Abstract
Previous studies showed that hepatitis B virus polymerase (HBV Pol) interacts with host factors such as the Hsp90 complex, which is a critical step in viral genome replication. In this report, we propose that another chaperone, Hsp60, interacts with human HBV Pol and that this is a very important step for maturation of human HBV Pol into the active state. In the immunoprecipitation of recombinant human HBV Pol expressed in insect cells with the recombinant baculovirus expression system, the 60-kDa protein was coimmunoprecipitated with Pol and the protein was identified as Hsp60 through peptide sequencing and immunogenic analysis with an anti-Hsp60 antibody. In vitro experiments showed that Hsp60 strongly affected human HBV Pol activity in that (i) blocking of Hsp60 by the protein-specific antibody reduced human HBV Pol activity, (ii) the activity was increased by addition of Hsp60 in the presence of ATP, and (iii) ATP synergistically activated human HBV Pol with Hsp60. In vivo experiments showed that inhibition of Hsp60 in cells by a mutant Hsp60, CΔ540, resulted in the reduction of human HBV Pol activity. In summary, our results indicate that the interaction is significant for conversion of human HBV Pol into the active state.
Hepatitis B virus (HBV), a member of the hepadnavirus family, is an enveloped virus with partially double-stranded DNA. It is also associated with the development of hepatocellular carcinoma and liver cirrhosis (10). Following the infection of hepatocytes, the partially double-stranded DNA genome is converted into a covalently closed circular DNA in the nucleus (21). HBV encodes four unspliced overlapping messages that terminate at a common polyadenylation signal (10). The transcript encoding the HBV polymerase (Pol) works as a replication intermediate, namely, as pregenomic RNA (30). HBV replicates through reverse transcription with the pregenomic RNA. Initiation of replication occurs via a priming reaction in which a nucleotide becomes covalently linked to the tyrosine residue within the terminal-protein domain of HBV Pol (38, 40). In this step, the 5′ epsilon stem-loop region is recognized by HBV Pol, and this process has a preference for cis pregenomic RNA, which appears to be cotranslational (11, 16, 24). After the coupling of 3 or 4 nucleotides that are linked to a tyrosine residue of HBV Pol, these oligonucleotides are translocated to a complementary sequence in the 3′ copy of DR1 and extended by HBV Pol (20, 23, 28, 33). The RNA template is degraded by the RNase H activity of HBV Pol. The synthesis of minus-strand DNA terminates at the 5′ end of pregenomic RNA, and plus-strand DNA is synthesized by HBV Pol. In this replication step, HBV Pol functions as a DNA-dependent DNA Pol. Although the 5′ epsilon stem-loop region of pregenomic RNA is used in priming, many other studies have reported that the 3′ epsilon stem-loop region of pregenomic RNA is enough for priming (17, 28, 31, 32). In vitro priming of competent duck HBV (DHBV) Pol has been expressed by in vitro translation and as an active fusion protein of DHBV Pol in a virus-like particle from the Saccharomyces cerevisiae retroposon Ty1 (14, 15, 31). However, in vitro priming of human HBV Pol was successful only if the protein was expressed in insect cells by using baculovirus expression systems (17, 18, 25, 29, 36). It was demonstrated that the complex between DHBV Pol and the epsilon stem-loop in the pregenomic RNA is stabilized by the 90-kDa heat shock protein (Hsp90) complex and that this complex facilitates the priming of DHBV Pol (14, 15). Additionally, p23 works as a chaperone partner of Hsp90 (15). These findings lend support to the concept that the interaction of molecular chaperones with HBV Pol plays a critical role in the maintenance of the enzyme in a conformational state that renders it competent for its various functions. Recently, it was found that the DHBV Pol expressed in E. coli also has in vitro priming activity assisted by the Hsp90 complex (13). These results show that chaperone assistance is needed for the proper function of HBV Pol.
In this report, we found that another molecular chaperone, Hsp60, participates in human HBV Pol activation. This Hsp60 is a common cellular protein that assists in the correct folding of proteins and stabilizes unfolded labile proteins (3). These functions maintain the activities of some cellular proteins and facilitate enzymatic maturation. The former is a well-known function of Hsp60 under stress conditions, and an example of the latter is activation of procaspase-3 and prion protein through conformational change by Hsp60 (8, 26, 39). Functioning as a chaperonin in eukaryotes, Hsp60 assembles into a heptamer and has ATPase activity for the release of bound protein (3, 34). In general, in in vitro experiments, ATP activated the Hsp60 function (3, 34). Under in vitro conditions, it is well known that Hsp60 interacts with many proteins, but recently under in vivo conditions, only a very small proportion of the total proteins were found to bind Hsp60 (7, 12). In some cases, the in vivo interaction of Hsp60 with its binding proteins is often very critical for the maturation of proteins such as pro-caspase-3 and prion protein (8, 26, 39).
This report shows that interaction enhances human HBV Pol activity in the presence of ATP and that ATP synergistically activates Pol. In addition to presenting findings from our in vitro experiments, we show that CΔ540, a deletion mutant Hsp60, affects the enzymatic activity of human HBV Pol by participation in oligomerization and inhibition of Hsp60's function (4, 22). The above results support the conclusion that Hsp60 is an essential factor for the activation of human HBV Pol.
MATERIALS AND METHODS
Plasmid constructions.
The baculovirus transfer vector pFPolE was created as follows (Fig. 1A). The sequence encoding the amino-terminal 346 amino acids of human HBV Pol was amplified by PCR using pMPLX (19) as the template, a 5′ primer designed to introduce a SacI site and the sequence encoding the FLAG tag (Met-Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys-Leu), and a 3′ primer spanning the XhoI site (amino acid 346). The XhoI/PstI fragment encoding the carboxyl-terminal part of human HBV Pol plus the 3′ nontranslated region (NTR) containing the epsilon stem-loop was subcloned into pFASTBAC, modified by PCR-mediated mutagenesis from pFASTBAC Ht (BAC-TO-BAC system; Life Technologies, Inc.). The amino-terminal part was cloned into the subcloned plasmid using the SacI/XhoI fragment. The fragment amplified by PCR was verified by DNA sequencing.
FIG. 1.
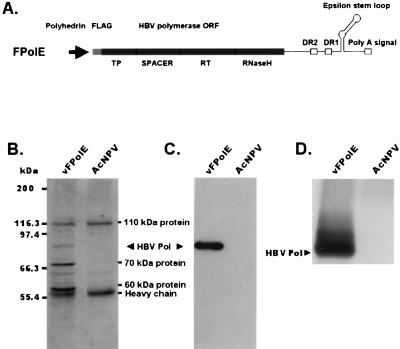
Expression and purification of recombinant human HBV Pol. (A) The transfer vector pFPolE contains the human HBV Pol open reading frame (ORF) as well as the 3′ NTR containing DR2, DR1, and the epsilon stem-loop region downstream of the polyhedrin promoter. This epsilon stem-loop region plays a role as the template for human HBV Pol priming. The recombinant baculovirus (vFPolE) was generated by the BAC-TO-BAC system. TP, terminal-protein domain; RT, reverse transcriptase domain. (B) FLAG-fused recombinant human HBV Pol was produced in insect cells by infection with vFPolE and then purified with M2 agarose beads. A detailed explanation of the procedure is presented in Materials and Methods. Protein samples obtained from the purification steps were analyzed by SDS–7.5% PAGE, and the gel was visualized by Coomassie blue staining. The recombinant human HBV Pol was copurified with three proteins: 110-, 70-, and 60-kDa proteins. The Pol band was located at the expected apparent molecular mass, 84 kDa. As a negative control, AcNPV-infected cells were used. (C) Purified recombinant human HBV Pol was analyzed by immunoblot analysis with an M2 monoclonal antibody that recognizes the FLAG epitope. This antibody was diluted 1:1,000 in PBS containing 0.3% Tween 20. Insect cells infected with wild-type virus, AcNPV, were used as a negative control. (D) An in vitro priming reaction was performed with purified recombinant human HBV Pol as described in Materials and Methods. The product of the reaction was analyzed by SDS–7.5% PAGE and autoradiographed.
Through reverse transcription-PCR, the Hsp60 gene was generated from HepG2 RNAs by using two oligonucleotides specific to the 5′ and 3′ ends of the Hsp60 open reading frame, identified in reference 37. The carboxyl-terminal deletions of Hsp60 were generated through PCR-mediated mutagenesis to include fragments spanning from the start codon to amino acids 560, 540, and 510. These fragments were cloned individually into the pFASTBAC Ht transfer vector.
Cells and infections.
The Spodoptera frugiperda Sf-9 cell line was maintained in TNM-FH (Sigma) supplemented with 5% certified fetal bovine serum (Life Technologies, Inc.). In the case of a spinner culture, TNM-FH medium was supplemented with 0.1% pluronic F68 (Life Technologies, Inc.) as well as 5% certified fetal bovine serum. Recombinant baculoviruses were generated with the plasmids that were cloned into the transfer vector pFASTBAC, using the BAC-TO-BAC system. For protein production, when Sf-9 cells reached about 70% confluence, recombinant baculoviruses (multiplicity of infection, 2 to 10) were added to the medium and incubated for 2 h at 22°C. The medium was changed, and virus-infected cells were incubated at 27°C. Two days later, the infected cells were harvested and stored at −70°C until use.
Purification of recombinant human HBV Pol using M2 beads.
Sf-9 cells infected with each recombinant baculovirus were lysed with 1 ml of lysis buffer (phosphate-buffered saline [PBS] containing 1 mM EDTA, 5 mM dithiothreitol, 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride, 100 μM leupeptin, and 50 U of recombinant RNasin [Promega] per ml) per 0.5 × 105 cells on ice for 15 min. After lysis, extracts were cleared by centrifugation at 30,000 × g for 15 min at 4°C. The cleared extracts were incubated with M2 agarose beads (Sigma) for 2 h on ice, and this mix was packed into a column. The column was washed sequentially with TNG (100 mM Tris · Cl [pH 7.5], 30 mM NaCl, and 10% glycerol), TNG with 1 M NaCl, and TNG. The bound proteins were eluted with 1× sodium dodecyl sulfate (SDS) sample buffer or elution buffer (0.1 M glycine [pH 3.0], 10% glycerol), using five times the bed volume of M2 agarose beads, and neutralized with 67 μl of neutralization buffer (0.8 M Tris · Cl [pH 8.4], 3% Triton X-100, and 80 mM dithiothreitol) per 1 ml. Purified proteins were frozen at −70°C until use.
Amino-terminal amino acid sequencing.
Partially purified recombinant human HBV Pol was separated by SDS-polyacrylamide gel electrophoresis (PAGE) and electrotransferred to a polyvinylidene difluoride (PVDF) membrane (Millipore). Protein bands were visualized by Coomassie blue staining, and the unknown 60-kDa protein band was excised. With the excised protein band, amino-terminal sequencing was carried out by Edman degradation and was performed by the Korea Basic Science Research Institute, with a Waters PicoTag workstation system and a Perkin-Elmer Procise 491 sequencing system (9). Identification of proteins with a region homologous to the amino-terminal peptides of the 60-kDa protein was performed using the BLAST program (1).
Immunoblot analysis.
Each protein sample was electrophoresed on an SDS-polyacrylamide gel of the appropriate percentage and subsequently transferred electrophoretically to a PVDF membrane in 25 mM Tris · Cl [pH 8.3], 192 mM glycine, and 20% methanol. Each blot was incubated with appropriate antibodies: anti-Hsp60 goat polyclonal N-20 (Santa Cruz) and M2 monoclonal antibodies (Sigma), each at the dilution recommended by the manufacturers. Incubation steps were performed as described previously (35). Antibody detection was performed by chemiluminescence (ECL system; Amersham Pharmacia Biotech).
In vitro priming assay.
The standard in vitro priming assays were performed with 200 to 300 ng of the purified recombinant human HBV Pol in the elution-neutralizing buffer containing 10 mM MgCl2; a 100 μM concentration each of unlabeled dATP, dGTP, and dCTP; and 5 μCi of [α-32P]TTP (3,000 Ci/mmol; ICN). The final reaction volume was adjusted to 50 μl, and assays were routinely performed at 30°C for 45 min. The standard in vitro priming reaction was performed as described for the above method unless otherwise stated. The reaction was stopped by addition of SDS sample buffer, and the sample was analyzed by SDS-PAGE and Coomassie blue staining. The stained polyacrylamide gel was dried, and the bands were detected by autoradiography with X-ray film (Fuji). The exposed X-ray films were analyzed with 1D Image Analysis software (Kodak Digital Science). When Hsp60 was added to the in vitro priming reaction mixture, the amount of purified human HBV Pol was divided in half (100 ng) to increase the difference between the amount of the preexisting and the amount of the added Hsp60.
RESULTS
A 60-kDa protein specifically interacts with human HBV Pol.
To express human HBV Pol in insect cells, a recombinant baculovirus (vFPolE) was generated by the BAC-TO-BAC system and this virus was used to infect Sf-9 cells to produce recombinant human HBV Pol (FPolE). The expressed recombinant human HBV Pol, which was active in in vitro priming (Fig. 1D), was purified by an M2 agarose bead method. Through this one-step purification column, five proteins were eluted: 110-, 84-, 70-, 65-, and 60-kDa proteins (Fig. 1B). The 110- and 65-kDa proteins were also observed in the negative control (AcNPV-infected cells). Perhaps these proteins were endogenous proteins nonspecifically but tightly bound to M2 or agarose beads. The purified 84-kDa protein was immunostained with the M2 monoclonal antibody, which is specific to the FLAG epitope, and represents recombinant human HBV Pol (Fig. 1C). Thus, the remaining two protein bands were copurified proteins that may bind with recombinant human HBV Pol. These two proteins were not removed by an excess of 1 M NaCl washing buffer, and among the two, only the 60-kDa protein was removed by treatment with SDS. Between the two coimmunoprecipitated proteins, the 60-kDa protein bound more specifically to human HBV Pol. This was elucidated from a binding site analysis that revealed that only the 60-kDa protein had specific binding sites on human HBV Pol, and we found the constructs that did not bind to Hsp60, like FPol177-345E (S. G. Park, S. O. Lim, K. G. Han, and G. Jung, unpublished data).
The 60-kDa protein was identified as Hsp60.
The 60-kDa protein was identified through amino-terminal amino acid sequencing. Following SDS-PAGE, the 60-kDa protein band was electroblotted onto a PVDF membrane and the blot was subjected to amino-terminal amino acid sequencing with 12 rounds of Edman degradation. Each of the 12 amino-terminal amino acids of the 60-kDa protein were identical to insect Hsp60 sequences and almost homologous to the human Hsp60 sequences (Table 1). To confirm that the 60-kDa protein was in fact Hsp60, immunogenic analyses were carried out with two different Hsp60 antibodies and the 60-kDa protein was immunostained with both antibodies (data not shown). This additional evidence clearly showed that the 60-kDa protein is in fact Hsp60.
TABLE 1.
Amino-terminal amino acid sequence of the copurified 60-kDa proteina
| Protein | NCBIb accession no. | Amino acid sequence |
|---|---|---|
| Obtained sequence | AKDVRFGADVRA | |
| Insect Hsp60 | P26317 | AKDVRFGADVRA |
| Human Hsp60 | P10809 | AKDVKFGADARA |
The sequence of the 60-kDa protein was determined by amino-terminal sequencing and alignment of homologus peptides from insect and human Hsp60s. Amino-terminal amino acid sequencing was performed by the Korea Basic Science Research Institute with a Waters PicoTag system and a Perkin-Elmer Procise, model 491.
NCBI, National Center for Biotechnology Information.
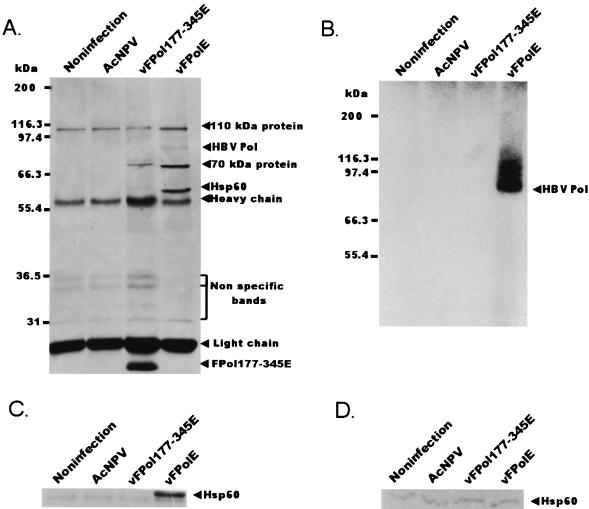
To confirm that the copurification of Hsp60 was caused by the binding of recombinant human HBV Pol and Hsp60, the following experiments were performed. The vFPolE-infected Sf-9 cells and three negative controls—uninfected cells, AcNPV-infected cells, and vFPol177-345E-infected cells—were lysed with 0.5% NP-40 lysis buffer. Through this step, the soluble fraction was obtained from each cell. Each fraction was incubated with M2 agarose beads, and then bound proteins were eluted with SDS sample buffer. Figure 2A and C indicate that the Hsp60 band appears only in the fraction originating from vFPolE-infected insect cells. The expressed protein of FPol177-345E is shown in Fig. 2A, where the protein band appears under the light chain of M2 antibody and the protein does not bind to Hsp60. If the binding of human HBV Pol to Hsp60 was due to the overproduction of the protein, FPol177-345E also bound to Hsp60 because the FPol177-345E protein was expressed more than the full-length human HBV Pol. In Fig. 2A, some bands appeared in all samples between the heavy chain and the light chain and these bands may be proteins that bind nonspecifically to M2 beads. Figure 2B indicates that purified recombinant human HBV Pol also has a priming activity. To confirm that the Hsp60 levels were similar to each other, a sample of each extract that had not gone through the M2 purification step was subjected to an immunoblot analysis with anti-Hsp60 (N-20). This confirmation was required, because if there was a large difference in the levels of Hsp60, it might have resulted in contamination of the M2 affinity column. It was found that the Hsp60 level of each sample was the same (Fig. 2D). Thus, human HBV Pol interacts with Hsp60 and we are able to prove that the presence of Hsp60 is not due to changes in the Hsp60 level or contamination. The results of recombinant human HBV Pol purification showed that the Hsp60 band was more intense than the Pol band because Hsp60 works as heptamer homo-multimer in eukaryotes (3). In the previous studies of DNA Pol II purification from yeast, the Hsp60 component of the stimulating factor I band was also more intense than the Pol band (2, 27). Additionally, the purification of recombinant human HBV Pol as described in Materials and Methods shows that the molar ratio of human HBV Pol to the copurified Hsp60 multimeric complex is about 1:0.7 as calculated with 1D Image Analysis software (Kodak Digital Science). To confirm that this binding occurs in mammalian cells, an MBP (40-kDa) fused human HBV Pol protein was expressed in HepG2 cells, which is a host cell line of human HBV. The expressed human HBV Pol was also coimmunoprecipitated with Hsp60 (data not shown).
FIG. 2.
The binding between human HBV Pol and Hsp60 is specific. Insect cells were infected by one of three baculoviruses: vFPolE, AcNPV, or vFPol177–345E. Soluble fractions were obtained from the three sets of virus-infected cells and control (uninfected) cells by incubating them on ice for 15 min with lysis buffer containing 0.5% NP-40 in PBS and then centrifuging them at 30,000 × g for 15 min. The soluble fractions were incubated with M2 agarose beads for 2 h, and the beads were washed once with 1 ml of TNG, two times with TNG plus 1 M NaCl, and once with 1 ml of TNG. (A) One-third of the M2 beads from each sample was analyzed by SDS–7.5% PAGE and visualized by silver staining. (B) Another third of each sample was subjected to an in vitro priming reaction. The reaction product was analyzed by SDS–7.5% PAGE and autoradiography. (C) The remaining third of each sample was fractionated by SDS–11% PAGE, and an immunoblot analysis was performed with anti-Hsp60 (N-20) antibody as described in Materials and Methods. (D) To check the level of Hsp60 in each sample, each soluble fraction was separated by SDS–7.5% PAGE and an immunoblot analysis was performed with the anti-Hsp60 N-20 antibody.
In vitro effect of Hsp60 on the enzymatic activity of human HBV Pol.
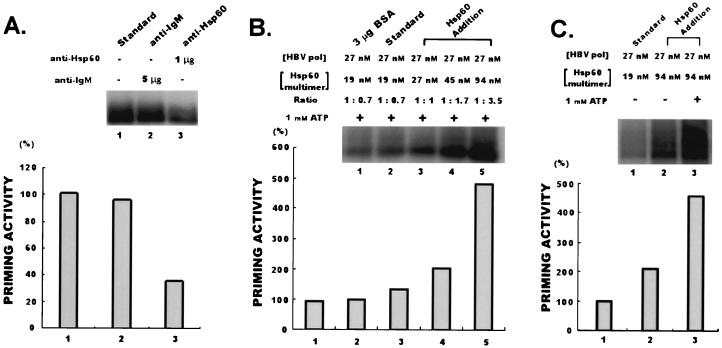
To assess the functional significance of the interaction between human HBV Pol and Hsp60, we performed several in vitro experiments. These experiments were designed to evaluate the influence of Hsp60 on the activation of human HBV Pol and to determine the significance of Hsp60 to human HBV Pol activities. Incubation of the in vitro priming reaction mixture with 1 μg of anti-Hsp60 (N-20) antibody reduced human HBV Pol activity (Fig. 3A), but with five times more anti-IgM control antibody, it did not (Fig. 3A). This result shows that the influence of an Hsp60-specific antibody on Hsp60 affects human HBV Pol activity. In other words, the inhibition of preexisting Hsp60 originating from the purified fraction of recombinant human HBV Pol induced the reduction of human HBV Pol activity. Thus, copurified Hsp60 is important for human HBV Pol activation. While an inhibitory effect on human HBV Pol is seen with anti-Hsp60 antibody, the addition of Hsp60 protein to up to five times the original molar ratio (94 nM) activated human HBV Pol activity in the presence of 1 mM ATP. Addition of Hsp60 to the in vitro priming reaction mixture induced a dose-dependent activation of human HBV Pol (Fig. 3B), while the addition of excess bovine serum albumin induced a slight reduction in human HBV Pol activity (Fig. 3B). The above results showed that Hsp60 activates human HBV Pol activity. Figure 3C shows that ATP synergistically activates human HBV Pol with Hsp60. Human HBV Pol was activated by Hsp60 alone but was further activated in the presence of both Hsp60 and ATP. The above results support the conclusion that Hsp60 activates human HBV Pol.
FIG. 3.
In vitro effect of Hsp60 on in vitro priming of human HBV Pol. (A) Blocking assay. One microgram of anti-Hsp60 (N-20) antibody was added into the in vitro priming reaction mixture, and this mixture was incubated on ice for 1 h prior to the initiation of the reaction. As a negative control, 5 μg of anti-IgM antibody (Sigma) was used. After incubation, the reaction was initiated by addition of unlabeled deoxyribonucleoside triphosphates (dATP, dGTP, and dCTP) plus 5 μCi of [α-32P]TTP. Following incubation for 45 min at 30°C, the reaction was stopped by the addition of SDS sample buffer. (B) Activation assays. Hsp60 (purchased from Stressgen) was added into the in vitro priming reaction mixture at different concentrations (27, 45, and 94 nM), and the mixture was incubated at 25°C for 30 min. After incubation, the reaction was initiated by addition of unlabeled deoxyribonucleoside triphosphates (dATP, dGTP, and dCTP) plus 5 μCi of [α-32P]TTP. As a negative control, 3 μg of bovine serum albumin was added to the reaction mixture. (C) In the presence of ATP or both ATP and Hsp60, the in vitro priming reaction was performed as described in Materials and Methods. The first lane shows a control reaction that was performed without addition of Hsp60 and ATP. In the second lane, the in vitro priming reaction was performed in the presence of 94 nM Hsp60. In the third lane, the in vitro priming reaction was performed in the presence of both 94 nM Hsp60 and 1 mM ATP. Products shown in panels A to C were analyzed by SDS-PAGE and autoradiography.
Human HBV Pol activity is affected by Hsp60 under in vivo conditions.
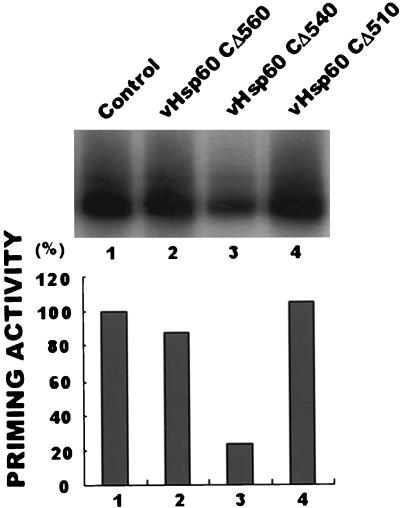
Studies of Hsp60 in the past have been based on GroEL, a bacterial Hsp60 family member, because bacterial cells are more accessible than eukaryotic cells. Previous studies showed that some mutant GroEL proteins with carboxyl-terminal deletions affected the chaperonin function of the GroEL complex (4, 22). These GroEL mutant proteins usually have a deletion of 28 amino acids but may have a few more amino acids deleted from the carboxyl terminus. These mutant proteins participate in the oligomerization of GroEL but inhibit chaperonin function. Deletions of 33 amino acids or more cause inhibition of the participation of the mutant GroEL in oligomerization, and therefore the mutant protein does not inhibit chaperonin function. For construction of a mutant Hsp60 with a deletion at the carboxyl terminus, we analyzed the sequence similarity between Hsp60 and GroEL using the DNAsis program (Hitachi Software Engineering Co.). Based on sequence similarity, it was possible to design three mutant Hsp60s with carboxyl-terminal deletions. The constructs of Hsp60 were as follows: a mutant protein with a deletion of 13 amino acids, CΔ560, which was the control because it was not expected to affect the chaperonin function of the Hsp60 complex; a mutant protein with a deletion of 33 amino acids, CΔ540, a function inhibitor mutant protein similar to the GroEL mutant protein with a deletion of 28 amino acids; and a mutant protein with a deletion of 63 amino acids, CΔ510, an inert construct similar to the GroEL mutant protein with a deletion of 33 amino acids. To determine the effect of Hsp60 on human HBV Pol under in vivo conditions, human HBV Pol and each mutant Hsp60 with a deletion of the carboxyl terminus were coexpressed and an in vitro priming assay was performed. The priming activity of human HBV Pol expressed with the CΔ540 Hsp60 construct was noticeably less than those of human HBV Pol expressed with the other mutant Hsp60s (Fig. 4). The inhibition of Hsp60 chaperonin function induced the reduction of human HBV Pol activity. SDS-PAGE analysis of the crude extracts of cells infected by recombinant baculovirus containing the CΔ540 mutant Hsp60 has shown that they are not different from cells infected by the other constructs (data not shown). This indicates that there have not been severe metabolic changes. The above-described inhibitory effect of human HBV Pol activity indicates that human HBV Pol is affected by Hsp60 under in vivo conditions because the enzymatic activity was decreased only in the presence of CΔ540, which is a functional inhibitor mutant Hsp60.
FIG. 4.
The carboxyl-terminal deletion mutant Hsp60 CΔ540 affects the activity of human HBV Pol under in vivo conditions. Insect cells were coinfected with vFPolE and one of three recombinant baculoviruses expressing a mutant Hsp60 with a carboxyl-terminal deletion: CΔ560, CΔ540, and CΔ510. Two days after the infection, each infected cell was harvested and lysed. The 0.5% NP-40 detergent-soluble fractions of each coinfected cell were incubated with M2 agarose beads on ice for 2 h. As described for Fig. 2, each sample was washed and subjected to the in vitro priming reaction as described in Materials and Methods. Following the addition of SDS sample buffer to the reaction mixture, each reaction product was analyzed by SDS-PAGE and autoradiography.
DISCUSSION
HBV Pol plays an essential role for HBV replication. The biochemical study of human HBV Pol has been hampered by its low expression level in heterologous expression systems (10). In the study of Lanford and coworkers (18), human HBV Pol was successfully expressed in insect cells and this expressed protein has been used in many studies. With the expressed protein, various approaches were tried for studying the mechanism of human HBV replication (17, 18, 25, 29, 36). In doing so, it was found that human HBV Pol was active in priming without capsid formation (17, 18). Also, several types of monoclonal antibodies were produced for structure-function analysis of human HBV Pol using the expressed protein (25). Human HBV Pol expressed in insect cells has a priming activity (Fig. 1D). In this report, we hypothesize that insect cells may provide host factors implicated in the maturing of human HBV Pol. For this reason, we expressed human HBV Pol in insect cells and found that human HBV Pol binds with 60- and 70-kDa proteins (Fig. 1B). We supposed that these proteins have the potential to assist in human HBV Pol maturations. Between the two proteins, we found by binding site analysis with the deletion mutant proteins of human HBV Pol that the 60-kDa protein had specific binding sites on human HBV Pol; the 70-kDa protein, however, did not have a specific binding site so its binding may be random. The protein was identified as a Hsp70, and the amount of Hsp70 that coimmunoprecipitated with human HBV Pol was related to the size of each expressed deletion mutant protein of human HBV Pol (S. G. Park, S. O. Lim, K. G. Han, and G. Jung, unpublished data). Furthermore, strong ionic strength, such as that of 1 M NaCl, did not break down the binding between human HBV Pol and the 60-kDa protein, demonstrating that this protein binds to human HBV Pol with high affinity. The 60-kDa protein was identified through peptide sequencing (Table 1) and immunoblot analysis (Fig. 2C) as a molecular chaperonin, Hsp60. The binding between human HBV Pol and Hsp60 was further confirmed in the HBV host cell line HepG2 using MBP-Pol produced by pCMV/MBP-Pol transfection of the cells (data not shown). The above results show that human HBV Pol binds to Hsp60.
In cells Hsp60 assists protein folding by its affinity for hydrophobic regions. Unlike Hsp70, Hsp60 interacts mainly with folding intermediates (3). Under in vitro conditions, Hsp60 can bind many kinds of proteins through low-affinity interactions (3). The analysis of all proteins influenced by Hsp60 indicates that only a fraction of the total adopts the Hsp60-assisted folding pathway under in vivo conditions (7, 12). This analysis showed that the true binding proteins were small in proportion under in vivo conditions. With some proteins, like pro-caspase-3 and prion protein, this event is critical for maturation into functional proteins in that activation of these proteins requires Hsp60 (8, 26, 39). Human HBV Pol was expressed in insect cells, and the protein was overexpressed in the cells. However, human deletion mutant HBV Pol proteins that did not bind to Hsp60 existed even though the mutant proteins were more highly expressed in the cells than the full-length human HBV Pol (Fig. 2A and S. G. Park, S. O. Lim, K. G. Han, and G. Jung, unpublished data). Additionally, the level of MBP-Pol expressed in HepG2 cells was so low that it was efficiently detected only by immunoblot analysis (5, 6). This low-level-expression protein also coimmunoprecipitated with Hsp60, and this result means that the interaction was not dependent on the Pol expression level in cells. These results indicate that Hsp60 should have interacted with human HBV Pol in its replication.
The above suggestion is supported by several in vitro experiments. One experiment entailed the blocking of Hsp60 by the protein-specific antibody (Fig. 3A). This experiment indicates that the inhibition of preexisting Hsp60 associated with purified human HBV Pol reduced the in vitro priming activity of human HBV Pol. Another experiment entailed the activation of human HBV Pol by the addition of Hsp60. The activation occurred in a dose-dependent manner (Fig. 3B) because the interaction rate was increased by the addition of Hsp60. Pol activation was further facilitated by ATP (Fig. 3C). For example, the activation of pro-caspase-3 is further facilitated by ATP (7, 39). For Hsp60 functions, ATP is needed to release binding proteins from Hsp60 (24), which implies that the activation process of human HBV Pol includes this step. Recent studies have shown that Hsp60 is implicated in protein maturation. With pro-caspase-3, the interaction between this protein and Hsp60 is a critical step for obtaining autoproteolytic activity (26, 39). In addition to pro-caspase-3, conformational changes of prion protein also require assistance by Hsp60 (8). These previous studies suggest that our conclusion of the human HBV Pol activation process by Hsp60 is due to conformational changes of Pol into active states.
The above in vitro results were further supported by in vivo experiments. Though Hsp60 and GroEL are not identical, in many cases the study of Hsp60 has been modeled after that of GroEL because of their structural similarity and high sequence homology. We designed a few carboxyl-terminally deleted mutant Hsp60s by determining sequence homology to GroEL: CΔ560, CΔ540, and CΔ510. It was expected that CΔ540 would inhibit Hsp60 function under in vivo conditions like the CΔ519 mutant GroEL (4, 22). The data here demonstrate that, as predicted, only the CΔ540 deletion construct of Hsp60 had an effect on human HBV Pol function (Fig. 4). Therefore, this result indicates that the Hsp60-human HBV Pol interaction is also significant under in vivo conditions.
It has been difficult to study human HBV Pol due to the low level of expression of this protein, as well as the difficulty of infecting in vitro-cultured cells. The use of insect cell systems was successful for the study of human-HBV replication because this system provides a relatively high level of expression of human HBV Pol and also provides conditions for priming and replication (17, 18, 25, 29). In this report, we focused on identifying proteins that interact with human HBV Pol. Our data enable us to conclude that Hsp60 specifically interacts with human HBV Pol and that this protein participates in the activation of human HBV Pol.
ACKNOWLEDGMENTS
This work was supported by a Genetic Engineering Research Grant, GE98, from the Korea Ministry of Education. Sung Gyoo Park is supported by research fellowship BK21 from the Ministry of Education and Human Resources Development.
We thank Kyung Goo Han and Seung Oe Lim for their assistance.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipma D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Brown W C, Smiley J K, Campbell J L. Purification of DNA polymerase II stimulatory factor I, a yeast single-stranded DNA-binding protein. Proc Natl Acad Sci USA. 1990;87:677–681. doi: 10.1073/pnas.87.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukau B, Horwich A L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 4.Burnett B P, Horwich A L, Low K B. A carboxy-terminal deletion impairs the assembly of GroEL and confers a pleiotropic phenotype in Escherichia coli K-12. J Bacteriol. 1994;176:6980–6985. doi: 10.1128/jb.176.22.6980-6985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho G, Park S G, Jung G. Localization of HSP90 binding sites in the human hepatitis B virus polymerase. Biochem Biophys Res Commun. 2000;269:191–196. doi: 10.1006/bbrc.2000.2240. [DOI] [PubMed] [Google Scholar]
- 6.Cho G, Suh S W, Jung G. HBV polymerase interacts independently with N-terminal and C-terminal fragments of Hsp90beta. Biochem Biophys Res Commun. 2000;274:203–211. doi: 10.1006/bbrc.2000.3119. [DOI] [PubMed] [Google Scholar]
- 7.Dubaquie Y, Looser R, Funfschilling U, Jeno P, Rospert S. Identification of in vivo substrates of the yeast mitochondrial chaperonins reveals overlapping but non-identical requirement for hsp60 and hsp10. EMBO J. 1998;17:5868–5876. doi: 10.1093/emboj/17.20.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edenhofer F, Rieger R, Famulok M, Wendler W, Weiss S, Winnacker E L. Prion protein PrPc interacts with molecular chaperones of the Hsp60 family. J Virol. 1999;70:4724–4728. doi: 10.1128/jvi.70.7.4724-4728.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edman P, Begg G. A protein sequenator. Eur J Biochem. 1967;1:80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- 10.Ganem D, Varmus H E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch R C, Loeb D D, Pollack J R, Ganem D. cis-acting sequences required for encapsidation of duck hepatitis B virus pregenomic RNA. J Virol. 1991;65:3309–3316. doi: 10.1128/jvi.65.6.3309-3316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houry W A, Frishman D, Eckerskorn C, Lottspeich F, Hartl F U. Identification of in vivo substrates of the chaperonin GroEL. Nature. 1999;402:147–154. doi: 10.1038/45977. [DOI] [PubMed] [Google Scholar]
- 13.Hu J, Anselmo D. In vitro reconstitution of a functional duck hepatitis B virus reverse transcriptase: posttranslational activation by hsp90. J Virol. 2000;74:11447–11455. doi: 10.1128/jvi.74.24.11447-11455.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu J, Seeger C. Hsp90 is required for the activity of a hepatitis B virus reverse transcriptase. Proc Natl Acad Sci USA. 1996;93:1060–1064. doi: 10.1073/pnas.93.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu J, Toft D O, Seeger C. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 1997;16:59–68. doi: 10.1093/emboj/16.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junker-Niepmann M, Bartenschlager R, Schaller H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990;9:3389–3396. doi: 10.1002/j.1460-2075.1990.tb07540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanford R E, Kim Y H, Lee H, Notvall L, Beames B. Mapping of the hepatitis B virus reverse transcriptase TP and RT domains by transcomplementation for nucleotide priming and by protein-protein interaction. J Virol. 1999;73:1885–1893. doi: 10.1128/jvi.73.3.1885-1893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanford R E, Notvall L, Beames B. Nucleotide priming and reverse transcriptase activity of hepatitis B virus polymerase expressed in insect cells. J Virol. 1995;69:4431–4439. doi: 10.1128/jvi.69.7.4431-4439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H J, Kwon Y T, Rho H M, Jung G. Expression of hepatitis B virus polymerase gene in E. coli. Biotechnol Lett. 1993;15:821–826. [Google Scholar]
- 20.Lien J M, Petcu D J, Aldrich C E, Mason W S. Initiation and termination of duck hepatitis B virus DNA synthesis during virus maturation. J Virol. 1987;61:3832–3840. doi: 10.1128/jvi.61.12.3832-3840.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason W S, Halpern M S, England J M, Seal G, Egan J, Coates L, Aldrich C, Summers J. Experimental transmission of duck hepatitis B virus. Virology. 1983;131:375–384. doi: 10.1016/0042-6822(83)90505-6. [DOI] [PubMed] [Google Scholar]
- 22.McLennan N F, McAteer S, Masters M. The tail of a chaperonin: the C-terminal region of Escherichia coli GroEL protein. Mol Microbiol. 1994;14:309–321. doi: 10.1111/j.1365-2958.1994.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 23.Molnar-Kimber K L, Summers J W, Mason W S. Mapping of the cohesive overlap of duck hepatitis B virus DNA and of the site of initiation of reverse transcription. J Virol. 1984;51:181–191. doi: 10.1128/jvi.51.1.181-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollack J R, Ganem D. An RNA stem-loop structure directs hepatitis B virus genomic RNA encapsidation. J Virol. 1993;67:3254–3263. doi: 10.1128/jvi.67.6.3254-3263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putlitz J, Lanford R E, Carlson R I, Notvall L, Monte S M, Wands J R. Properties of monoclonal antibodies directed against hepatitis B virus polymerase protein. J Virol. 1999;73:4188–4196. doi: 10.1128/jvi.73.5.4188-4196.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samali A, Cai J, Zhivotovsky B, Jones D P, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. 1999;18:2040–2048. doi: 10.1093/emboj/18.8.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seeger C, Ganem D, Varmus H E. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science. 1986;232:477–484. doi: 10.1126/science.3961490. [DOI] [PubMed] [Google Scholar]
- 28.Seifer M, Hamatake R, Bifano M, Standring D N. Generation of replication-competent hepatitis B virus nucleocapsids in insect cells. J Virol. 1998;72:2765–2776. doi: 10.1128/jvi.72.4.2765-2776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smiley J K, Brown W C, Campbell J L. The 66 kDa component of yeast SFI, stimulatory factor I, is Hsp60. Nucleic Acids Res. 1992;20:4913–4918. doi: 10.1093/nar/20.18.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Summers J, Mason W S. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- 31.Tavis J E, Ganem D. Expression of functional hepatitis B virus polymerase in yeast reveals it to be the sole viral protein required for correct initiation of reverse transcription. Proc Natl Acad Sci USA. 1993;90:4107–4111. doi: 10.1073/pnas.90.9.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavis J E, Ganem D. Evidence for activation of the hepatitis B virus polymerase by binding of its RNA template. J Virol. 1996;70:5741–5750. doi: 10.1128/jvi.70.9.5741-5750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavis J E, Perri S, Ganem D. Hepadnavirus reverse transcription initiates within the stem-loop of the RNA packaging signal and employs a novel strand transfer. J Virol. 1994;68:3536–3543. doi: 10.1128/jvi.68.6.3536-3543.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todd M J, Viitanen P V, Lorimer G H. Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Science. 1994;265:659–666. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- 35.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urban M, McMillan D J, Canning G, Newell A, Brown E, Mills J S, Jupp R. In vitro activity of hepatitis B virus polymerase: requirement for distinct metal ions and the viral epsilon stem-loop. J Gen Virol. 1998;79:1121–1131. doi: 10.1099/0022-1317-79-5-1121. [DOI] [PubMed] [Google Scholar]
- 37.Venner T J, Singh B, Gupta R S. Nucleotide sequences and novel structural features of human and Chinese hamster hsp60 (chaperonin) gene families. DNA Cell Biol. 1990;9:545–552. doi: 10.1089/dna.1990.9.545. [DOI] [PubMed] [Google Scholar]
- 38.Wang G H, Seeger C. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell. 1992;71:663–670. doi: 10.1016/0092-8674(92)90599-8. [DOI] [PubMed] [Google Scholar]
- 39.Xanthoudakis S, Roy S, Rasper D, Hennessey T, Aubin Y, Cassady R, Tawa P, Ruel R, Rosen A, Nicholson D W. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 1999;18:2049–2056. doi: 10.1093/emboj/18.8.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoulim F, Seeger C. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase. J Virol. 1994;68:6–13. doi: 10.1128/jvi.68.1.6-13.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]