Abstract
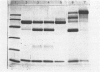
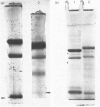
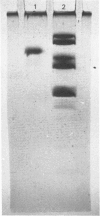
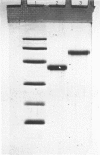
The subunit composition of human myeloperoxidase was studied with the use of sodium dodecyl sulphate/polyacrylamide-gel electrophoresis and gel filtration. The subunit pattern observed depended on the manner in which the enzyme was treated before analysis. Reduction before heat treatment in detergent led to two main protein species (Mr 57 000 and 10 500), whereas reduction during or after heat treatment yielded an additional species of Mr 39 000. Heating without any reductive pretreatment yielded the 39 000-Mr form as the major electrophoretic species. Carbohydrate staining showed large amounts of sugar on the 57 000-Mr species and little on the 10 500-Mr form. Significant amounts of haem were associated with this latter subunit. Haem also seemed to be associated with the 57 000-Mr form but not with the 39 000-Mr one. These three subunit forms were isolated and their amino acid composition analysed. The 57 000-Mr and 39 000-Mr forms had very similar amino acid composition and yielded an apparently identical collection of fragments on incubation with CNBr. Once separated, the subunits could not be interconverted. Generally, minor amounts of other molecular-mass forms were observed. The nature of the various molecular-mass forms originating from myeloperoxidase is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. R., Atkin C. L., Eyre H. J. Intact form of myeloperoxidase from normal human neutrophils. Arch Biochem Biophys. 1982 Mar;214(1):273–283. doi: 10.1016/0003-9861(82)90031-5. [DOI] [PubMed] [Google Scholar]
- Andrews P. C., Krinsky N. I. The reductive cleavage of myeloperoxidase in half, producing enzymically active hemi-myeloperoxidase. J Biol Chem. 1981 May 10;256(9):4211–4218. [PubMed] [Google Scholar]
- Atkin C. L., Andersen M. R., Eyre H. J. Abnormal neutrophil myeloperoxidase from a patient with chronic myelocytic leukemia. Arch Biochem Biophys. 1982 Mar;214(1):284–292. doi: 10.1016/0003-9861(82)90032-7. [DOI] [PubMed] [Google Scholar]
- Bakkenist A. R., Wever R., Vulsma T., Plat H., van Gelder B. F. Isolation procedure and some properties of myeloperoxidase from human leucocytes. Biochim Biophys Acta. 1978 May 11;524(1):45–54. doi: 10.1016/0005-2744(78)90101-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dahlgren C., Stendahl O. Role of myeloperoxidase in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1983 Feb;39(2):736–741. doi: 10.1128/iai.39.2.736-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Harrison J. E., Pabalan S., Schultz J. The subunit structure of crystalline canine myeloperoxidase. Biochim Biophys Acta. 1977 Aug 23;493(2):247–259. doi: 10.1016/0005-2795(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Myeloperoxidase: confirmation and nature of heme-binding inequivalence. Resolution of a carbonyl-substituted heme. Biochim Biophys Acta. 1978 Oct 23;536(2):341–349. doi: 10.1016/0005-2795(78)90492-0. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelhoch S. R., Evans W. H., Mage M. G., Peterson E. A. Purification of myeloperoxidases from the bone marrow of the guinea pig. Biochemistry. 1969 Mar;8(3):914–921. doi: 10.1021/bi00831a022. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Kokriakov V. N., Borisov A. I., Slepenkov S. V., Lyzlova S. N. Sravnitel'noe issledovanie nekotorykh fiziko-khimicheskikh svoistv mieloperoksidaz svin'i i korovy. Biokhimiia. 1982 Jan;47(1):100–107. [PubMed] [Google Scholar]
- Kowit J. D., Maloney J. Protein cleavage by boiling in sodium dodecyl sulfate prior to electrophoresis. Anal Biochem. 1982 Jun;123(1):86–93. doi: 10.1016/0003-2697(82)90627-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Isolation and properties of human neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):325–330. doi: 10.1021/bi00505a015. [DOI] [PubMed] [Google Scholar]
- Nauseef W. M., Root R. K., Malech H. L. Biochemical and immunologic analysis of hereditary myeloperoxidase deficiency. J Clin Invest. 1983 May;71(5):1297–1307. doi: 10.1172/JCI110880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K., Kubota Y., Ooi T. Classification of proteins into groups based on amino acid composition and other characters. I. Angular distribution. J Biochem. 1983 Sep;94(3):981–995. doi: 10.1093/oxfordjournals.jbchem.a134442. [DOI] [PubMed] [Google Scholar]
- Odajima T. Myeloperoxidase of the leukocyte of normal blood. Nature of the prosthetic group of myeloperoxidase. J Biochem. 1980 Feb;87(2):379–391. doi: 10.1093/oxfordjournals.jbchem.a132758. [DOI] [PubMed] [Google Scholar]
- Odajima T., Yamazaki I. Myeloperoxidase of the leukocyte of normal blood. I. Reaction of myeloperoxidase with hydrogen peroxide. Biochim Biophys Acta. 1970 Apr 22;206(1):71–77. doi: 10.1016/0005-2744(70)90083-5. [DOI] [PubMed] [Google Scholar]
- Olsen R. L., Little C. Purification and some properties of myeloperoxidase and eosinophil peroxidase from human blood. Biochem J. 1983 Mar 1;209(3):781–787. doi: 10.1042/bj2090781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Odeberg H. Myeloperoxidase-mediated iodination in granulocytes. Scand J Haematol. 1972;9(5):483–491. doi: 10.1111/j.1600-0609.1972.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Pember S. O., Shapira R., Kinkade J. M., Jr Multiple forms of myeloperoxidase from human neutrophilic granulocytes: evidence for differences in compartmentalization, enzymatic activity, and subunit structure. Arch Biochem Biophys. 1983 Mar;221(2):391–403. doi: 10.1016/0003-9861(83)90158-3. [DOI] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- SCHULTZ J., KAMINKER K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962 Mar;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- SCHULTZ J., SHMUKLER H. W. MYELOPEROXIDASE OF THE LEUCOCYTE OF NORMAL HUMAN BLOOD. II. ISOLATION, SPECTROPHOTOMETRY, AND AMINO ACID ANALYSIS. Biochemistry. 1964 Sep;3:1234–1238. doi: 10.1021/bi00897a009. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Yamada M., Mori M., Sugimura T. Purification and characterization of small molecular weight myeloperoxidase from human promyelocytic leukemia HL-60 cells. Biochemistry. 1981 Feb 17;20(4):766–771. doi: 10.1021/bi00507a018. [DOI] [PubMed] [Google Scholar]