Abstract
Acute myocardial infarction caused by coronary artery aneurysms typically occurs within 1 to 2 years after Kawasaki disease onset. We report a rare case of sudden death from acute myocardial infarction caused by thrombotic occlusion in a coronary artery aneurysm in a 41-year-old patient diagnosed Kawasaki disease at age 5 years.
Key Words: acute myocardial infarction, coronary artery aneurysm, Kawasaki disease, sudden cardiac death
Graphical Abstract
Kawasaki disease (KD) is an acute vasculitis mainly affecting infants and young children. The most significant complication of KD is the development of coronary artery aneurysms (CAAs), occurring in approximately 25% of untreated cases.1 KD, through the sequelae of CAA, is the leading cause of acquired heart disease in children within developed countries, contributing not only to acute myocardial infarction (AMI) in the acute phase but also increasing the risk of AMI among young adults.2,3 Contrary to the common understanding that AMI associated with KD predominantly occurs within 2 years of the diagnosis,4 we present a rare case of sudden death caused AMI in an adult man caused by thrombotic occlusion of the CAA, more than 3 decades after the diagnosis of KD.
Take-Home Messages
-
•
This case emphasize that AMI in young individuals can result from thrombosis of giant CAA from KD that occurred in childhood.
-
•
Continuous monitoring and awareness of late cardiovascular complications of KD are important for minimizing its long-term cardiovascular risks.
Case Presentation
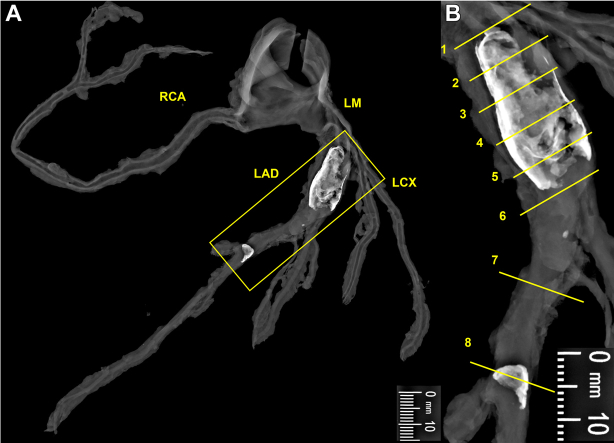
A 41-year-old White male (180 cm, 90 kg), diagnosed with KD at the age of 5 years, presented with a history of hyperlipidemia, smoking, and intermittent chest pain that quickly subsided. The episodes of pain had been ongoing for 1 year. No medications were documented, and the detailed treatment history for KD was unclear. The patient experienced chest pain and dizziness, and during an attempt by his wife to transport him to the emergency department, he exhibited rigidity and seizures and experienced a cardiac arrest. Emergency medical services administered advanced cardiac life support while en route to the hospital, though resuscitation was unsuccessful. At autopsy, the heart weighed 420 g (predicted normal heart weight: 371 g [95% CI: 281-489 g] based on sex and body weight5), with normal ventricular wall thickness, chamber dimensions, and valves. The examination of the epicardial coronary arteries revealed a CAA with calcification, measuring 20 mm in length and 12 mm in maximum diameter (Z score: 17.2), located in the proximal left anterior descending (LAD) artery (Figure 1). The coronary system exhibited a right dominance with a total occlusion of the lumen caused by a thrombus within the CAA in the proximal LAD. Histologic sections showed occlusive acute fibrin-platelet rich thrombus and underlying fibrocalcific plaque without a necrotic core (Figures 2 and 3). The lack of endothelium in the CAA suggested endothelial dysfunction (Figure 4). The rest of the coronary tree showed adaptive intimal thickening except for a single lesion in the middle LAD consisting of a fibrocalcific plaque.
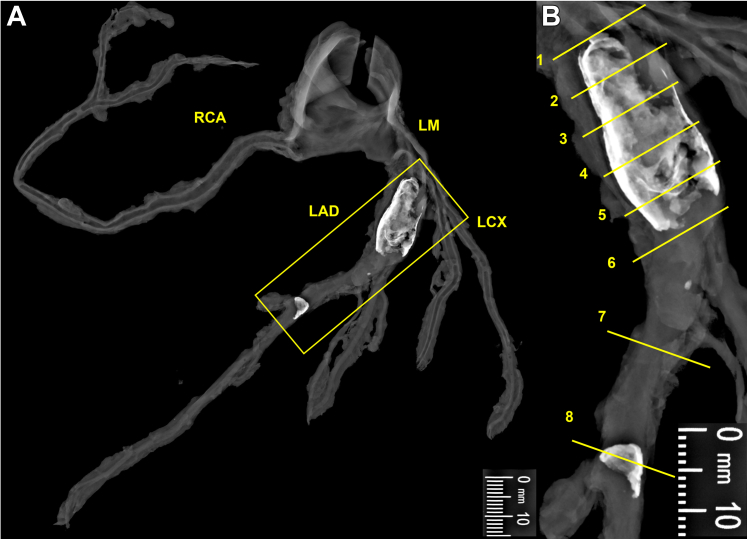
Figure 1.
Radiographic Examination of the Coronary Artery Aneurysm
(A) Faxitron (Hologic, Inc) radiograph of epicardial coronary arteries dissected from the heart demonstrating aneurysmal dilation and calcification of the proximal LAD artery and focal calcification of the middle LAD artery (yellow boxed area). (B) An enlarged view of the yellow boxed area in A, with yellow lines marking the levels at which histologic sections (Figures 2 and 3) were taken. LAD = left anterior descending; LCX = left circumflex artery; LM = left main; RCA = right coronary artery.
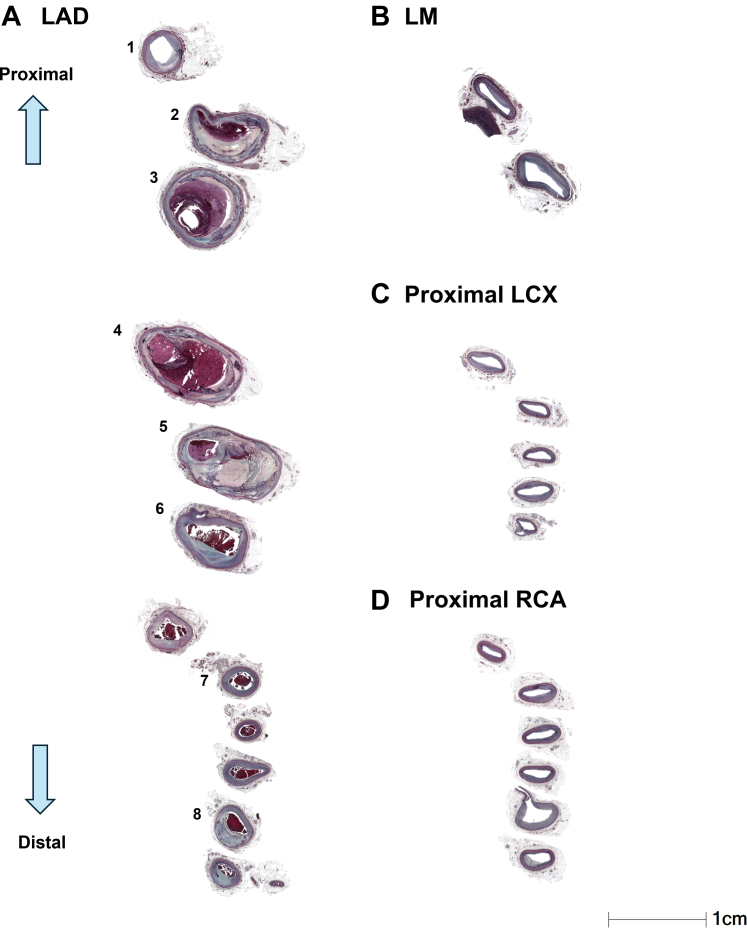
Figure 2.
Comparative Histologic Examination and Vessel Diameter of Coronary Arteries
(A to D) Sections of the coronary arteries are presented at identical magnification. (A) The maximum vessel diameter within the LAD aneurysm (numbers 2-6) was 12 mm × 10 mm (number 4). The numbers on each section align with those marked by yellow lines in Figure 1B, as well as the numbers in Figure 3. (B to D) All sections are stained with Movat pentachrome stain. Abbreviations as in Figure 1.
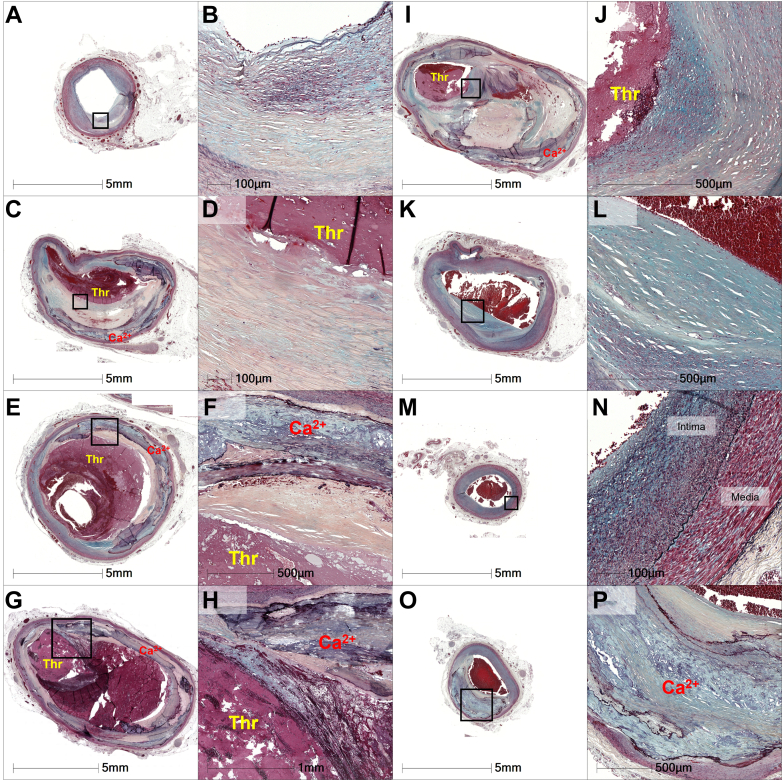
Figure 3.
Histologic Examination of the Proximal LAD Artery
(A) Proximal LAD artery without aneurysm formation. (B) High-power image of the boxed area in A, showing smooth muscle cells enriched with proteoglycans and collagen. (C) Occlusive acute thrombus and underlying fibrocalcific plaque. (D) Boxed area from C, showing the underlying plaque rich in smooth muscle cells embedded in a matrix rich in proteoglycans and collagen. (E) Occlusive acute thrombus and underlying fibrocalcific plaque. (F) Boxed area from E, highlighting the plaque rich in smooth muscle cells within a proteoglycan-collagen–rich matrix, between acute thrombus and sheet calcification. (G, I) Sheet calcification, organizing thrombus, and overlying acute occlusive thrombus. (H, J) High-power image from the boxed area in G and I, respectively, showing acute luminal thrombus and underlying healing plaque rich in proteoglycans, without necrotic core. Sections within the aneurysm show pronounced thinning of the media layer. (K, L) Intima is composed of smooth muscle cells that are rich in proteoglycans and collagen. (M, N) Section without aneurysm, with a vessel maximum diameter of 3.5 mm and media preserved. (O, P) Middle LAD artery with approximately 60% narrowing by fibrocalcific plaque. All sections were stained with Movat pentachrome stain. Ca2+ = calcification; LAD = left anterior descending; Thr = thrombus.
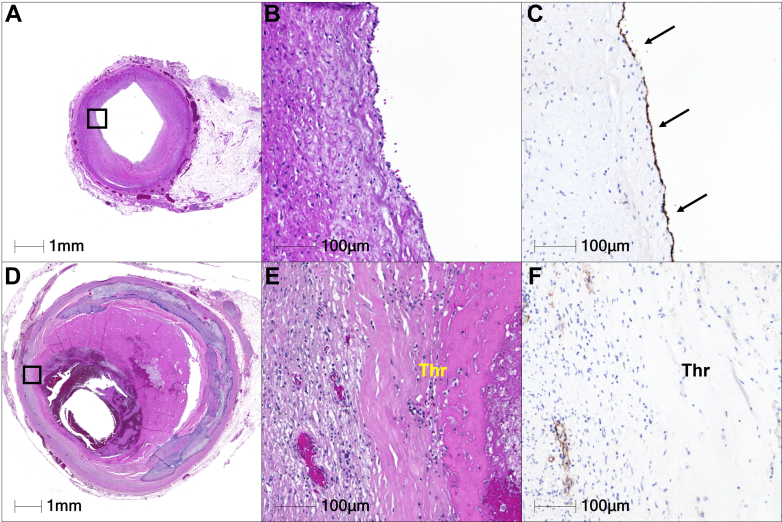
Figure 4.
Immunostaining for Endothelial Marker
Hematoxylin and eosin–stained sections of the proximal LAD artery (A, B) without CAA and (D, E) with CAA. (C, F) The presence of endothelium on the luminal surface is confirmed by CD34 immunostaining. (C) A high-power image of an adjacent section without CAA shows normal endothelialized surface (arrows). (F) Conversely, this image reveals the absence of endothelium on the luminal surface with CAA, highlighting luminal surface in direct contact with acute thrombus. CAA = coronary artery aneurysm; LAD = left anterior descending; Thr = thrombus.
Discussion
Coronary arteritis caused by KD begins approximately a week after onset as infiltration of inflammatory cells in the arterial intima and adventitia. Around the 10th day, the inflammation extends to the entire arterial wall, including media. The main components of the arterial wall, that is, the internal elastic lamina and smooth muscle cells of the media, are damaged by neutrophils and macrophages, leading to arterial dilation starting around day 12.6 Most CAAs persisting beyond day 30 show a tendency to shrink, with regression typically occurring within 2 years of onset. Despite regression or pseudonormalization of CAAs in certain patients, there remains a greater risk for cardiac disease in adulthood.7 Giant CAAs generally do not regress, and if aneurysms persist, the risk of thrombus formation increases because of turbulent blood flow and endothelial dysfunction, resulting in coronary artery thrombotic occlusion, primarily within the early onset of KD.4
Long-term follow-up studies reveal that even many years after KD, the risk of AMI in young adults is significant. Indeed, Daniels et al2 reported that 5% of individuals younger than 40 years old presenting with AMI had CAAs from KD during childhood. In addition, computed tomography studies on long-term follow-up of CAAs show that coronary artery calcification becomes detectable approximately 10 years post-KD.8 A recent pathologic study, however, showed that calcification occurred in 58% of CAAs in KD patients who died during the follow-up period between 40 days and 3 years.9 Calcification may be induced by acute phase inflammation, and it is suggested that the induction of calcification continues for an extended period. For the long-term management of persisting giant CAAs, not only is the control of cardiovascular risk factors important, but medical therapy with antiplatelet agents or anticoagulants is highly recommended.1,10 Additionally, regular assessments for inducible myocardial ischemia and imaging evaluations are also considered essential.1,10 Adult cardiologists need to be familiar with KD and its complications to ensure timely follow-up of patients as they transition to adulthood.
In summary, individuals can remain at risk for occlusive acute thrombosis decades after the onset of KD. Persistent endothelial dysfunction and complex plaque formation with calcification within CAAs may be contributing factors.
Conclusions
We report a rare case of sudden death caused by AMI from thrombotic occlusion within a giant CAA in a 41-year-old man, occurring more than 35 years after KD.
Funding Support and Author Disclosures
Drs Virmani and Finn have received institutional research support from NIH-HL141425, Leducq Foundation Grant, 4C Medical, 4Tech, Abbott Vascular, Ablative Solutions, Absorption Systems, Advanced NanoTherapies, Aerwave Medical, Alivas, Amgen, Asahi Medical, Aurios Medical, Avantec Vascular, BD, Biosensors, Biotronik, Biotyx Medical, Bolt Medical, Boston Scientific, Canon, Cardiac Implants, Cardiawave, CardioMech, Cardionomic, Celonova, Cerus, EndoVascular, Chansu Vascular Technologies, Children’s National, Concept Medical, Cook Medical, Cooper Health, Cormaze, CRL, Croivalve, CSI, Dexcom, Edwards, Elucid Bioimaging, eLum Technologies, Emboline, Endotronix, Envision, Filterlex, Imperative Care, Innovalve, Innovative Cardiovascular Solutions, Intact Vascular, Interface Biologics, Intershunt Technologies, Invatin, Lahav, Limflow, L&J Bio, Lyra Therapeutics, Mayo Clinic, Maywell, MedAlliance, Medanex, Medtronic, Mercator, Microport, Microvention, Neovasc, Nephronyx, Nova Vascular, Nyra Medical, Occultech, Olympus, Ohio Health, OrbusNeich, Ossiso, Phenox, Pi-Cardia, Polares Medical, Polyvascular, Profusa, ProKidney, LLC, Protembis, Pulse Biosciences, Qool Therapeutics, Recombinetics, Recor, Regencor, Renata Medical, Restore Medical, Ripple Therapeutics, Rush University, Sanofi, Shockwave, SMT, SoundPipe, Spartan Micro, Spectrawave, Surmodics, Terumo, The Jacobs Institute, Transmural Systems, Transverse Medical, TruLeaf, UCSF, UPMC, Vascudyne, Vesper, Vetex Medical, Whiteswell, W. L. Gore, and Xeltis. Dr Finn has received honoraria from Abbott Vascular (stents), Biosensors (stents), Boston Scientific (stents), Celonova (stents), Cook Medical (stents), CSI (catheters), Lutonix Bard (balloons), Sinomed (stents), and Terumo Corporation (stents) and has served as a consultant to Amgen, Amgen (Farma), Abbott Vascular (stents), Boston Scientific (stents, valves, and balloons), Celonova (stents), Cook Medical (stents), Lutonix Bard (stents and balloons), and Sinomed (stents). Dr Virmani has received honoraria from Abbott Vascular, Biosensors, Boston Scientific, Celonova, Cook Medical, Cordis, CSI, Lutonix Bard, Medtronic, OrbusNeich Medical, CeloNova, SINO Medical Technology, ReCor, Terumo Corporation, W. L. Gore, and Spectranetics and has served as a consultant for Abbott Vascular, Boston Scientific, Celonova, Cook Medical, CSI, Edwards Lifesciences, Bard BD, Medtronic, OrbusNeich Medical, ReCor Medical, SinoMedical Technology, Surmodics, Terumo Corporation, W. L. Gore, and Xeltis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.McCrindle B.W., Rowley A.H., Newburger J.W., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 2.Daniels L.B., Tjajadi M.S., Walford H.H., et al. Prevalence of Kawasaki disease in young adults with suspected myocardial ischemia. Circulation. 2012;125:2447–2453. doi: 10.1161/CIRCULATIONAHA.111.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato H., Ichinose E., Kawasaki T. Myocardial infarction in Kawasaki disease: clinical analyses in 195 cases. J Pediatr. 1986;108:923–927. doi: 10.1016/s0022-3476(86)80928-3. [DOI] [PubMed] [Google Scholar]
- 4.Tsuda E., Hamaoka K., Suzuki H., et al. A survey of the 3-decade outcome for patients with giant aneurysms caused by Kawasaki disease. Am Heart J. 2014;167:249–258. doi: 10.1016/j.ahj.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Kitzman D.W., Scholz D.G., Hagen P.T., Ilstrup D.M., Edwards W.D. Age-related changes in normal human hearts during the first 10 decades of life. Part II (Maturity): a quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988;63:137–146. doi: 10.1016/s0025-6196(12)64946-5. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K., Oharaseki T., Yokouchi Y. Histopathological aspects of cardiovascular lesions in Kawasaki disease. Int J Rheum Dis. 2018;21:31–35. doi: 10.1111/1756-185X.13207. [DOI] [PubMed] [Google Scholar]
- 7.Iemura M., Ishii M., Sugimura T., Akagi T., Kato H. Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart. 2000;83:307–311. doi: 10.1136/heart.83.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahn A.M., Budoff M.J., Daniels L.B., et al. Calcium scoring in patients with a history of Kawasaki disease. JACC Cardiovasc Imaging. 2012;5:264–272. doi: 10.1016/j.jcmg.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokouchi Y., Oharaseki T., Asakawa N., Makino H., Takahashi K. Histological studies shed new light on the initiation and characteristics of calcification of coronary artery aneurysms in Kawasaki disease. Cardiovasc Pathol. 2022;61 doi: 10.1016/j.carpath.2022.107456. [DOI] [PubMed] [Google Scholar]
- 10.Brogan P., Burns J.C., Cornish J., et al. Lifetime cardiovascular management of patients with previous Kawasaki disease. Heart. 2020;106:411–420. doi: 10.1136/heartjnl-2019-315925. [DOI] [PMC free article] [PubMed] [Google Scholar]