Abstract
The urinary normetanephrine to creatinine ratio (uNMN/Cr) and urinary metanephrine to creatinine ratio (uMN/Cr) are commonly evaluated for the diagnosis of pheochromocytomas (PCC) in dogs. This study aimed to establish reference intervals for uNMN/Cr and uMN/Cr in 56 healthy dogs in Japan and to investigate the effect of urine collection methods on these measurements in 15 non-PCC dogs. The upper limits of reference intervals for uNMN/Cr and uMN/Cr were 124.4 nmol/mmol (90% confidence interval [CI] 107.7–137.0) and 121.1 nmol/mmol (90% CI 102.8–145.1), respectively. Both uNMN/Cr and uMN/Cr were significantly higher when urine was collected in the hospital compared to at home. Several factors, including the method of urine collection, should be considered when utilizing these reference intervals.
Keywords: canine, metanephrines, pheochromocytomas, reference interval, urine
Pheochromocytomas (PCCs) are tumors of the chromaffin cells in the adrenal medulla, producing catecholamines such as epinephrine and norepinephrine [6]. Common clinical signs of PCC in dogs include weakness, tachyarrhythmias, tachypnea, anorexia, collapse, and polyuria/polydipsia [1]. However, approximately 50% of cases are nonsymptomatic [1, 8], which makes the diagnosis difficult. Diagnosis of PCC in dogs is based on clinical signs, diagnostic imaging, and increased catecholamine or metanephrine levels in plasma or urine [9, 10, 12, 13, 16,17,18,19]. Metanephrines represent metanephrine and normetanephrine, which are the metabolites of epinephrine and norepinephrine. Previous studies showed that urinary normetanephrine to creatinine ratio (uNMN/Cr) and urinary metanephrine to creatinine ratio (uMN/Cr) were useful for the diagnosis of PCC in dogs [13, 16,17,18]. However, reference intervals for uNMN/Cr and uMN/Cr using healthy controls have not been fully established. Although a recent study proposed the reference intervals for uNMN/Cr and uMN/Cr in dogs [19], the small number of samples was one of the limitations of the study. In addition, the effect of urine collection methods on the measurement of uNMN/Cr and uMN/Cr in diseased dogs has not been adequately evaluated. This study aimed to establish the reference intervals for uNMN/Cr and uMN/Cr using larger samples of healthy dogs in Japan and to clarify the effect of urine collection methods on its measurement in dogs.
Residual urine samples from 56 dogs presented at a primary veterinary hospital for health check-up purposes were used to establish the reference intervals for uNMN/Cr and uMN/Cr. All urine samples were collected at home by the owners, immediately brought to the hospital, and the residual supernatants were then stored at −20°C until analyzed. According to their history, physical examination, complete blood count, biochemistry profile, and urinalyses, all 56 dogs were determined to be healthy. In addition, urine samples from 15 dogs without PCC were used to evaluate the effect of urine collection methods on the measurements of uNMN/Cr and uMN/Cr. These 15 dogs were evaluated at Hokkaido University Veterinary Teaching Hospital under suspicion of PCC or hyperadrenocorticism based on clinical signs and/or imaging findings. For these dogs, urine samples were collected at home and in the hospital. Free-catch urine samples were collected at home by the owners and immediately brought to the hospital. Additionally, urine samples were collected in the hospital using cystocentesis or catheterization when veterinarians considered them necessary for clinical purposes. In 11 of the 15 dogs, urine samples were collected both at home and in the hospital on the same day. For the remaining four dogs, urine samples were collected at home 2 to 6 days after the collection in the hospital. The residual supernatants of these urine samples were stored at −80°C until analyzed. No dogs included in this study were treated with glucocorticoids or medications that affect normetanephrine and metanephrine concentrations. Informed written consent was obtained from all dog owners for the usage of residual urine samples for the study’s purpose.
Free NMN, free MN, and Cr concentrations in urine were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS) as previously described [18], and the ratios of NMN (nmol/L) and MN (nmol/L) to Cr (mmol/L) were calculated. In healthy dogs, the results of the urinary corticoid to creatinine ratio (UCCR) from a part of a previous study were available [14].
The reference intervals for uNMN/Cr and uMN/Cr were determined in 56 healthy dogs using robust methods with the software program Reference Value Advisor V2.1 (downloaded from https://www.biostat.envt.fr/reference-value-advisor/), in accordance with the guidelines of the American Society for Veterinary Clinical Pathology (ASVCP) [5, 7]. All other statistical analyses were performed using JMP Pro version 17.0 (SAS Institute Inc., Cary, NC, USA). The Mann-Whitney U test was used to compare values between groups. Spearman’s rank correlation test was used for determining correlations between values. Wilcoxon signed-rank test was used to compare values between urine collection methods. A P value of less than 0.05 was considered statistically significant.
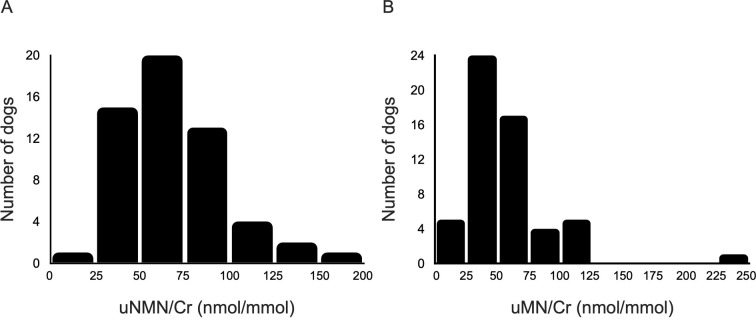
The median age and body weight of the 56 healthy dogs were 4.3 years (range: 0.4–14.7 years) and 5.5 kg (range: 2.1–27 kg), respectively. These dogs consisted of 20 different breeds, with mixed breeds (n=8) and toy poodles (n=8) being the most common. Thirty-four were female (1 intact) and 22 were male (1 intact). The median uNMN/Cr and uMN/Cr were 65.4 (range: 19.6–171.4) and 49.4 (range: 16.4–231.3), respectively, and the distributions of uNMN/Cr and uMN/Cr for these healthy dogs were shown in Fig. 1. Neither uNMN/Cr nor uMN/Cr was significantly different between female and male dogs (P=0.45 and P=0.38, respectively). There was a significant negative correlation between both uNMN/Cr and body weight (rs=−0.37, P=0.005), and uMN/Cr and body weight (rs=−0.41, P=0.002). Additionally, a significant positive correlation was observed between uMN/Cr and age (rs=0.39, P=0.003), and a significant negative correlation between uMN/Cr and plasma creatinine concentrations (n=33, rs=−0.41, P=0.019). Significant positive correlations were detected between uNMN/Cr and UCCR (rs=0.38, P=0.004), and between uMN/Cr and UCCR (rs=0.50, P<0.001). The reference intervals for uNMN/Cr and uMN/Cr in 56 healthy dogs are presented in Table 1. When the healthy dogs were divided into two groups based on body weight (<5 kg and >5 kg), uNMN/Cr in dogs weighing <5 kg was significantly higher than in those weighing >5 kg (P=0.049), while uMN/Cr did not significantly differ between the groups (P=0.059). The upper limits of the reference intervals for uNMN/Cr in dogs weighing <5 kg (n=25) and those weighing >5 kg (n=31) were 148.8 (90% CI: 123.2–170.2) and 102.8 (90% CI: 91.4–112.1), respectively.
Fig. 1.
Distribution of the (A) urinary normetanephrine to creatinine ratio (uNMN/Cr) and (B) urinary metanephrine to creatinine ratio (uMN/Cr) in 56 healthy dogs.
Table 1. Reference intervals for the urinary normetanephrine to creatinine ratio (uNMN/Cr) and urinary metanephrine to creatinine ratio (uMN/Cr) in healthy dogs.
| Initial number of dogs | Number of removed outliers | P-value for symmetry test | Reference interval | Methods | ||
|---|---|---|---|---|---|---|
| Lower limit (90% CI) | Upper limit (90% CI) | |||||
| uNMN/Cr (nmol/mmol) | 56 | 0 | 0.30 | 6.9 (0–20.8) | 124.4 (107.7–137.0) | Robust (untransformed) |
| uMN/Cr (nmol/mmol) | 56 | 1 | 0.038 | 17.9 (15.6–21.2) | 121.1 (102.8–145.1) | Robust (Box-Cox transformed) |
CI, confidence interval.
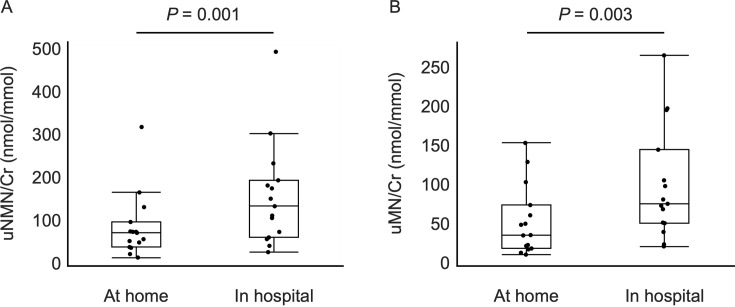
The 15 dogs for which both urine samples collected at home and in the hospital were available had diagnoses including hyperadrenocorticism (n=9), non-functional adrenal tumor (n=4), renal glucosuria (n=1), and dietary hyperlipidemia (n=1). The median age and body weight of these dogs were 12.5 years (range: 3.8–14.9 years) and 5.0 kg (range: 3.04–16.7 kg), respectively. These dogs consisted of 10 different breeds, with mixed breeds (n=3) being the most common. Eight were female (all spayed) and 7 were male (1 intact). The uNMN/Cr obtained from urine sample collected in the hospital was significantly higher than the value obtained from urine sample collected at home (mean difference 71.1, 95% CI 34.3–107.9, P=0.001) as shown in Fig. 2A. Similarly, the uMN/Cr in the hospital was significantly higher than that at home (mean difference 45.2, 95% CI 18.4–72.0, P=0.003), as indicated in Fig. 2B. Three of the uNMN/Cr at home exceeded the upper limit of the reference interval (124.4), while eight of the uNMN/Cr in the hospital exceeded this limit. For uMN/Cr, two samples at home and four in the hospital surpassed the upper limit of the reference interval (121.1).
Fig. 2.
Box and whisker plots of the (A) urinary normetanephrine to creatinine ratio (uNMN/Cr) and (B) urinary metanephrine to creatinine ratio (uMN/Cr) measured using urine collected at home and in hospital.
In this study, we established the reference intervals for uNMN/Cr and uMN/Cr using urine samples collected at home in 56 healthy dogs in Japan. A recent study conducted in the Netherlands with 37 healthy dogs reported that the upper limits of the reference intervals (90% CI) for uNMN/Cr were 97.4 (78.6–120), while those for uMN/Cr were 65.6 (50.1–83.0) [19], which are lower than the results of our study. Although the LC-MS/MS methods used in this study and the previous study differed, measurements of metanephrines using LC-MS/MS are reported to be well harmonized between laboratories in human medicine [15]. Therefore, these discrepancies could be due to differences in the sample numbers and the populations. The median age of the healthy dogs in this study was 4.3 years, comparable to that in the previous study (median 4.6 years) [19]. However, the median body weight was smaller in our dog group (5.5 kg) compared to that in the previous study (19.8 kg) [19]. Since both uNMN/Cr and uMN/Cr had negative correlations with body weight, the inclusion of smaller dogs in this study could explain the higher values of uNMN/Cr and uMN/Cr. Decreased muscle mass and reduced creatinine excretion in smaller dogs may result in increased values of uNMN/Cr and uMN/Cr, similar to UCCR [14, 20]. The uNMN/Cr of dogs weighing <5 kg was significantly higher than that of dogs weighing >5 kg, indicating the need to establish reference intervals based on body weight. Despite the small number of samples in each weight group in this study, weight-specific reference intervals can be valuable for the diagnosis of PCC in Japan.
The methods of collecting urine clearly influenced the values of uNMN/Cr and uMN/Cr. Values measured using urine collected in the hospital were higher than those collected at home, which is consistent with previous studies [11,12,13]. Since diurnal variations in uNMN/Cr and uMN/Cr are unlikely [3, 11], this difference could partly be due to the direct effect of stress on catecholamine production. Additionally, stress-induced production of cortisol can also induce catecholamine production, as glucocorticoids have various effects on catecholamine synthesis and metabolism [2]. Among the 15 non-PCC dogs, four urine samples collected at home exceeded the upper limit of the reference interval for uNMN/Cr and/or uMN/Cr. All four of these dogs were diagnosed with hyperadrenocorticism, a condition associated with increased urinary epinephrines and metanephrines [16]. Interestingly, both uNMN/Cr and uMN/Cr were positively correlated with UCCR in healthy dogs in this study, indicating that even physiological variations in cortisol levels can affect catecholamine levels. In clinical settings, increased values of uNMN/Cr and uMN/Cr measured using urine collected in the hospital can lead to false positive results. In fact, a larger number of samples in this study exceeded the reference intervals when urine collected in the hospital was used, especially for uNMN/Cr. To avoid the stress-induced increase of uNMN/Cr and uMN/Cr, urine should ideally be collected at home.
One limitation of this study was the small number of samples used to establish reference intervals. Although the number of samples in this study was larger than that in a previous study, which included 37 dogs [19], the confidence intervals for the lower and upper limits for uNMN/Cr and the upper limit for uMN/Cr were wide. Specifically, the ratio of the width of the confidence interval to the width of the reference interval (WCI/WRI) was greater than 0.2. Further efforts are needed to establish more reliable reference intervals with a larger number of samples, in accordance with the ASVCP guidelines [5]. Additionally, not all healthy dogs underwent abdominal ultrasound, which may have led to the overlooking of asymptomatic PCC. However, PCC in dogs is relatively rare (0.01–0.1% of all canine tumors) [6] and is typically diagnosed in older dogs (mean age of 10.5–12 years) [1, 4, 8], indicating that the likelihood of healthy dogs in this study having PCC was low.
In conclusion, we established the reference intervals for uNMN/Cr and uMN/Cr using urine collected at home in healthy dogs in Japan. Because several factors, such as body weight, can influence the values, these factors should be taken into account when evaluating the results of uNMN/Cr and uMN/Cr. Additionally, urine collection methods clearly affect the values. We propose that urine collected at home should be used for accurate assessment when applying the reference intervals established in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The authors are grateful to the staff of the Poplar Animal Hospital for generosity in the collection of urine samples. This work was partly supported by JSPS KAKENHI Grant Number 22K05980.
REFERENCES
- 1.Barthez PY, Marks SL, Woo J, Feldman EC, Matteucci M. 1997. Pheochromocytoma in dogs: 61 cases (1984-1995). J Vet Intern Med 11: 272–278. doi: 10.1111/j.1939-1676.1997.tb00464.x [DOI] [PubMed] [Google Scholar]
- 2.Bechmann N, Berger I, Bornstein SR, Steenblock C. 2021. Adrenal medulla development and medullary-cortical interactions. Mol Cell Endocrinol 528: 111258. doi: 10.1016/j.mce.2021.111258 [DOI] [PubMed] [Google Scholar]
- 3.Cameron KN, Monroe WE, Panciera DL, Magnin-Bissel GC. 2010. The effects of illness on urinary catecholamines and their metabolites in dogs. J Vet Intern Med 24: 1329–1336. doi: 10.1111/j.1939-1676.2010.0595.x [DOI] [PubMed] [Google Scholar]
- 4.Enright D, Dickerson VM, Grimes JA, Townsend S, Thieman Mankin KM. 2022. Short- and long-term survival after adrenalectomy in 53 dogs with pheochromocytomas with or without alpha-blocker therapy. Vet Surg 51: 438–446. doi: 10.1111/vsu.13771 [DOI] [PubMed] [Google Scholar]
- 5.Friedrichs KR, Harr KE, Freeman KP, Szladovits B, Walton RM, Barnhart KF, Blanco-Chavez J. American Society for Veterinary Clinical Pathology.2012. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 41: 441–453. doi: 10.1111/vcp.12006 [DOI] [PubMed] [Google Scholar]
- 6.Galac S, Korpershoek E. 2017. Pheochromocytomas and paragangliomas in humans and dogs. Vet Comp Oncol 15: 1158–1170. doi: 10.1111/vco.12291 [DOI] [PubMed] [Google Scholar]
- 7.Geffré A, Concordet D, Braun JP, Trumel C. 2011. Reference Value Advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet Clin Pathol 40: 107–112. doi: 10.1111/j.1939-165X.2011.00287.x [DOI] [PubMed] [Google Scholar]
- 8.Gilson SD, Withrow SJ, Wheeler SL, Twedt DC. 1994. Pheochromocytoma in 50 dogs. J Vet Intern Med 8: 228–232. doi: 10.1111/j.1939-1676.1994.tb03222.x [DOI] [PubMed] [Google Scholar]
- 9.Gostelow R, Bridger N, Syme HM. 2013. Plasma-free metanephrine and free normetanephrine measurement for the diagnosis of pheochromocytoma in dogs. J Vet Intern Med 27: 83–90. doi: 10.1111/jvim.12009 [DOI] [PubMed] [Google Scholar]
- 10.Green BA, Frank EL. 2013. Comparison of plasma free metanephrines between healthy dogs and 3 dogs with pheochromocytoma. Vet Clin Pathol 42: 499–503. doi: 10.1111/vcp.12093 [DOI] [PubMed] [Google Scholar]
- 11.Höglund K, Palmqvist H, Ringmark S, Svensson A. 2022. Quantification of normetanephrine in canine urine using ELISA: evaluation of factors affecting results. J Vet Diagn Invest 34: 28–35. doi: 10.1177/10406387211052984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kook PH, Boretti FS, Hersberger M, Glaus TM, Reusch CE. 2007. Urinary catecholamine and metanephrine to creatinine ratios in healthy dogs at home and in a hospital environment and in 2 dogs with pheochromocytoma. J Vet Intern Med 21: 388–393. doi: 10.1111/j.1939-1676.2007.tb02980.x [DOI] [PubMed] [Google Scholar]
- 13.Kook PH, Grest P, Quante S, Boretti FS, Reusch CE. 2010. Urinary catecholamine and metadrenaline to creatinine ratios in dogs with a phaeochromocytoma. Vet Rec 166: 169–174. doi: 10.1136/vr.b4760 [DOI] [PubMed] [Google Scholar]
- 14.Nagata N, Sawamura H, Morishita K, Hosoya K, Yokoyama N, Sasaoka K, Sasaki N, Nakamura K, Ikenaka Y, Takiguchi M. 2022. Urinary corticoid to creatinine ratios using IMMULITE 2000 XPi for diagnosis of canine hypercortisolism. J Vet Med Sci 84: 954–959. doi: 10.1292/jvms.22-0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peitzsch M, Novos T, Kaden D, Kurlbaum M, van Herwaarden AE, Müller D, Adaway J, Grouzmann E, McWhinney B, Hoad K, Woollard G, Kema I, Boot C, Fassnacht M, Sweep F, Loh TP, Horvath AR, Eisenhofer G. 2021. Harmonization of LC-MS/MS Measurements of Plasma Free Normetanephrine, Metanephrine, and 3-Methoxytyramine. Clin Chem 67: 1098–1112. doi: 10.1093/clinchem/hvab060 [DOI] [PubMed] [Google Scholar]
- 16.Quante S, Boretti FS, Kook PH, Mueller C, Schellenberg S, Zini E, Sieber-Ruckstuhl N, Reusch CE. 2010. Urinary catecholamine and metanephrine to creatinine ratios in dogs with hyperadrenocorticism or pheochromocytoma, and in healthy dogs. J Vet Intern Med 24: 1093–1097. doi: 10.1111/j.1939-1676.2010.0578.x [DOI] [PubMed] [Google Scholar]
- 17.Salesov E, Boretti FS, Sieber-Ruckstuhl NS, Rentsch KM, Riond B, Hofmann-Lehmann R, Kircher PR, Grouzmann E, Reusch CE. 2015. Urinary and plasma catecholamines and metanephrines in dogs with pheochromocytoma, hypercortisolism, nonadrenal disease and in healthy dogs. J Vet Intern Med 29: 597–602. doi: 10.1111/jvim.12569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki N, Ikenaka Y, Inoue Y, Ichise T, Nagata N, Ishizuka M, Nakayama SM, Nakamura K, Takiguchi M. 2021. Urinary free metanephrines measurement in dogs with adrenal gland diseases using a new simple liquid chromatography tandem mass spectrometry method. J Vet Med Sci 83: 648–655. doi: 10.1292/jvms.20-0508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Berg MF, Kooistra HS, Grinwis GCM, van Nimwegen SA, van Faassen M, Kema IP, Teske E, Galac S. 2023. Reference intervals for plasma, urinary, and salivary concentrations of free metanephrines in dogs: Relevance to the diagnosis of pheochromocytoma. J Vet Intern Med 37: 173–183. doi: 10.1111/jvim.16624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeugswetter F, Bydzovsky N, Kampner D, Schwendenwein I. 2010. Tailored reference limits for urine corticoid:creatinine ratio in dogs to answer distinct clinical questions. Vet Rec 167: 997–1001. doi: 10.1136/vr.c4010 [DOI] [PubMed] [Google Scholar]