Abstract
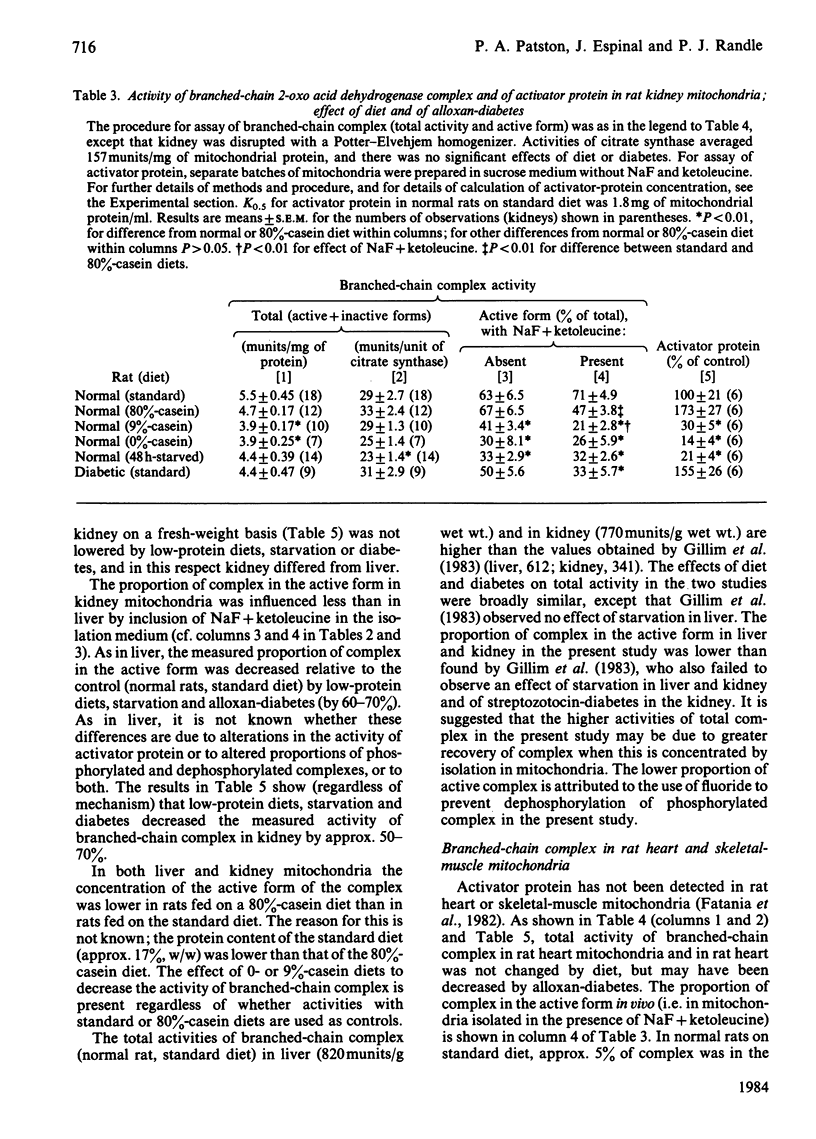
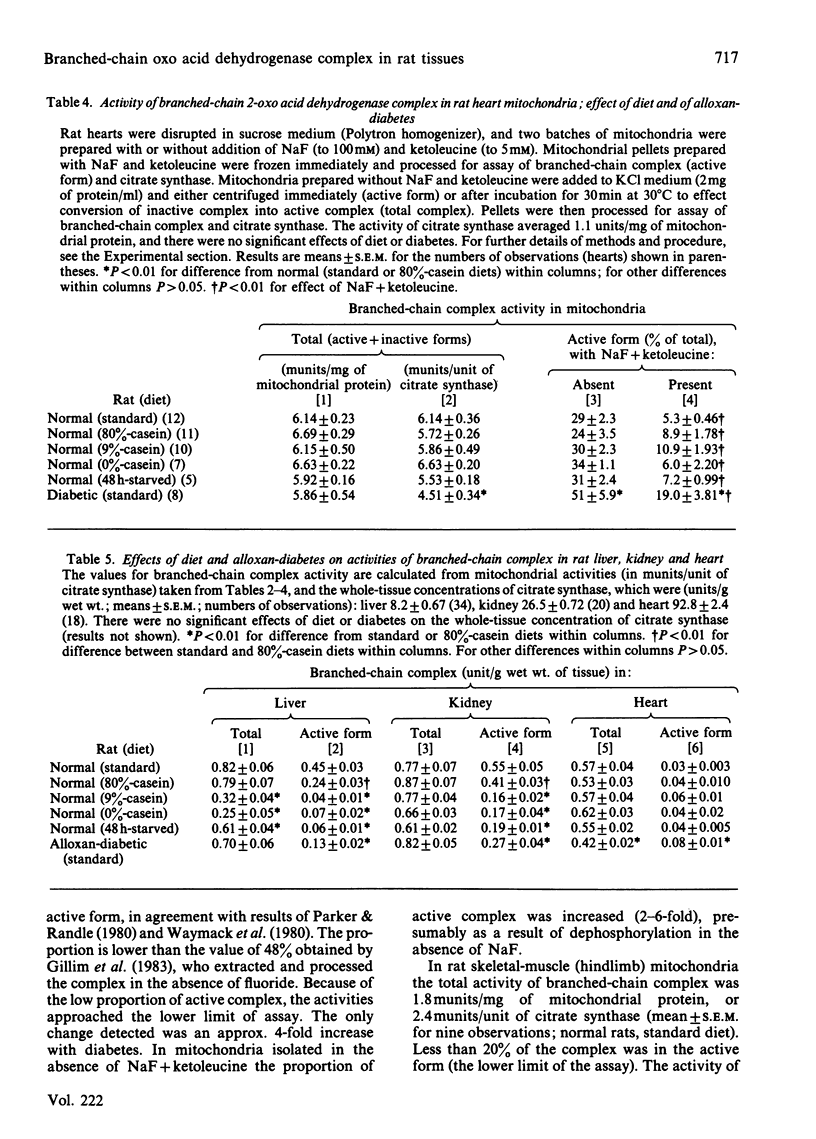
The total activities (sum of active and inactive forms) of branched-chain 2-oxo acid dehydrogenase complex in tissues of normal rats fed on a standard diet were (unit/g wet wt.): liver, 0.82; kidney, 0.77; heart, 0.57; hindlimb skeletal muscles, 0.034. Total activity was decreased in liver by 9%- or 0%-casein diets and by 48 h starvation, but not by alloxan-diabetes. Total activities were unchanged in kidney and heart. The amount of active form of the complex (in unit/g wet wt. and as % of total) in tissues of normal rats fed on standard diet was: liver, 0.45, 55%; kidney, 0.55, 71%; heart, 0.03, 5%; skeletal muscle less than 0.007, less than 20% (below lower limit of assay). The concentration of the active form of the complex was decreased in liver and kidney, but not in heart, by low-protein diets, 48 h starvation and alloxan-diabetes. In heart muscle alloxan-diabetes increased the concentration of active complex. The concentration of activator protein (which activates phosphorylated complex without dephosphorylation) in liver and kidney was decreased by 70-90% by low-protein diets and 48 h starvation. Alloxan-diabetes decreased activator protein in liver, but not in kidney. Evidence is given that in tissues of rats fed on a normal diet approx. 70% of whole-body active branched chain complex is in the liver and that the major change in activity occasioned by low-protein diets is also in the liver.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAPPELL J. B., PERRY S. V. Biochemical and osmotic properties of skeletal muscle mitochondria. Nature. 1954 Jun 5;173(4414):1094–1095. doi: 10.1038/1731094a0. [DOI] [PubMed] [Google Scholar]
- Chuang D. T., Niu W. L., Cox R. P. Activities of branched-chain 2-oxo acid dehydrogenase and its components in skin fibroblasts from normal and classical-maple-syrup-urine-disease subjects. Biochem J. 1981 Oct 15;200(1):59–67. doi: 10.1042/bj2000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. G., Lawson R., Yeaman S. J., Aitken A. Amino acid sequence at the major phosphorylation site on bovine kidney branched-chain 2-oxoacid dehydrogenase complex. FEBS Lett. 1983 Nov 28;164(1):47–50. doi: 10.1016/0014-5793(83)80016-7. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. J., Lemmon S. K., Elsas L. J., 2nd Substrate specificity and stabilization by thiamine pyrophosphate of rat liver branched chain alpha-ketoacid dehydrogenase. Biochem Med. 1978 Feb;19(1):27–38. doi: 10.1016/0006-2944(78)90004-2. [DOI] [PubMed] [Google Scholar]
- Fatania H. R., Lau K. S., Randle P. J. Activation of phosphorylated branched chain 2-oxoacid dehydrogenase complex. FEBS Lett. 1982 Oct 4;147(1):35–39. doi: 10.1016/0014-5793(82)81006-5. [DOI] [PubMed] [Google Scholar]
- Fatania H. R., Lau K. S., Randle P. J. Inactivation of purified ox kidney branched-chain 2-oxoacid dehydrogenase complex by phosphorylation. FEBS Lett. 1981 Sep 28;132(2):285–288. doi: 10.1016/0014-5793(81)81180-5. [DOI] [PubMed] [Google Scholar]
- Fatania H. R., Patston P. A., Randle P. J. Dephosphorylation and reactivation of phosphorylated purified ox-kidney branched-chain dehydrogenase complex by co-purified phosphatase. FEBS Lett. 1983 Jul 25;158(2):234–238. doi: 10.1016/0014-5793(83)80585-7. [DOI] [PubMed] [Google Scholar]
- Fuller S. J., Randle P. J. Reversible phosphorylation of pyruvate dehydrogenase in rat skeletal-muscle mitochondria. Effects of starvation and diabetes. Biochem J. 1984 Apr 15;219(2):635–646. doi: 10.1042/bj2190635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillim S. E., Paxton R., Cook G. A., Harris R. A. Activity state of the branched chain alpha-ketoacid dehydrogenase complex in heart, liver, and kidney of normal, fasted, diabetic, and protein-starved rats. Biochem Biophys Res Commun. 1983 Feb 28;111(1):74–81. doi: 10.1016/s0006-291x(83)80119-3. [DOI] [PubMed] [Google Scholar]
- Harper A. E., Benevenga N. J., Wohlhueter R. M. Effects of ingestion of disproportionate amounts of amino acids. Physiol Rev. 1970 Jul;50(3):428–558. doi: 10.1152/physrev.1970.50.3.428. [DOI] [PubMed] [Google Scholar]
- Heffelfinger S. C., Sewell E. T., Danner D. J. Identification of specific subunits of highly purified bovine liver branched-chain ketoacid dehydrogenase. Biochemistry. 1983 Nov 22;22(24):5519–5522. doi: 10.1021/bi00293a011. [DOI] [PubMed] [Google Scholar]
- Hutson N. J., Randle P. J. Enhanced activity of pyruvate dehydrogenase kinase in rat heart mitochondria in alloxan-diabetes or starvation. FEBS Lett. 1978 Aug 1;92(1):73–76. doi: 10.1016/0014-5793(78)80724-8. [DOI] [PubMed] [Google Scholar]
- Kerbey A. L., Radcliffe P. M., Randle P. J. Diabetes and the control of pyruvate dehydrogenase in rat heart mitochondria by concentration ratios of adenosine triphosphate/adenosine diphosphate, of reduced/oxidized nicotinamide-adenine dinucleotide and of acetyl-coenzyme A/coenzyme A. Biochem J. 1977 Jun 15;164(3):509–519. doi: 10.1042/bj1640509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Cooper R. H., Whitehouse S., Pask H. T., Denton R. M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976 Feb 15;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Lund P. Aspects of the regulation of the metabolism of branched-chain amino acids. Adv Enzyme Regul. 1976;15:375–394. doi: 10.1016/0065-2571(77)90026-7. [DOI] [PubMed] [Google Scholar]
- Lau K. S., Fatania H. R., Randle P. J. Inactivation of rat liver and kidney branched chain 2-oxoacid dehydrogenase complex by adenosine triphosphate. FEBS Lett. 1981 Apr 6;126(1):66–70. doi: 10.1016/0014-5793(81)81034-4. [DOI] [PubMed] [Google Scholar]
- Lau K. S., Fatania H. R., Randle P. J. Regulation of the branched chain 2-oxoacid dehydrogenase kinase reaction. FEBS Lett. 1982 Jul 19;144(1):57–62. doi: 10.1016/0014-5793(82)80568-1. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey G., Lund P. Enzymic determination of branched-chain amino acids and 2-oxoacids in rat tissues. Transfer of 2-oxoacids from skeletal muscle to liver in vivo. Biochem J. 1980 Jun 15;188(3):705–713. doi: 10.1042/bj1880705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odessey R. Direct evidence for the inactivation of branched-chain oxo-acid dehydrogenase by enzyme phosphorylation. FEBS Lett. 1980 Dec 1;121(2):306–308. doi: 10.1016/0014-5793(80)80369-3. [DOI] [PubMed] [Google Scholar]
- Odessey R. Purification of rat kidney branched-chain oxo acid dehydrogenase complex with endogenous kinase activity. Biochem J. 1982 Apr 15;204(1):353–356. doi: 10.1042/bj2040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Active and inactive forms of branched-chain 2-oxoacid dehydrogenase complex in rat heart and skeletal muscle. FEBS Lett. 1980 Apr 7;112(2):186–190. doi: 10.1016/0014-5793(80)80176-1. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Randle P. J. Inactivation of rat heart branched-chain 2-oxoacid dehydrogenase complex by adenosine triphosphate. FEBS Lett. 1978 Nov 1;95(1):153–156. doi: 10.1016/0014-5793(78)80072-6. [DOI] [PubMed] [Google Scholar]
- Paxton R., Harris R. A. Isolation of rabbit liver branched chain alpha-ketoacid dehydrogenase and regulation by phosphorylation. J Biol Chem. 1982 Dec 10;257(23):14433–14439. [PubMed] [Google Scholar]
- Wohlhueter R. M., Harper A. E. Coinduction of rat liver branched chain alpha-keto acid dehydrogenase activities. J Biol Chem. 1970 May 10;245(9):2391–2401. [PubMed] [Google Scholar]


