], P = 0.036), patients in higher-tier hospitals (58.4% vs 34.0%; OR [95% CI]: 2.73 [1.58-4.68], P <0.0001), and patients enrolled after NAP-AMR (56.1% vs 38.7%; OR [95% CI]: 2.09 [1.23-3.55], P = 0.006). Conversely, current antibiotic use during sample collection was found to be a protective factor against UTIs caused by ESBL-PE (33.3% vs 51.7%; OR [95% CI]: 0.47 [0.25-0.88], P = 0.019). However, the multivariate logistic regression analysis showed that UTIs caused by ESBL-PE remained significantly common in patients in higher-tier hospitals (58.4% vs 34.0%; OR [95% CI]: 2.51 [1.41-4.48], P = 0.002) (Supplementary Table 2).
Discussion
The current study aimed to evaluate the epidemiology and antimicrobial resistance trends of pathogens causing UTIs during and after the implementation of the NAP-AMR 2017-2022 in Mwanza, Tanzania. The cumulative prevalence of culture-positive UTIs in both periods was 22.8%, which is in line with previous studies among HIV-positive pregnant women (21.4%) in 2017 [15], pregnant women (28.0%) in 2019 [16], and outpatients (26.5%) in 2022 [6] in the same region. Our observations indicate that UTIs are still a health care concern in our region. Although there was a slight decrease in UTIs in high-tier hospitals, there was also slight increase in UTIs in the lower-tier hospitals when comparing the two periods. The changing trends of UTIs in higher-tier and lower-tier hospitals may be attributed to various factors such as variations in patient demographics, such as patients’ age (23 [5-40] vs 29 [8-44] years, P <0.0001), clinical characteristics, and health care practices [17,18]. Patients in the higher-tier hospitals are more likely to be exposed to antibiotics in the cascade of referral systems and hence, have fewer positive urine cultures but more resistant isolates.
It is worth noting that the proportion of patients using antibiotics at the time of enrollment decreased from 47.1% during NAP-AMR to 0.3% after NAP-AMR (P = 0.0526). This observation could be attributed to the fact that the second half of the first cohort was done amid COVID-19 pandemic, which may have resulted in an increase in antibiotic use in health care facilities and the community for prevention and treatment of symptoms related to COVID-19. This differs from the second cohort, which took place after the pandemic and most of its participants were enrolled as outpatients, unlike those enrolled during NAP-AMR. After the pandemic, the high demand for antibiotic use (due to COVID-19) in the general community was absent. In addition, it is well noted that inpatients are more likely to be exposed to antibiotics than outpatients. However, almost the same proportion of participants enrolled during and after NAP-AMR had previously used antibiotics within 3 months before enrolling in the current study.
GNB remained the predominant causative agents of UTIs in both periods. However, their proportion decreased significantly (from 88.1% to 74.3%, P <0.0001). This shift may be attributed by increased antibiotics use during NAP-AMR from COVID-19 pandemic, which may have suppressed wild-type GNB, resulting in the emergence of resistant GPB. For instance, we observed an increased proportions of E. faecalis and E. faecium, which are previously reported to be intrinsically resistant toward multiple antibiotics [19]. Similar to previous studies in the same settings [6,15,16] and elsewhere [20], E. coli remained the most prevalent agent of UTIs, highlighting its persistence as a major pathogen, followed by K. pneumoniae [15,16]. The predominance of these two pathogens is not surprising because these are gut microbiota which can easily contaminate perineum and genital regions, resulting into ascending infections in the urinary tract mostly among women.
The detection of rare bacteria species such as Citrobacter braakii, Pseudomonas putida, Proteus terrae, Acinetobacter haemolyticus, Acinetobacter schindleri, Enterococcus avium, Empedobacter brevis, Leclercia adecarboxylata, Paenibacillus alvei, and Streptococcus vestibularis is largely linked to the matrix-assisted laser desorption ionization-time of flight Vitek MS advanced identification technology used in this study. These species were isolated exclusively after NAP-AMR, suggesting that these bacteria are more resilient to the changes in antibiotic usage patterns or have become more pathogenic due to the removal of competitive strains during the NAP-AMR period. In addition, these rare bacterial species have been reported as emerging human pathogens [21], harboring virulence genes [13] and intrinsic antibiotic resistance [22], and are associated with multidrug-resistance (MDR) and extensive drug-resistance infections [23,24]. For instance, the role of CoNS in UTIs, along with their virulence genes and AMR genes, has been previously documented in the same context [13]. In addition, P. alvei was reported to cause UTI [25], A. calcoaceticus was reported to cause bloodstream infection [24], P. putida was reported to cause UTI and bloodstream infection [23], L. adecarboxylata was reported to cause UTI and cellulitis [21], and R. stabekisii was reported to cause dental root canal infection [26].
AST showed that there has been a significant increase in antibiotic resistance toward cefpodoxime, ceftazidime, and ciprofloxacin among GNB. Similar findings were reported elsewhere, particularly, after COVID-19, associated with increased antibiotic use [27]. Remarkably, nitrofurantoin has demonstrated its continued efficacy as an antibiotic for treating uncomplicated UTIs in lower- and higher-tier hospital settings, as recommended by the Tanzanian standard treatment guidelines [9]. The most notable finding in our study is the significant increase in the proportion of ESBL-PE (38.7% vs 56.9%, P = 0.0307). This observation may imply the impact of increased use of antibiotics during the COVID-19 pandemic [8]. The increase in ESBL-PE underscores the urgency of addressing the AMR crisis and the need for comprehensive AMS programs in our setting. Similar to a previous UTIs study in the cascade of referral health care systems [28], the resistance trend in higher-tier hospitals is more critical than in lower-tier hospitals, suggesting that lower-tier hospitals use less antibiotics and, therefore, have less resistance or have a different profile of UTI-causing GNB. These findings suggest that higher-tier hospitals face more severe antibiotic resistance challenges, attributed by patient load, more complex and severe cases, and excessive use of broad-spectrum antibiotics, which exert greater selective pressure [29].
On the other hand, GPB increased high resistance toward trimethoprim-sulfamethoxazole, erythromycin, tetracycline, levofloxacin, and fosfomycin. Interestingly, we observed no resistance of GPB toward linezolid, daptomycin, mupirocin, rifampicin, and vancomycin in both periods, which is an appealing finding because these are last-resort antibiotics for GPB. Similar findings were reported previously [15,16]. However, it is essential to remain vigilant in monitoring the resistance patterns for these antibiotics because misuse or overuse can lead to emerging resistance in the future [29].
Moreover, we observed that UTIs caused by ESBL-PE were significantly common among inpatients, patients in higher-tier hospitals, and patients enrolled after NAP-AMR. Inpatients are more likely to be infected by MDR bacteria due to high exposure to antibiotics and reservoirs (viz. hospital environment) of MDR bacteria. Frequent exposure to antibiotics enhances selective pressure on the normal microbiota, leading to the proliferation of MDR bacteria, resulting in subsequent endogenous MDR infections [29]. In addition, higher-tier hospitals are linked with infections by MDR bacteria because they are often dealing with complex cases and use a broad range of antibiotics, which may contribute to the increased prevalence of ESBL-PE-associated UTIs. This finding emphasizes the urgent need for stringent AMS programs and infection control measures within higher-tier hospitals to curb the rise of resistant strains. Furthermore, the increased proportion of UTIs caused by ESBL-PE after NAP-AMR can be linked to several factors associated with the COVID-19 pandemic in 2020 and 2021: a significant rise in the use of antibiotics, disinfectants, and antiseptics in the community and health care settings; compromised AMS programs and infection prevention and control measures; and changes in microbial ecology due to the extensive and prolonged use of broad-spectrum antibiotics [8,30].
Due to budget constraints, the data collection for the second cohort was shorter than the first cohort. However, we addressed issues raised and observed during the first cohort. Therefore, in the second cohort, we made significant improvements by disseminating the research findings of the first cohort, sensitizing doctors to utilize microbiology laboratories, and re-training staff to improve case screening and laboratory diagnosis. This was particularly crucial in district hospitals (lower-tier hospitals) where culture and AST were initially lacking. As a result, the total number of study participants in the second cohort was relatively similar to the first cohort, despite being conducted in a relatively shorter period.
Study limitations
The limited number of GPB in this study has limited plausible extrapolation of the resistant trends in this group of pathogens, underscoring a need for long-term AMR surveillance targeting GPB.
Conclusion
The implementation of the NAP-AMR (2017-2022) encountered significant challenges exacerbated by heightened antibiotic use during the COVID-19 pandemic, particularly, in 2020 and 2021. This increase in antibiotic consumption raised concerns about the acceleration of AMR, undermining the efforts of the NAP-AMR in combating this pressing public health concern. Our study shows increasing AMR from lower- to higher-tier hospitals and after the implementation of NAP-AMR, notably, in third-generation cephalosporins and ciprofloxacin. However, nitrofurantoin has maintained its effectiveness in treating uncomplicated UTIs in lower- and higher-tier hospital settings. Furthermore, the lower resistance to last-resort antibiotics in higher-tier hospitals is promising but necessitates strengthening AMS programs to prolong their efficacy. In addition, future endeavors should focus on characterizing predominant UTI pathogens to better understand intra- and inter-hospital transmission dynamics, thereby guiding effective infection prevention and control measures.
Declarations of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
The Antimicrobial Resistance Cross-Council Initiative provided funding for SNAP-AMR during NAP-AMR via a grant from the Medical Research Council, a Council of UK Research and Innovation, as well as the National Institute for Health Research (Grant Number: MRC/AMR/MR/S004815/1). Subsequent data collection after NAP-AMR was funded by the Catholic University of Health and Allied Sciences (Mwanza, Tanzania). The work of VS was funded by the Else Kröner-Fresenius Foundation in the framework of the Else Kröner Center Würzburg-Mwanza.
Ethical approval
The first cohort of this study was cleared by the joint Catholic University of Health and Allied Sciences and Bugando Medical Centre Research Ethics and Review Committee (CREC) with certificate number CREC/318/2018 and the National Health Research Ethics Review Committee, with certificate number NIMR/HQ/R.8a/Vol.IX/3017. The second cohort of the study was also ethically cleared by CREC, with a certificate number CREC/654/2023, and the National Health Research Ethics Review Committee, with a certificate number NIMR/HQ/R.8a/Vol.IX/4613.
Acknowledgments
The authors would like to acknowledge all health care workers who provided technical or intellectual support and all patients who participated in this study.
Authors contributions
The conception and design of the study: VS, KO, LM, SEM, HC, and JS; acquisition of data: VS; analysis and interpretation of data: VS and JS; drafting the article: VS; revising the article critically for important intellectual content: VS, KO, LM, SEM, HC, and JS; and final approval of the version to be submitted: VS, KO, LM, SEM, HC, and JS.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2024.107208.
Appendix. Supplementary materials
References
- 1.Salam MA, Al-Amin MY, Salam MT, Pawar JS, Akhter N, Rabaan AA, et al. Antimicrobial resistance: a growing serious threat for global public health. Healthcare. 2023;11:1946. doi: 10.3390/healthcare11131946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva, Switzerland: 2016. Global action plan on antimicrobial resistance. [Google Scholar]
- 3.URT . URT; Dar es Salaam: 2017. The national action plan on antimicrobial resistance 2017 - 2022. [Google Scholar]
- 4.Zeng Z, Zhan J, Zhang K, Chen H, Cheng S. Global, regional, and national burden of urinary tract infections from 1990 to 2019: an analysis of the global burden of disease study 2019. World J Urol. 2022;40:755–763. doi: 10.1007/s00345-021-03913-0. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; Geneva: 2017. WHO publishes list of bacteria for which new antibiotics are urgently needed. [Google Scholar]
- 6.Silago V, Moremi N, Mtebe M, Komba E, Masoud S, Mgaya FX, et al. Multidrug-resistant uropathogens causing community acquired urinary tract infections among patients attending health facilities in Mwanza and Dar es Salaam, Tanzania. Antibiotics (Basel) 2022;11:1718. doi: 10.3390/antibiotics11121718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarimo CS, Wu J. The first confirmed case of COVID-19 in Tanzania: recommendations based on lesson learned from China. Trop Med Health. 2020;48:25. doi: 10.1186/s41182-020-00214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olamijuwon E, Konje E, Kansiime C, Kesby M, Keenan K, Neema S, et al. Antibiotic dispensing practices during COVID-19 and implications for antimicrobial resistance (AMR): parallel mystery client studies in Uganda and Tanzania. Antimicrob Resist Infect Control. 2023;12:10. doi: 10.1186/s13756-022-01199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MoHT . 6th ed. Ministry of Health; Tanzania: 2021. Standard treatment guidelines and national essential medicines list for Tanzania mainland. [Google Scholar]
- 10.Procop GW, Church DL, Hall GS, Janda WM. Jones & Bartlett Publishers Learning; Burlington: 2020. Koneman's color atlas and textbook of diagnostic microbiology. [Google Scholar]
- 11.CLSI. CLSI supplement M100 . 33rd ed. Clinical and Laboratory Standards Institute; Berwyn: 2023. Performance standard for antimicrobial susceptibility testing. [Google Scholar]
- 12.EUCAST . EUCAST; Copenhagen: 2023. The European committee on antimicrobial susceptibility testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 13.0, 2023. [Google Scholar]
- 13.Phillip S, Mushi MF, Decano AG, Seni J, Mmbaga BT, Kumburu H, et al. Molecular characterizations of the coagulase-negative staphylococci species causing urinary tract infection in Tanzania: a laboratory-based cross-sectional study. Pathogens. 2023;12:180. doi: 10.3390/pathogens12020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu T, Li L, Zhao Q, Wang P, Zuo X. Complete genome sequence of bile-isolated Enterococcus avium strain 352. Gut Pathog. 2019;11:16. doi: 10.1186/s13099-019-0294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaula T, Seni J, Ng'walida N, Kajura A, Mirambo MM, DeVinney R, et al. Urinary tract infections among HIV-positive pregnant women in Mwanza city, Tanzania, are high and predicted by low CD4+ Count. Int J Microbiol. 2017;2017 doi: 10.1155/2017/4042686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaduma J, Seni J, Chuma C, Kirita R, Mujuni F, Mushi MF, et al. Urinary tract infections and preeclampsia among pregnant women attending two hospitals in Mwanza City, Tanzania: a 1:2 matched case-control study. BioMed Res Int. 2019;2019 doi: 10.1155/2019/3937812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey JA, Rudolph KE, Robinson SC, Bruxvoort K, Raphael E, Hong V, et al. Sociodemographic inequalities in urinary tract infection in 2 large California health systems. Open Forum Infect Dis. 2021;8:ofab276. doi: 10.1093/ofid/ofab276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansåker F, Li X, Sundquist K. Sociodemographic factors and uncomplicated cystitis in women aged 15–50 years: a nationwide Swedish cohort registry study (1997–2018) Lancet Reg Health Eur. 2021;4 doi: 10.1016/j.lanepe.2021.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaheer R, Cook SR, Barbieri R, Goji N, Cameron A, Petkau A, et al. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Sci Rep. 2020;10:3937. doi: 10.1038/s41598-020-61002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mlugu EM, Mohamedi JA, Sangeda RZ, Mwambete KD. Prevalence of urinary tract infection and antimicrobial resistance patterns of uropathogens with biofilm forming capacity among outpatients in morogoro, Tanzania: a cross-sectional study. BMC Infect Dis. 2023;23:660. doi: 10.1186/s12879-023-08641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keyes J, Johnson EP, Epelman M, Cadilla A, Ali S. Leclercia adecarboxylata: an emerging pathogen among pediatric infections. Cureus. 2020;12:e8049. doi: 10.7759/cureus.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhoads DD. Stenotrophomonas maltophilia susceptibility testing challenges and strategies. J Clin Microbiol. 2021;59:e0109421. doi: 10.1128/JCM.01094-21. p. 10.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan G, Xi Y, Yuan P, Sun Z, Yang D. Risk factors and antimicrobial resistance profiles of Pseudomonas putida infection in Central China, 2010–2017. Medicine. 2019;98:e17812. doi: 10.1097/MD.0000000000017812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancilla-Rojano J, Ochoa SA, Reyes-Grajeda JP, Flores V, Medina-Contreras O, Espinosa-Mazariego K, et al. Molecular epidemiology of Acinetobacter calcoaceticus-Acinetobacter baumannii complex isolated from children at the Hospital Infantil de México Federico Gómez. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.576673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padhi S, Dash M, Sahu R, Panda P. Urinary tract infection due to Paenibacillus alvei in a chronic kidney disease: a rare case report. J Lab Physicians. 2013;5:133–135. doi: 10.4103/0974-2727.119872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson AC, Hellwig E, Vespermann R, Wittmer A, Schmid M, Karygianni L, et al. Comprehensive analysis of secondary dental root canal infections: a combination of culture and culture-independent approaches reveals new insights. PLoS One. 2012;7:e49576. doi: 10.1371/journal.pone.0049576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaspar GG, Ferreira LR, Feliciano CS, Campos CP, Júnior, Molina FMR, Vendruscolo ACS, et al. Pre-and post-COVID-19 evaluation of antimicrobial susceptibility for healthcare-associated infections in the intensive care unit of a tertiary hospital. Rev Soc Bras Med Trop. 2021;54:e0090–e2021. doi: 10.1590/0037-8682-0090-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seni J, Tito JN, Makoye SJ, Mbena H, Alfred HS, van der Meer F, et al. Multicentre evaluation of significant bacteriuria among pregnant women in the cascade of referral healthcare system in North-Western Tanzania: bacterial pathogens, antimicrobial resistance profiles and predictors. J Glob Antimicrob Resist. 2019;17:173–179. doi: 10.1016/j.jgar.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 29.Mittal AK, Bhardwaj R, Mishra P, Rajput SK. Antimicrobials misuse/overuse: adverse effect, mechanism, challenges and strategies to combat resistance. TOBIOTJ. 2020;14:107–112. doi: 10.2174/1874070702014010107. [DOI] [Google Scholar]
- 30.Subramanya SH, Czyż DM, Acharya KP, Humphreys H. The potential impact of the COVID-19 pandemic on antimicrobial resistance and antibiotic stewardship. Virusdisease. 2021;32:330–337. doi: 10.1007/s13337-021-00695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.