Abstract
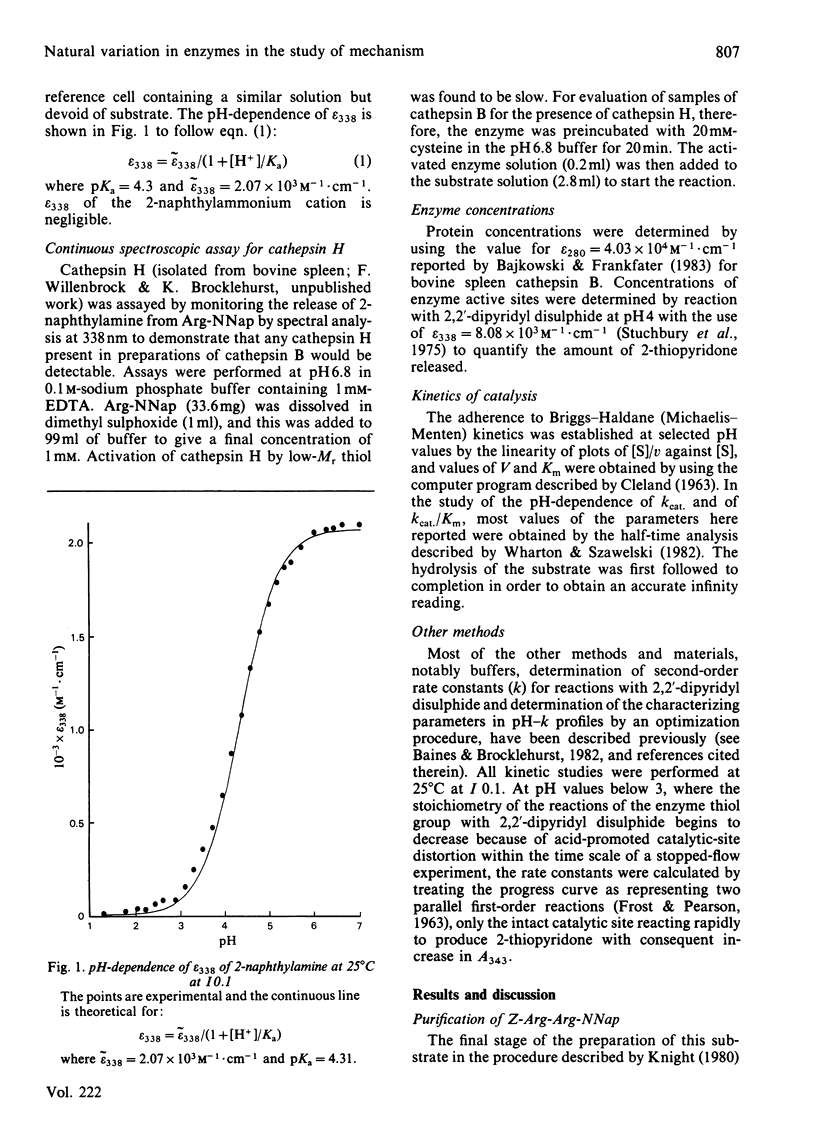
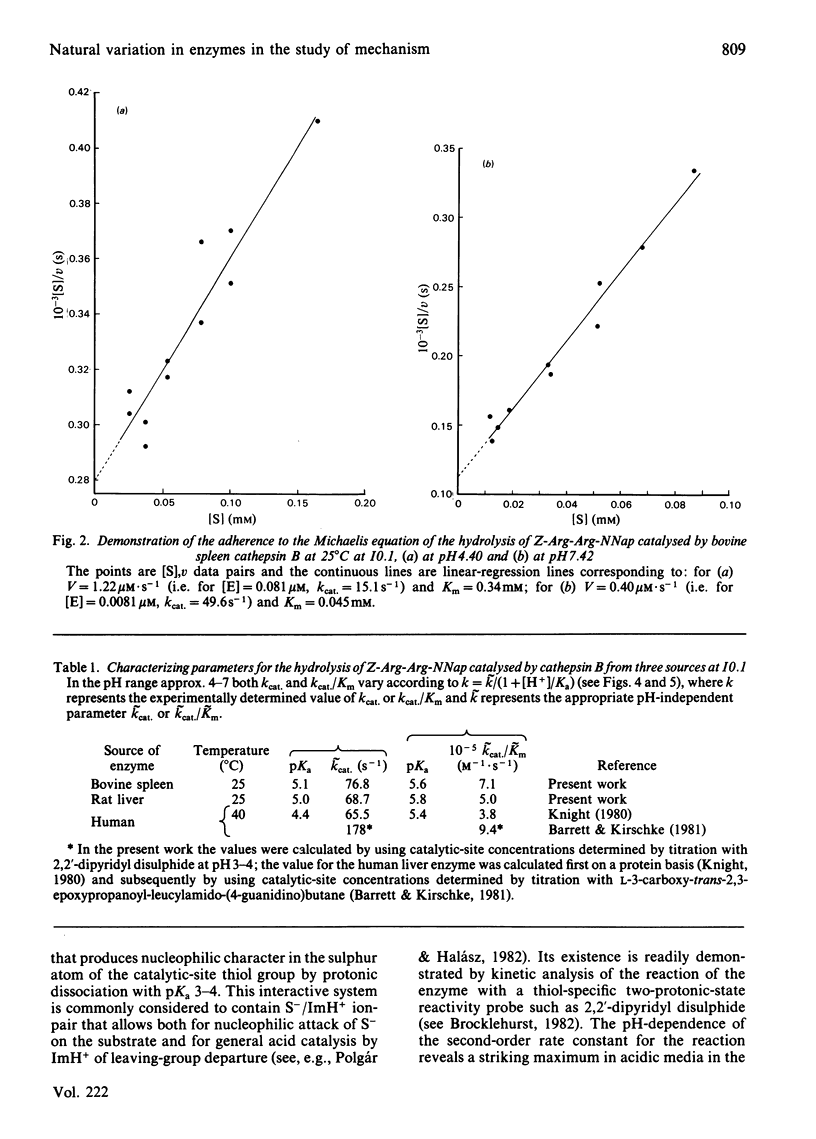
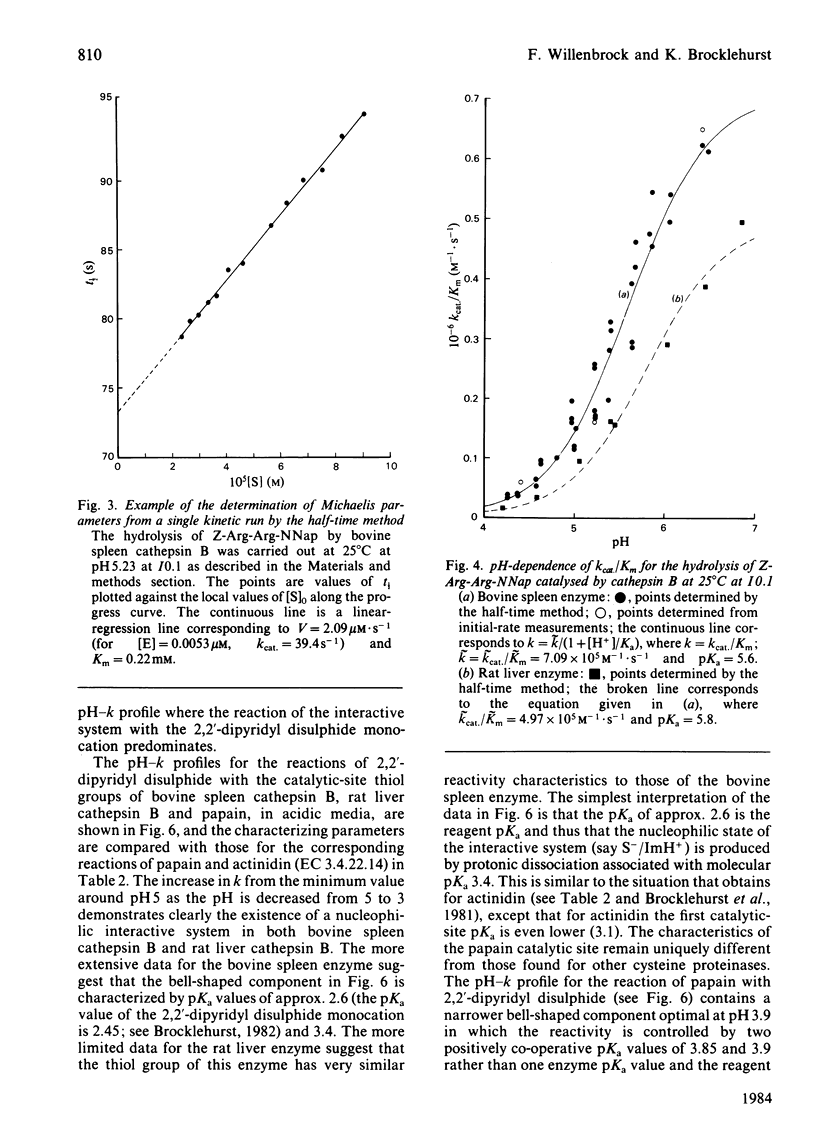
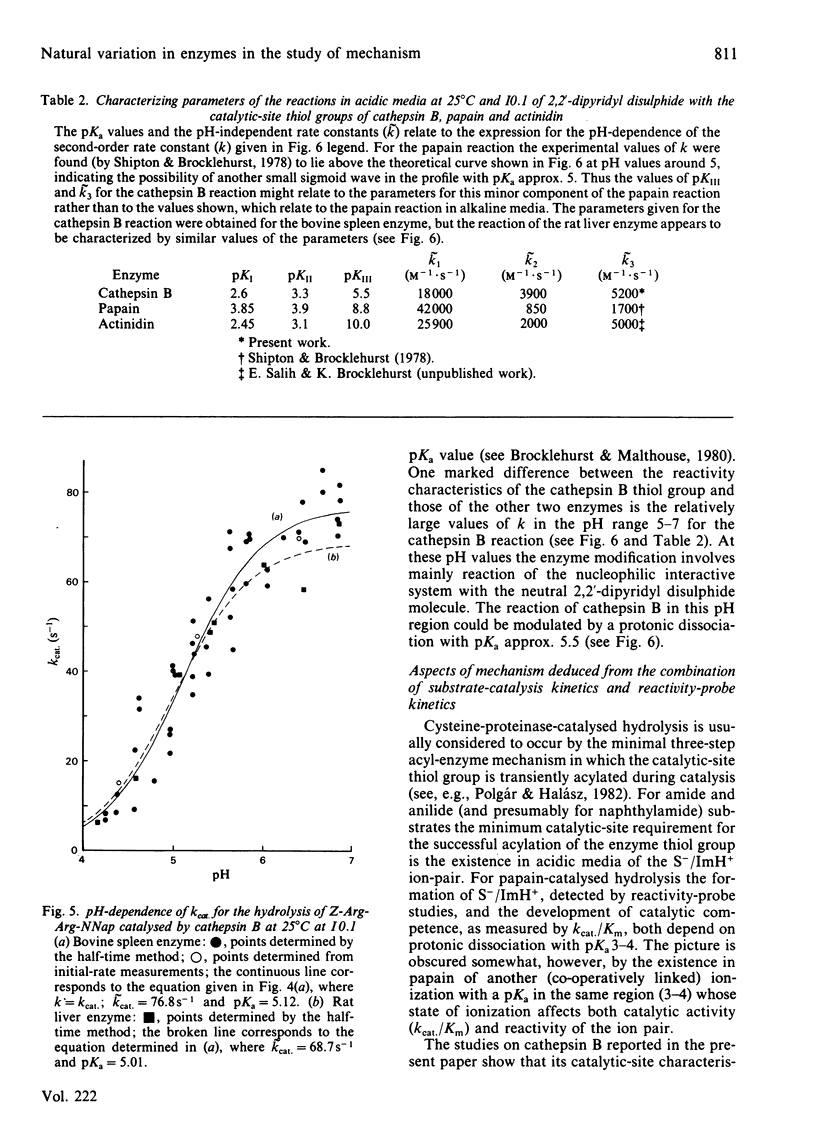
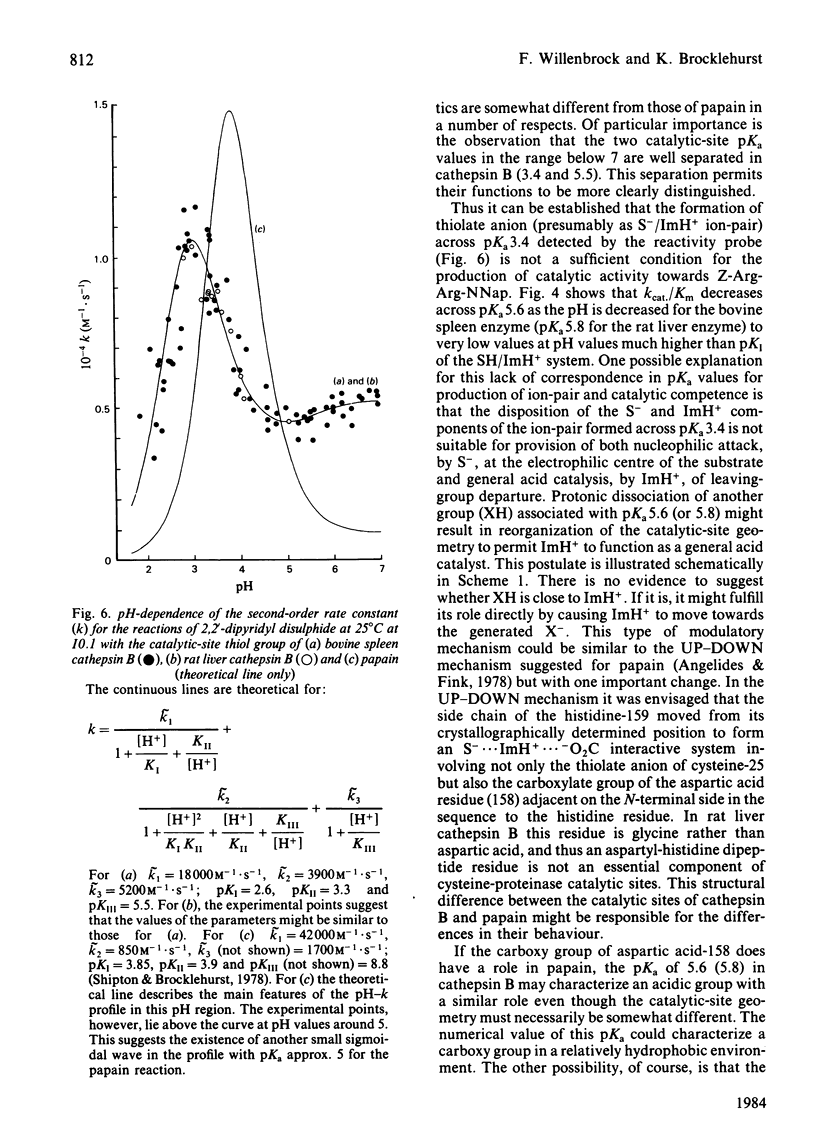
Cathepsin B (EC 3.4.22.1) from bovine spleen and the analogous enzyme from rat liver were investigated at 25 degrees C at I0.1 in acidic media by kinetic study of (a) the reactions of their catalytic-site thiol groups towards the two-protonic-state reactivity probe 2,2'-dipyridyl disulphide and (b) their catalysis of the hydrolysis of N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide. Reactivity-probe kinetics showed that nucleophilic character is generated in the sulphur atom of cathepsin B by protonic dissociation with pKa 3.4, presumably to form an S-/ImH+ ion-pair. Substrate-catalysis kinetics showed that ion-pair formation is not sufficient to generate catalytic competence in cathepsin B, because catalytic activity is not generated as the pH is raised across pKa 3.4 but rather as it is raised across pKa 5-6 (5.1 for kcat; 5.6 for kcat./Km for the bovine spleen enzyme and 5.8 for kcat./Km for the rat liver enzyme). The implications of these results and of known structural differences between the catalytic sites of the rat liver enzyme and papain (EC 3.4.22.2) for the mechanism of cysteine-proteinase-catalysed hydrolysis are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelides K. J., Fink A. L. Cryoenzymology of papain: reaction mechanism with an ester substrate. Biochemistry. 1978 Jun 27;17(13):2659–2668. doi: 10.1021/bi00606a032. [DOI] [PubMed] [Google Scholar]
- Baines B. S., Brocklehurst K. Characterization of papaya peptidase A as a cysteine proteinase of Carica papaya L. with active-centre properties that differ from those of papain by using 2,2'-dipyridyl disulphide and 4-chloro-7-nitrobenzofurazan as reactivity probes. Use of two-protonic-state electrophiles in the identification of catalytic-site thiol groups. Biochem J. 1982 Jul 1;205(1):205–211. doi: 10.1042/bj2050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajkowski A. S., Frankfater A. Steady state kinetic evidence for an acyl-enzyme intermediate in reactions catalyzed by bovine spleen cathepsin B. J Biol Chem. 1983 Feb 10;258(3):1645–1649. [PubMed] [Google Scholar]
- Barrett A. J. A new assay for cathepsin B1 and other thiol proteinases. Anal Biochem. 1972 May;47(1):280–293. doi: 10.1016/0003-2697(72)90302-8. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. An improved color reagent for use in Barrett's assay of Cathepsin B. Anal Biochem. 1976 Nov;76(50):374–376. doi: 10.1016/0003-2697(76)90298-0. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Baines B. S., Malthouse J. P. Differences in the interaction of the catalytic groups of the active centres of actinidin and papain. Rapid purification of fully active actinidin by covalent chromatography and characterization of its active centre by use of two-protonic-state reactivity probes. Biochem J. 1981 Sep 1;197(3):739–746. doi: 10.1042/bj1970739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P. Evidence for a two-state transition in papain that may have no close analogue in ficin. Differences in the disposition of cationic sites and hydrophobic binding areas in the active centres of papain and ficin. Biochem J. 1980 Dec 1;191(3):707–718. doi: 10.1042/bj1910707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P. Mechanism of the reaction of papain with substrate-derived diazomethyl ketones. Implications for the difference in site specificity of halomethyl ketones for serine proteinases and cysteine proteinases and for stereoelectronic requirements in the papain catalytic mechanism. Biochem J. 1978 Nov 1;175(2):761–764. doi: 10.1042/bj1750761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Mushiri S. M., Patel G., Willenbrock F. A marked gradation in active-centre properties in the cysteine proteinases revealed by neutral and anionic reactivity probes. Reactivity characteristics of the thiol groups of actinidin, ficin, papain and papaya peptidase A towards 4,4'-dipyridyl disulphide and 5,5'-dithiobis-(2-nitrobenzoate) dianion. Biochem J. 1983 Mar 1;209(3):873–879. doi: 10.1042/bj2090873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. Two-protonic-state electrophiles as probes of enzyme mechanisms. Methods Enzymol. 1982;87:427–469. doi: 10.1016/s0076-6879(82)87026-2. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- Knight C. G. Human cathepsin B. Application of the substrate N-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide to a study of the inhibition by leupeptin. Biochem J. 1980 Sep 1;189(3):447–453. doi: 10.1042/bj1890447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., LaRue J. N., Wilson I. B. A simple spectrophotometric assay for amino acyl arylamidases (naphthylamidases, aminopeptidases). Anal Biochem. 1971 Jun;41(2):397–401. doi: 10.1016/0003-2697(71)90157-6. [DOI] [PubMed] [Google Scholar]
- Polgár L., Halász P. Current problems in mechanistic studies of serine and cysteine proteinases. Biochem J. 1982 Oct 1;207(1):1–10. doi: 10.1042/bj2070001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Brochlehurst K. Characterization of the papain active centre by using two-protonic-state electrophiles as reactivity probes. Evidence for nucleophilic reactivity in the un-interrupted cysteine-25-histidine-159 interactive system. Biochem J. 1978 May 1;171(2):385–401. doi: 10.1042/bj1710385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Teller D. C., Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towatari T., Kawabata Y., Katunuma N. Crystallization and properties of cathepsin B from rat liver. Eur J Biochem. 1979 Dec;102(1):279–289. doi: 10.1111/j.1432-1033.1979.tb06290.x. [DOI] [PubMed] [Google Scholar]
- Wharton C. W., Szawelski R. J. Half-time analysis of the integrated Michaelis equation. Simulation and use of the half-time plot and its direct linear variant in the analysis of some alpha-chymotrypsin, papain- and fumarase-catalysed reactions. Biochem J. 1982 May 1;203(2):351–360. doi: 10.1042/bj2030351. [DOI] [PMC free article] [PubMed] [Google Scholar]


