Abstract
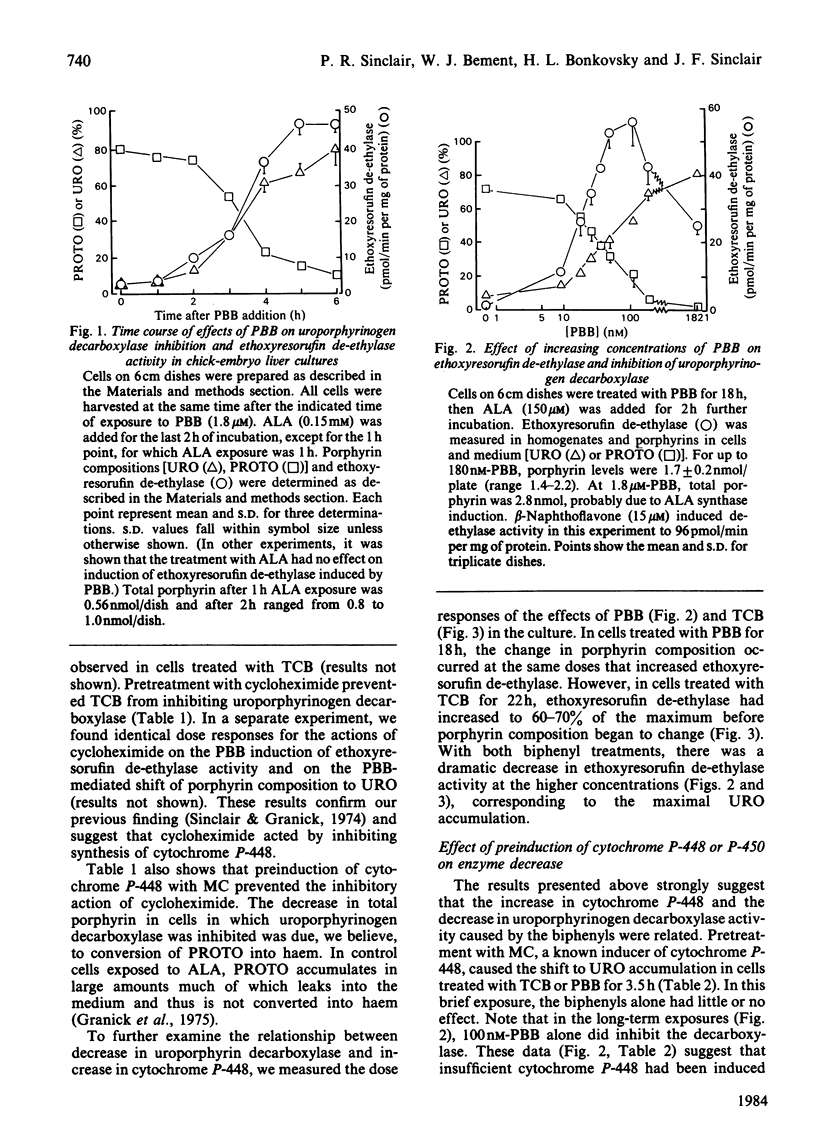
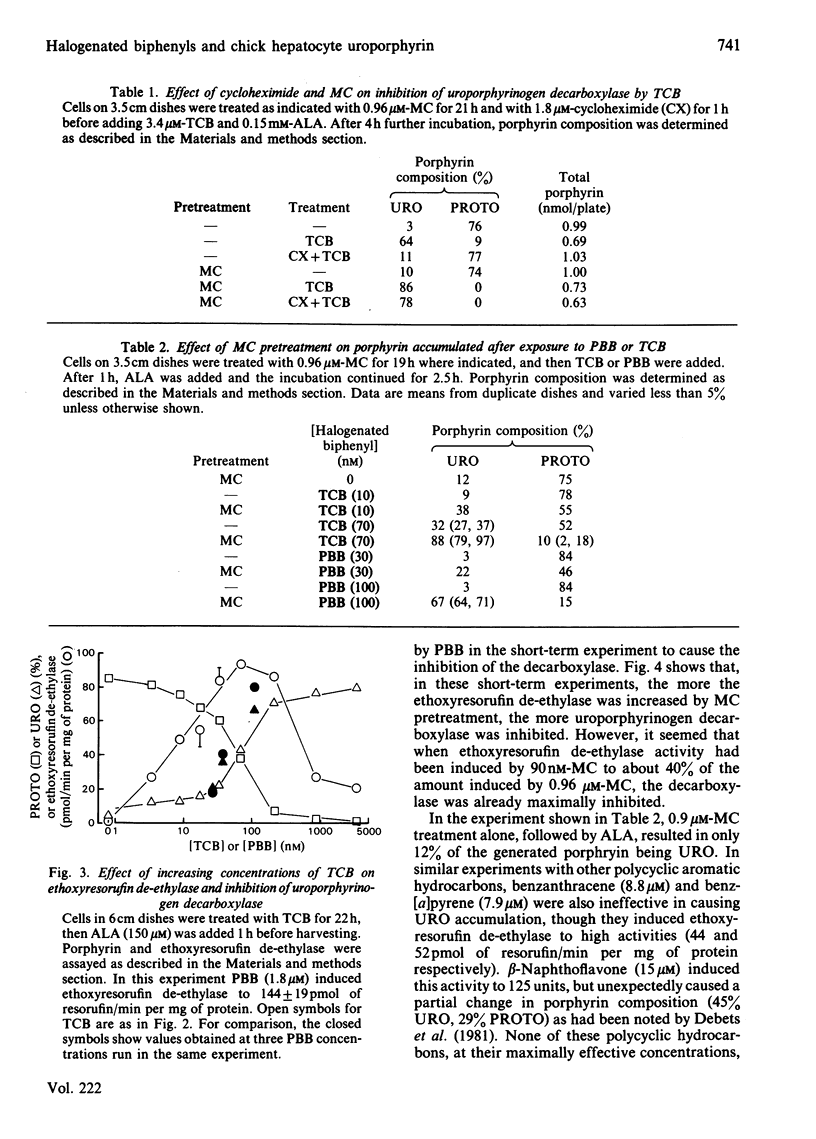
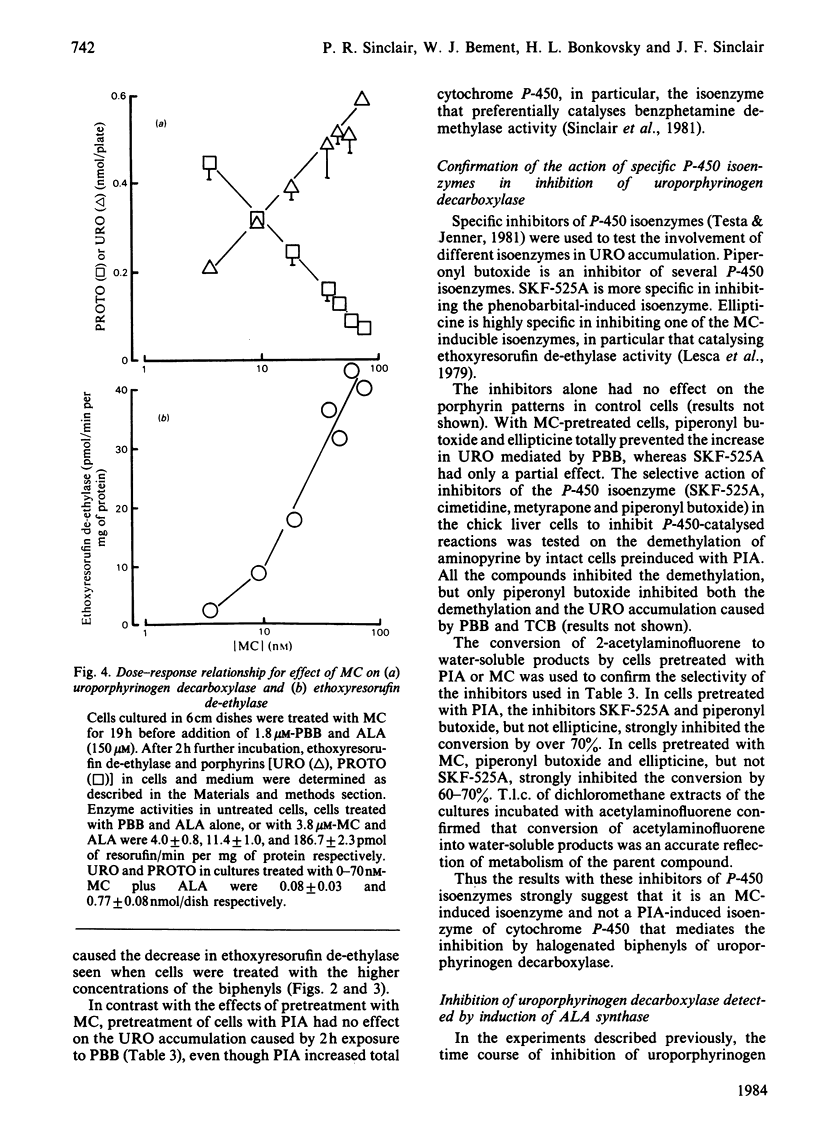
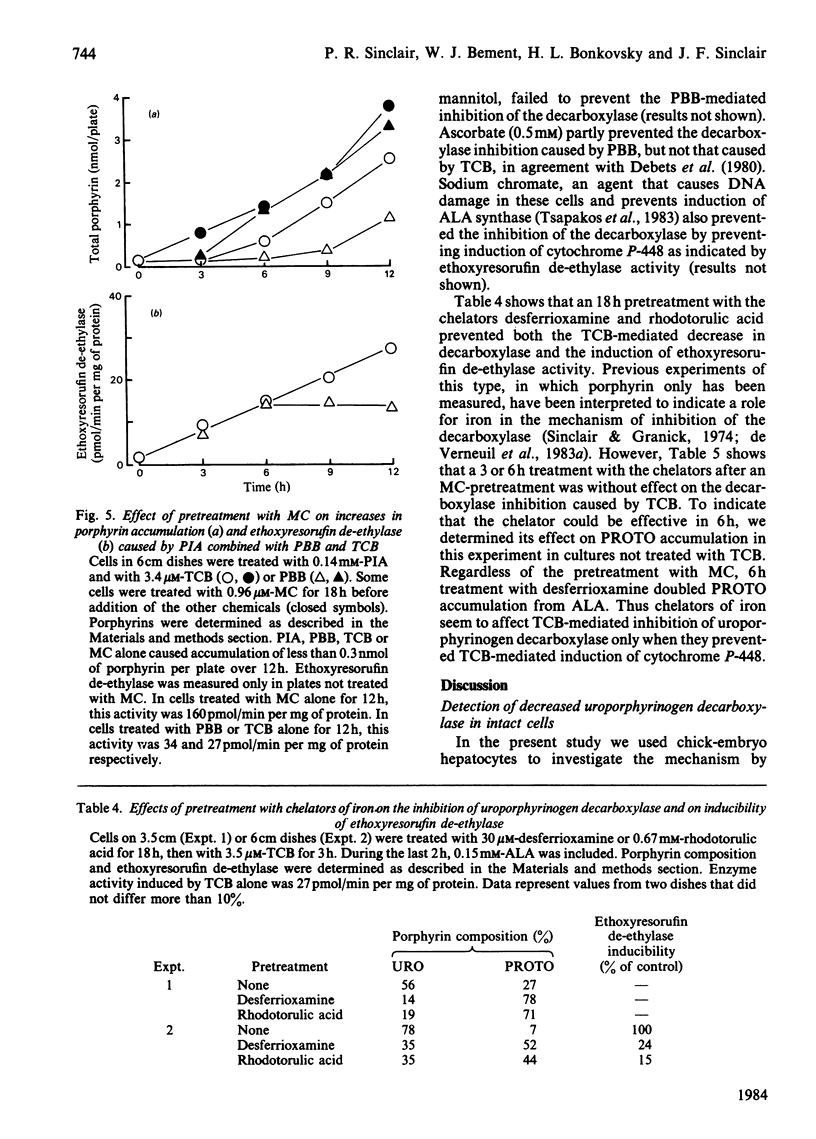
Uroporphyrinogen decarboxylase (EC 4.1.1.37) activity was assayed in cultures of chick-embryo hepatocytes by the changes in composition of porphyrins accumulated after addition of excess 5-aminolaevulinate. Control cells accumulated mainly protoporphyrin, whereas cells treated with 3,4,3',4'-tetrachlorobiphenyl or 2,4,5,3',4'-pentabromobiphenyl accumulated mainly uroporphyrin, indicating decreased activity of the decarboxylase. 3-Methylcholanthrene and other polycyclic-hydrocarbon inducers of the P-448 isoenzyme of cytochrome P-450, did not affect the decarboxylase in the absence of the biphenyls. Induction of P-448 was detected as an increase in ethoxyresorufin de-ethylase activity. Pretreatment of cells with methylcholanthrene decreased the time required for the halogenated biphenyls to inhibit the decarboxylase. The dose response of methylcholanthrene showed that less than 40% of the maximal induction of cytochrome P-448 was needed to produce the maximum biphenyl-mediated inhibition of the decarboxylase. In contrast, induction of the cytochrome P-450 isoenzyme by propylisopropylacetamide had no effect on the biphenyl-mediated decrease in decarboxylase activity. Use of inhibitors of the P-450 and P-448 isoenzymes (SKF-525A, piperonyl butoxide and ellipticine) supported the concept that only the P-448 isoenzyme is involved in the inhibition of the decarboxylase by the halogenated biphenyls. The effect of preinduction with methylcholanthrene to enhance inhibition of the decarboxylase was also shown by the increased rate at which porphyrin accumulated from endogenously synthesized 5-aminolaevulinate after treatment of cells with the combination of propylisopropylacetamide and the biphenyls. Antioxidants, chelators of iron, and chromate affected the decrease in decarboxylase activity only if they prevented the induced increase in cytochrome P-448. We conclude that the P-448 and not the P-450 isoenzyme of cytochrome P-450 plays an obligatory role in the inhibition of uroporphyrinogen decarboxylase caused by halogenated biphenyls.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus F. R., Sinclair J. F., Sinclair P., Meyers U. A. Drug-mediated induction of cytochrome(s) P-450 and drug metabolism in cultured hepatocytes maintained in chemically defined medium. J Biol Chem. 1979 Mar 25;254(6):2148–2153. [PubMed] [Google Scholar]
- De Verneuil H., Sassa S., Kappas A. Effects of polychlorinated biphenyl compounds, 2,3,7,8-tetrachlorodibenzo-p-dioxin, phenobarbital and iron on hepatic uroporphyrinogen decarboxylase. Implications for the pathogenesis of porphyria. Biochem J. 1983 Jul 15;214(1):145–151. doi: 10.1042/bj2140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debets F. M., Hamers W. J., Strik J. J. Metabolism as a prerequisite for the porphyrinogenic action of polyhalogenated aromatics, with special reference to hexachlorobenzene and polybrominated biphenyls (Firemaster BP-6). Int J Biochem. 1980;12(5-6):1019–1025. doi: 10.1016/0020-711x(80)90205-0. [DOI] [PubMed] [Google Scholar]
- Debets F. M., Reinders J. H., Debets A. J., Lössbroek T. G., Strik J. J., Koss G. Biotransformation and porphyringogenic action of hexachlorobenzene and its metabolites in a primary liver cell culture. Toxicology. 1981;19(3):185–196. doi: 10.1016/0300-483x(81)90128-1. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O., Matlin S. A. The effect of the porphyrogenic compound, hexachlorobenzene, on the activity of hepatic uroporphyrinogen decarboxylase in the rat. Clin Sci Mol Med. 1976 Jul;51(1):71–80. doi: 10.1042/cs0510071. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Sheppard D. M. Immunoreactive uroporphyrinogen decarboxylase is unchanged in porphyria caused by TCDD and hexachlorobenzene. Biochem Biophys Res Commun. 1982 Nov 16;109(1):113–120. doi: 10.1016/0006-291x(82)91573-x. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Wyvill P. C. Measurement of uroporphyrinogen decarboxylase using porphyrinogens prepared by chemical reduction. Enzyme. 1982;28(2-3):186–195. doi: 10.1159/000459101. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Immunologic abnormalities in the acquired immunodeficiency syndrome (AIDS). Clin Res. 1984 Dec;32(5):491–499. [PubMed] [Google Scholar]
- Grandchamp B., Deybach J. C., Grelier M., de Verneuil H., Nordmann Y. Studies of porphyrin synthesis in fibroblasts of patients with congenital erythropoietic porphyria and one patient with homozygous coproporphyria. Biochim Biophys Acta. 1980 May 22;629(3):577–586. doi: 10.1016/0304-4165(80)90163-4. [DOI] [PubMed] [Google Scholar]
- Granick S., Sinclair P., Sassa S., Grieninger G. Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem. 1975 Dec 25;250(24):9215–9225. [PubMed] [Google Scholar]
- Jones K. G., Cole F. M., Sweeney G. D. The role of iron in the toxicity of 2,3,7,8-tetrachlorodibenzo-(p)-dioxin (TCDD). Toxicol Appl Pharmacol. 1981 Oct;61(1):74–88. doi: 10.1016/0041-008x(81)90009-0. [DOI] [PubMed] [Google Scholar]
- Jones K. G., Sweeney G. D. Dependence of the porphyrogenic effect of 2,3,7,8-tetrachlorodibenzo(p)dioxin upon inheritance of aryl hydrocarbon hydroxylase responsiveness. Toxicol Appl Pharmacol. 1980 Mar 30;53(1):42–49. doi: 10.1016/0041-008x(80)90379-8. [DOI] [PubMed] [Google Scholar]
- Kawanishi S., Seki Y., Sano S. Polychlorobiphenyls that induce delta-aminolevulinic acid synthetase inhibit uroporphyrinogen decarboxylase in cultured chick embryo liver cells. FEBS Lett. 1981 Jun 29;129(1):93–96. doi: 10.1016/0014-5793(81)80763-6. [DOI] [PubMed] [Google Scholar]
- Kawanishi S., Seki Y., Sano S. Uroporphyrinogen decarboxylase. Purification, properties, and inhibition by polychlorinated biphenyl isomers. J Biol Chem. 1983 Apr 10;258(7):4285–4292. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lesca P., Rafidinarivo E., Lecointe P., Mansuy D. A class of strong inhibitors of microsomal monooxygenases: the ellipticines. Chem Biol Interact. 1979 Feb;24(2):189–197. doi: 10.1016/0009-2797(79)90007-3. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., West S. B. Multiplicity of mammalian microsomal cytochromes P-45. Pharmacol Rev. 1979 Dec;31(4):277–295. [PubMed] [Google Scholar]
- Marks G. S., Follows S. B., Zelt D. T., Cole S. P. Patterns of porphyrin accumulation in response to chemicals in chick embryo liver cells. Can J Physiol Pharmacol. 1983 Jun;61(6):546–553. doi: 10.1139/y83-084. [DOI] [PubMed] [Google Scholar]
- Mühlebach S., Bickel M. H. Pharmacokinetics in rats of 2,4,5,2',4',5'-hexachlorobiphenyl, an unmetabolizable lipophilic model compound. Xenobiotica. 1981 Apr;11(4):249–257. doi: 10.3109/00498258109045299. [DOI] [PubMed] [Google Scholar]
- Pimstone N. R. Porphyria cutanea tarda. Semin Liver Dis. 1982 May;2(2):132–142. doi: 10.1055/s-2008-1040703. [DOI] [PubMed] [Google Scholar]
- Poland A., Kappas A. The metabolism of aminopyrine in chick embryo hepatic cell culture: effects of competitive substrates and carbon monoxide. Mol Pharmacol. 1971 Nov;7(6):697–705. [PubMed] [Google Scholar]
- SWELL L., TROUT E. C., Jr, HOPPER J. R., FIELD H., Jr, TREADWELL C. R. Mechanism of cholesterol absorption. I. Endogenous dilution and esterification of fed cholesterol-4-C14. J Biol Chem. 1958 May;232(1):1–8. [PubMed] [Google Scholar]
- Schoenfeld N., Greenblat Y., Epstein O., Atsmon A. The effects of succinylacetone (4,6-dioxoheptanoic acid) on delta-aminolevulinate synthase activity and the content of heme in monolayers of chick embryo liver cells. Biochim Biophys Acta. 1982 Dec 30;721(4):408–417. doi: 10.1016/0167-4889(82)90096-9. [DOI] [PubMed] [Google Scholar]
- Shedlofsky S. I., Bonkowsky H. L., Sinclair P. R., Sinclair J. F., Bement W. J., Pomeroy J. S. Iron loading of cultured hepatocytes. Effect of iron on 5-aminolaevulinate synthase is independent of lipid peroxidation. Biochem J. 1983 May 15;212(2):321–330. doi: 10.1042/bj2120321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. F., Sinclair P. R., Healey J. F., Smith E. L., Bonkowsky H. L. Decrease in hepatic cytochrome P-450 by cobalt. Evidence for a role of cobalt protoporphyrin. Biochem J. 1982 Apr 15;204(1):103–109. doi: 10.1042/bj2040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. F., Sinclair P. R., Smith E. L., Bement W. J., Pomeroy J., Bonkowsky H. Ethanol-mediated increase in cytochrome P-450 in cultured hepatocytes. Biochem Pharmacol. 1981 Oct;30(20):2805–2809. doi: 10.1016/0006-2952(81)90418-4. [DOI] [PubMed] [Google Scholar]
- Sinclair P. R., Elder G. H., Bement W. J., Smith S. G., Bonkowsky H. L., Sinclair J. F. Decreased activity of uroporphyrinogen decarboxylase caused by 2,4,5,3',4'-pentabromobiphenyl in chick embryo hepatocyte cultures. Difference in activity in intact or homogenized cells. FEBS Lett. 1983 Feb 21;152(2):217–221. doi: 10.1016/0014-5793(83)80383-4. [DOI] [PubMed] [Google Scholar]
- Sinclair P. R., Granick S. Uroporphyrin formation induced by chlorinated hydrocarbons (lindane, polychlorinated biphenyls, tetrachlorodibenzo-p-dioxin). Requirements for endogenous iron, protein synthesis and drug-metabolizing activity. Biochem Biophys Res Commun. 1974 Nov 6;61(1):124–133. doi: 10.1016/0006-291x(74)90543-9. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Francis J. E., Kay S. J., Greig J. B. Hepatic toxicity and uroporphyrinogen decarboxylase activity following a single dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin to mice. Biochem Pharmacol. 1981 Oct;30(20):2825–2830. doi: 10.1016/0006-2952(81)90421-4. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Francis J. E. Synergism of iron and hexachlorobenzene inhibits hepatic uroporphyrinogen decarboxylase in inbred mice. Biochem J. 1983 Sep 15;214(3):909–913. doi: 10.1042/bj2140909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain M. G., Follows S. B., Marks G. S. Inhibition of uroporphyrinogen decarboxylase by 3,3',4,4'-tetrachlorobiphenyl in chick embryo liver cell culture. Can J Physiol Pharmacol. 1983 Jan;61(1):105–108. doi: 10.1139/y83-014. [DOI] [PubMed] [Google Scholar]
- Taljaard J. J., Shanley B. C., Joubert S. M. Decreased uroporphyrinogen decarboxylase activity in 'experimental symptomatic porphyria'. Life Sci II. 1971 Aug;10(15):887–893. [PubMed] [Google Scholar]
- Testa B., Jenner P. Inhibitors of Cytochrome P-450s and their mechanism of action. Drug Metab Rev. 1981;12(1):1–117. doi: 10.3109/03602538109011082. [DOI] [PubMed] [Google Scholar]
- Tsapakos M. J., Hampton T. H., Sinclair P. R., Sinclair J. F., Bement W. J., Wetterhahn K. E. The carcinogen chromate causes DNA damage and inhibits drug-mediated induction of porphyrin accumulation and glucuronidation in chick embryo hepatocytes. Carcinogenesis. 1983 Aug;4(8):959–966. doi: 10.1093/carcin/4.8.959. [DOI] [PubMed] [Google Scholar]
- de Verneuil H., Sassa S., Kappas A. Purification and properties of uroporphyrinogen decarboxylase from human erythrocytes. A single enzyme catalyzing the four sequential decarboxylations of uroporphyrinogens I and III. J Biol Chem. 1983 Feb 25;258(4):2454–2460. [PubMed] [Google Scholar]


