Abstract
A 79-year-old man presented with acute-onset coldness and severe pain in his left foot 4 hours prior. His foot (distal to the left Lisfranc joint) was pale and cold with slight motor and sensory deficits. Angiography demonstrated occlusion of the lateral plantar artery and plantar metatarsal arteries (PMAs). Angioplasty using balloons for each PMA and lateral plantar artery was conducted, but failed to achieve satisfactory blood flow. The foot condition subsequently worsened. A 22-gauge cannula was then inserted into the dorsalis pedis artery, and continuous local intra-arterial infusion of heparin, alprostadil, and nicorandil was administered. A marked reduction in the cyanotic areas of the foot was observed, with improved motor and sensory deficits post–continuous local intra-arterial infusion therapy. Follow-up angiography via the cannula on day 3 of hospitalization demonstrated significant flow improvement in the first to third PMAs. Foot salvage was achieved without tissue necrosis or amputation.
Key Words: continuous intra-arterial infusion therapy, endovascular treatment, limb ischemia, plantar metatarsal artery
Graphical Abstract
History of Presentation
A 79-year-old man who complained of acute-onset coldness and severe pain in his left foot 4 hours prior was transported to our hospital by ambulance.
Learning Objectives
-
•
To recognize acute limb ischemia due to plantar metatarsal artery occlusion, which cannot be treated with surgery or endovascular treatment.
-
•
To understand the effects of continuous intra-arterial infusion therapy for acute limb ischemia when revascularization is challenging.
Past Medical History
The patient had diabetes mellitus and hypertension, and diverticular bleeding, and had undergone surgery for colon cancer 7 years ago but had not received chemotherapy. His daily medications included antihypertensive agents (amlodipine 5 mg/d, valsartan 80 mg/d) and an antidiabetic agent (sitagliptin 50 mg/d).
Investigations
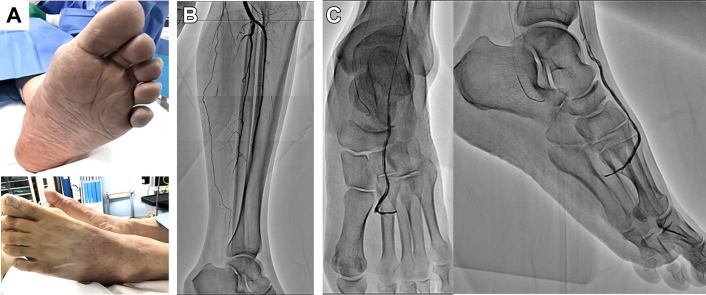
The patient’s left foot (distal to the left Lisfranc joint) was pale and cold, with slight motor and sensory deficits (Figure 1A). Although a posterior tibial pulse was absent, the right femoral, popliteal, and dorsalis pedis pulses were palpable. There was no Doppler signal in the posterior tibial artery (PTA); however, femoral, popliteal, and pedal pulses were detectable. Electrocardiography revealed sinus rhythm, and the left ankle-brachial index was 1.18. Computed tomography angiography revealed PTA and dorsalis pedis artery (DPA) occlusion. Laboratory results were as follows: white blood cell count 8,500/μL, creatine kinase level 79 U/L, C-reactive protein level 0.55 mg/dL, serum creatinine level 0.9 mg/dL, and D-dimer level 1.6 μg/mL. Protein C, protein S, and antithrombin III levels were normal. Antinuclear antibodies, lupus anticoagulant antibodies, anticardiolipin antibodies, antiphospholipid antibodies, anti-beta2 glycoprotein, myeloperoxidase antineutrophil cytoplasmic antibodies, proteinase-3 antineutrophil cytoplasmic antibodies, lipoprotein(a), and complement levels were within normal ranges.
Figure 1.
Initial Assessment of the Patient’s Foot
(A) The patient’s foot demonstrates prominent pallor and coldness. (B) Initial angiography reveals flow limitation in the distal portion of the anterior and posterior tibial artery without significant stenosis. (C) Microcatheter injection introduced to the pedal arch demonstrates occlusion of the lateral plantar artery and plantar metatarsal arteries.
Management
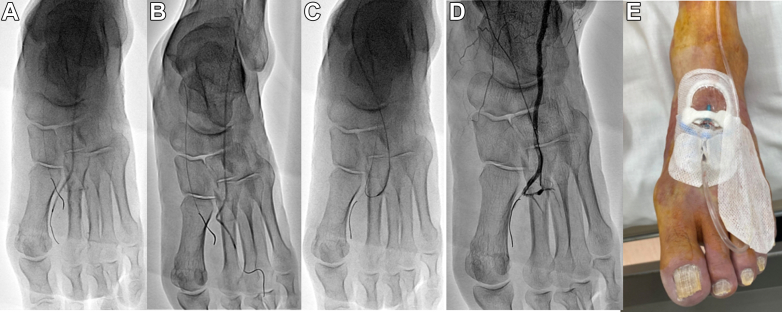
Acute limb ischemia (ALI) (Rutherford class II) was diagnosed, and emergency endovascular treatment (EVT) was performed. Intravenous unfractionated heparin (5,000 U) was administered before EVT. A 4-F sheath was inserted anterogradely through the left common femoral artery. Initial angiography revealed flow limitation in the distal portion of the anterior tibial artery (ATA) and PTA without significant stenosis (Figure 1B). The 4-F sheath was replaced with a 7-F Destination 45-cm sheath (Terumo). A Cruise guidewire (Asahi Intec) was crossed over the ATA, and a Jupiter SFC guidewire (Boston Scientific Japan) was crossed over the PTA. Intravascular ultrasound (OptiCross; Boston Scientific) did not show a thrombus in the distal portion of the ATA and PTA. Aspiration thrombectomy with the 6-F Eliminate SL (Terumo) could not aspirate a thrombus. Although a 2.0- × 220-mm balloon (Coyote, Boston Scientific) angioplasty of the left PTA was performed, satisfactory blood flow to the pedal artery was not achieved. Contrast injection from the Prominent BTA microcatheter (Tokai Medical Products) introduced into the pedal arch demonstrated occlusion of the lateral plantar artery (LPA) and plantar metatarsal arteries (PMAs) (Figure 1C). Alprostadil injection through the microcatheter did not improve blood flow. A Cruise guidewire was advanced to the first and second PMAs, and angioplasty using a 2.0- × 220-mm balloon for each PMA was conducted (Figures 2A and 2B). The Jupiter SFC guidewire was advanced to the DPA and distal ATA with retrograde wire manipulation via the LPA. Although combined angioplasty using a 3.0- × 220-mm balloon (Coyote, Boston Scientific) for the LPA to the PTA and alprostadil injection through the microcatheter were performed (Figure 2C), satisfactory blood flow to the PMAs and LPA could not be achieved, and the distal portion of the PTA was not depicted (Figure 2D). His left foot condition worsened after the procedure, and we decided to perform continuous local intra-arterial infusion (CLAI) therapy. A 22-gauge cannula was inserted into the DPA under ultrasound guidance, and CLAI of heparin (15,000 U/d), alprostadil (80 μg/d), nicorandil (48 mg/d), and acetated Ringer’s solution (80 mL/h) was administered (Figure 2E).
Figure 2.
Intraoperative Findings
(A and B) Angioplasty using a 2.0-mm balloon for the first and second plantar metatarsal arteries was conducted. (C and D) Although combined angioplasty using a 3.0-mm balloon for lateral plantar artery to the posterior tibial artery and alprostadil injection through the microcatheter was performed, satisfactory blood flow to the plantar metatarsal arteries and for lateral plantar artery could not be achieved, and the distal portion of the posterior tibial artery is not depicted. (E) A 22-gauge cannula is inserted in the dorsal pedal artery under ultrasound guidance.
Discussion
ALI presents as a sudden lower limb ischemia that threatens the viability of the limb.1 The rate of amputation during hospitalization and mortality rate within 1 year range from 10% to 15% and 15% to 20%, respectively.2,3 Treatments for ALI include surgical treatment (Fogarty thrombectomy and bypass surgery), EVT (percutaneous aspiration thrombectomy, catheter-directed thrombolysis, thrombus crushing by balloon inflation, and stent deployment), and hybrid EVT.4 Here, a lysis catheter (a multiside holed end-hole occluded infusion catheter or an end-hole catheter) could not be used because the diameter of the PMA was too small, and the lysis catheter may not be positioned within the thrombus of PMA. Fogarty thrombectomy for LPA and DPA is possible but technically challenging and carries a risk of vessel injury. Therefore, it cannot be performed for PMAs. Acute ischemia causes tissue edema, which reduces the vascular bed by increasing pressure and causing loss of sensitivity to vasorelaxing factors, leading to elevated peripheral resistance.5 In this situation, continuous intravenous infusion may not be effective for drug delivery due to elevated peripheral resistance and reduced prostaglandin E1 clearance rates due to intrapulmonary metabolism. Compared with continuous intravenous infusion, CLAI has limited and high-dose drug delivery to the targeted area. When considering CLAI, the catheter could be advanced to the DPA through the sheath inserted into the common femoral artery. However, this carries the risk of foot exercise restriction, vascular injury, and thrombus formation around the catheter compared with the 22-gauge cannula inserted into the DPA. A 22-gauge cannula can be inserted with an ultrasound-guided puncture, which has a lower rate of access site bleeding, and puncture site can be selected. Here, heparin was used as an anticoagulant because alteplase and monteplase could not be used due to insurance restrictions in Japan. Additionally, there was urokinase shortage. Catheter-directed thrombolysis without thrombolytic agents may not be effective and requires bed rest, which may lead to a decline in activities of daily living and a prolonged hospital stay. Although minor amputation should be considered if the cyanotic areas and motor and sensory deficits worsen during CLAI, CLAI is a potential treatment for ALI with below-the-ankle arterial occlusions that are difficult to revascularize.
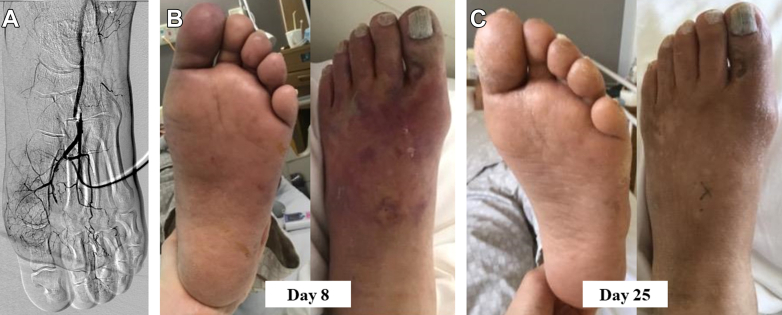
Follow-Up
The patient was immediately prescribed antiplatelet therapy (aspirin, 100 mg/d), and a marked reduction in the cyanotic areas of the foot was observed, with improved motor and sensory deficits post-CLAI therapy. The peak creatine kinase level was 4,210 U/L. Follow-up angiography via the cannula on day 3 of hospitalization demonstrated significant flow improvement in the first to third PMAs and LPA. However, the proximal portion of the LPA was not visible (Figure 3A). CLAI therapy was completed on day 4 of hospitalization, and an anticoagulant (edoxaban 60 mg/d) was prescribed. Contrast-enhanced computed tomography revealed atherosclerotic plaque ulceration in the ascending aorta, suspected to be the source of arterial thrombosis from the ruptured atherosclerotic plaque. Because atrial fibrillation was not detected during hospitalization, we discontinued edoxaban and continued aspirin administration. Foot salvage was achieved without tissue necrosis or amputation (Figures 3B and 3C), and the patient was discharged on postoperative day 26. At 6-month follow-up, the patient was free of thrombotic events.
Figure 3.
Follow-Up
(A) Follow-up angiography via the cannula demonstrates improved flow in the first to third plantar metatarsal arteries and lateral plantar artery; however, the proximal portion of the lateral plantar artery is not depicted. (B and C) Skin color has improved without cyanotic and gangrene progression.
Conclusions
ALI due to PMA occlusion is extremely rare, and no similar reports have been documented in the past. Continuous intravenous infusion of heparin and alprostadil is generally administered. However, it may not be effective in most cases, and early amputation should be considered. CLAI for ALI can provide limited drug delivery (eg, heparin, vasodilators) to the targeted area, making this report novel in its approach. Further research and clinical experience will continue to refine this technique, potentially leading to advancements in treating ALI.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Norgren L., Hiatt W.R., Dormandy J.A., Nehler M.R., Harris K.A., Fowkes F.G. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Eliason J.L., Wainess R.M., Proctor M.C., et al. A national and single institutional experience in the contemporary treatment of acute lower extremity ischemia. Ann Surg. 2003;238:382–389. doi: 10.1097/01.sla.0000086663.49670.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earnshaw J.J., Whitman B., Foy C. National audit of thrombolysis for acute leg ischemia (NATALI): clinical factors associated with early outcome. J Vasc Surg. 2004;39:1018–1025. doi: 10.1016/j.jvs.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Obara H., Matsubara K., Kitagawa Y. Acute limb ischemia. Ann Vasc Dis. 2018;11:443–448. doi: 10.3400/avd.ra.18-00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon F., Oberhuber A., Floros N., et al. Acute limb ischemia-much more than just a lack of oxygen. Int J Mol Sci. 2018;19:374. doi: 10.3390/ijms19020374. [DOI] [PMC free article] [PubMed] [Google Scholar]