Abstract
The metabolic syndrome (MetS) is a group of diseases conceptualized as a clustering of risk factors, with the risks of developing MetS in women varying significantly before and after menopause. This study investigated MetS clustering patterns and their association with cardiovascular disease (CVD) risk among post-menopausal women (n = 2479) using data from the Korean Genome Epidemiology Study. Using latent class analysis, three groups emerged: diabetic (5.6%), hypertensive (35.2%), and low-risk (59.2%). Relative to the low-risk group, the diabetic group demonstrated associations with older age, a family history of chronic disease, an increased Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), an elevated white blood cell (WBC) count, experience with hysterectomy, being a non-drinker, more physical activity, and excessive sleep. The hypertensive group was associated with older age, lower monthly income, a family history of chronic disease, increased HOMA-IR, a higher WBC count, more physical activity, and excessive sleep. The diabetic and hypertensive groups had a significantly higher CVD risk than the low-risk group (diabetic: odds ratio [OR] = 2.41 [1.11, 5.27]; hypertensive: OR = 2.46 [1.33, 4.55]). This study identified potential markers for MetS screening in post-menopausal women, highlighting the need for early intervention and personalized healthcare for middle-aged women to reduce CVD risk following menopause.
Keywords: Metabolic syndrome, Latent class analysis, National Cholesterol Education Program Adult Treatment Panel III, Post-menopause, Cardiovascular disease
Subject terms: Cardiology, Health care, Risk factors
Introduction
Metabolic syndrome (MetS) is diagnosed when an individual exhibits a cluster of conditions including hyperglycemia, abdominal obesity, dyslipidemia, and hypertension. Reaven first coined the term ‘Syndrome X’ to describe this phenomenon, with a common cause among affected patients being a decline in beta cell function due to insulin resistance 1. The primary goal of preventing and treating MetS is to mitigate the morbidity and mortality associated with these conditions 2. The prevalence of MetS in the Republic of Korea has been rising steadily over time, affecting both men and women 3. Consequently, medical expenses and national economic losses due to MetS are expected to increase, highlighting the urgent need for its management4.
Although MetS is a predictor of various diseases, its pathophysiology remains elusive5. Several studies have utilized factor analysis to examine the structural connections between the components of MetS and identify common underlying causes6–15. The literature review findings suggest that between two and four factors can explain the relationships between the components of MetS, indicating that there is no single pathophysiological pathway for its development. However, factor analysis has limitations such as the arbitrariness of factor extraction criteria and the requirement for continuous variables to follow a normal distribution16. Furthermore, it is essential to consider the normal range for each MetS component when applying these results to clinical practice. Latent class analysis (LCA) is a person-centered statistical method that examines non-continuous and categorical variables to classify individuals into distinct groups and create personalized interventions based on their response patterns17.
MetS is acknowledged as a lifestyle disease shaped by factors such as socioeconomic status and health behaviors, with its effects differing according to gender and menopausal status18. Middle-aged women, particularly those who are post-menopausal, face an increased risk of MetS owing to a decline in female sex hormones, leading to heightened vulnerability throughout a considerable portion of their lives19,20. Studies utilizing LCA16,21–24 have typically identified between two and four groups of clustering patterns of MetS components. However, they did not account for the unique characteristics of post-menopausal women, who are particularly vulnerable to MetS owing to hormonal changes unlike women of other age groups. As the risk of developing MetS increases considerably after middle age, particularly in women owing to factors such as menopause19,25, it is essential to closely monitor MetS occurrence and patterns in post-menopausal women.
MetS is associated with an increased risk of cardiovascular disease (CVD), particularly in women26,27. However, research on MetS has primarily focused on the presence or total number of MetS risk factors, which has not led to a profound understanding of the heterogeneous nature of the condition’s development28. Consequently, comprehensive studies are necessary to understand the multifaceted nature of MetS and its intricate relationship with CVD. Given the elevated risk faced by post-menopausal women with MetS, this study aimed to (1) identify the clustering patterns of MetS components among women aged 40 to 69 who were post-menopausal at the time of the baseline survey conducted from 2001 to 2002; (2) pinpoint the characteristics of participants associated with MetS class membership; and (3) explore how the risk of CVD varies across MetS classes.
Methods
We performed a secondary analysis using the dataset from the Korean Genome Epidemiology Study (KoGES), an ongoing population-based cohort study of adults aged 40 to 69 living in Ansan and Anseong provinces in 2000.
Data source
We employed a secondary analysis using KoGES, which comprehensively represents the characteristics of the South Korean population. Initiated by the Korea Centers for Disease Control and Prevention Agency in 2001, KoGES aimed to create a framework and guidelines for monitoring and addressing risk factors for the development of non-communicable diseases in South Koreans. It commenced with a baseline survey from 2001 to 2002, which was subsequently administered every two years for eight waves. Focusing on non-communicable diseases, such as diabetes, hypertension, and CVD, KoGES encompasses a wide array of data, including physical examinations, laboratory analyses (e.g. blood and urine tests), and interviews detailing lifestyle habits and disease history. Given its comprehensive coverage of noncommunicable diseases that are closely linked to MetS and the depth of data pertaining to physiological measurements and lifestyle factors, KoGES is well-suited for fulfilling the present study’s objectives. The dataset is accessible to approved researchers, and detailed information on the cohort profile has been presented elsewhere29.
Participants and sample size
The initial sample consisted of 10,030 adults, of whom 5272 were women aged 40 to 69, living in both rural and urban areas in central South Korea. Written informed consent was obtained from all participants. All methods used in this study were performed by the guidelines and regulations of the Declaration of Helsinki. This study utilized a baseline survey conducted from 2001 to 2002, which had the least missing data and was currently available for research. This study was approved by the Institutional Review Board of Yonsei University Medical Center (IRB #4-2021-0373).
For this analysis, we included women aged 40 to 69 who were not menstruating at the time of the baseline survey. Among 5272 women aged 40 to 69, we classified 3299 as menopausal as they answered ‘No’ to the question ‘Are you currently menstruating?’ Thus, we excluded participants who had been previously diagnosed with CVD at baseline (n = 63), reported current use of female sex hormones at baseline (n = 388), or were unresponsive to the questionnaire (n = 369) from the analysis. Supplementary Table 1 presents the baseline characteristics of the excluded and included participants. Finally, we included 2479 samples in the analysis.
Measures
Measurement and definition of MetS
MetS components were diagnosed using the definition based on the criteria from the 2004 revised version of the National Cholesterol Education Program Adult Treatment Panel III, including the waist circumference (WC) cut-off point suggested by the Korean Society of Obesity to account for the typical features of South Korea’s population. The cut-off points for MetS components were as follows: WC ≥ 85 cm for women, diastolic/systolic blood pressure (DBP/SBP) ≥ 85/130 mmHg, high-density lipoprotein cholesterol (HDL-C) < 50 mg/dL in women, triglycerides (TG) ≥ 150 mg/dL, and fasting blood glucose (FBG) ≥ 100 mg/dL. We considered the medication status for diabetes, hypertension, or hyperlipidemia to be a separate MetS component. Using these eight components, we statistically derived MetS clustering patterns using LCA.
Characteristics considered for LCA
We considered general, biological, fertility-related, and health-related behavioral characteristics to be correlates of MetS latent class membership.
General characteristics included age, marital status, family history, monthly income, profession, and education level. Marital status was a binary variable set based on whether the respondent was married or single. Family history was a binary variable based on whether the participant’s parents or siblings had been diagnosed with hypertension, diabetes, or hyperlipidemia. We categorized monthly income as less or more than 2 million won. We classified homemakers as unemployed, factory workers as manual laborers, farmers as agricultural workers, and people such as office workers, the self-employed, salespeople, and professionals as non-manual laborers. We classified education level as their final level of schooling (high school and below or college and above).
Biological characteristics included an increased Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), white blood cell (WBC) count, and C-reactive protein (CRP). HOMA-IR is a reliable marker for MetS as it can be calculated from the FBG and insulin blood concentrations, as proposed by Matthews et al.30. We assessed CRP levels through a blood test and categorized them as normal if they were below 1 mg/dL and moderately increased if they were 1 mg/dL or above following the standard proposed by Nehring et al.31. We measured WBC count as a continuous variable, determined using a blood test.
Fertility-related characteristics included age at menarche, gravidity, age at first delivery, and history of hysterectomy. In the Republic of Korea, with an average age at menarche of 12.4 years32,33, it was categorized as < 13 years old or 13 years and older. Previous studies34–39 that demonstrated a significant relationship between gravidity and MetS used different methods to process the gravidity variable. To better reflect the distribution of gravidity in our study population (median 5, mean 5.39), we categorized them into three groups: no births, one to five births, and six or more births. A birth was defined as either a live birth or a stillbirth, excluding miscarriages. The age at first delivery, sourced from open-ended baseline survey responses, denotes the age at which a participant first gave birth; we coded it as 0 if they had never been pregnant. We assessed experience with hysterectomy using a ‘yes’ or ‘no’ answer to the question, ‘Have you ever had surgery to remove your uterus (i.e. hysterectomy)?’.
Health behavioral characteristics included smoking, alcohol consumption, physical activity, unrecommended dietary habits, stress, and sleep duration. We categorized smoking status as a non-smoker (‘Never smoked’), former smoker (‘Used to smoke but do not currently smoke’), and current smoker (‘Occasionally smoke’ or ‘Habitually smoke’). Based on a meta-analysis study40, which found that the prevalence of the metabolic syndrome is lowest in women consuming less than 20 g of alcohol per day, we classified individuals who consume less than 20 g of alcohol per day as 'moderate drinkers' and those who consume 20 g or more per day as 'heavy drinkers'. We quantified physical activity using the following:
We assessed unrecommended dietary habits based on three criteria: (1) consuming more than 130 g of carbohydrates, (2) consuming more than 2000 mg of sodium, and (3) consuming less than 20 g of fiber per day, each earning one point41. We measured stress using a 30-question self-assessment tool developed by the Ministry of Health and Welfare, with scores ranging from 0 to 30 and higher scores signifying greater stress42. We assessed sleep duration based on self-reported average daily sleep over the past year, including naps, and categorized it as adequate (6–8 h), insufficient (< 6 h), or excessive (> 8 h) based on National Sleep Foundation's guidelines43 and research findings43–47.
Risk of CVD
For this study, we defined CVD as including myocardial infarction, congestive heart failure, coronary artery disease, peripheral vascular disease, and cerebrovascular disease (stroke, hemorrhage, etc.); CVD occurs in the heart and cerebral blood vessels. We considered CVD to have occurred if a participant responded ‘No’ to the question ‘Have you ever been diagnosed with CVD by a doctor in the past?’ in the 2001–2002 baseline survey and also responded ‘Yes’ at least once during the eight follow-ups to the question ‘Have you been diagnosed with CVD since the last survey [in the past 2 years]?’.
Statistical analysis
LCA is a statistical method used to estimate unobserved heterogeneity within a population; it classifies participants with similar response patterns into groups and identifies their characteristics and differences48. In this study, we employed the bias-adjusted 3-step approach proposed by Asparouhov and Muthén49. Previously, researchers included covariates directly in the same model to identify the class solution, a method known as the one-step approach, but this often led to flawed models50,51. The one-step approach has been replaced with newer methodologies, with the three-step approach now widely recommended49,52. This approach involves first identifying the measurement model, adding covariates separately, and fixing measurement parameters to those from the initial model to achieve more unbiased results51,53. This model consists of the following sequential steps.
Step 1: We determined the optimal number of latent classes by considering various indices, including information criteria, quality of classification, model fit comparisons, latent class proportions, and their theoretical/clinical meaningfulness. We explored the number of latent classes, ranging from two to five; covariates were not included in this step. We used the Akaike information criterion (AIC) , the Bayesian information criterion (BIC), and the sample size-adjusted Bayesian information criterion (SABIC) to assess model fit. We evaluated classification quality using entropy, which ranged from 0 to 1, with higher values denoting lower classification errors54. Additionally, we employed the Lo-Mendell-Rubin-adjusted likelihood ratio test (LMR LRT) and the bootstrap-adjusted likelihood ratio test (BLRT) to determine whether a model with k latent classes performed better than a model with k − 1 latent classes55. Best practices advise careful consideration of each of these indices when selecting the best-fit model.
Step 2: Given the latent class distribution identified in Step 1 and the response patterns of each individual, we estimated the posterior probabilities of membership in specific latent classes using Bayes’ rule49. This approach calibrates the classification uncertainty rate, which is essential when examining covariates to predict latent class membership in Step 3. We assigned individuals to the class classified in Step 1 to which they had the highest estimated probability of belonging.
Step 3: We performed a multinomial logistic regression analysis by incorporating the covariates and correcting the uncertainty classification errors that occurred in Step 2. We performed this step to identify the characteristics that determine class membership. Multinomial regression involves configuring binomial logistic regression models, which predict the probability of an individual belonging to each category relative to the reference group56.
Step 4: We conducted a discrete-time survival analysis to explore the differences in the hazard probability of a CVD event among classes, which is the conditional probability of an event occurring at a particular time for a specific individual57.
We placed more than half of the participants (59.2%) in the low-risk group; they exhibited the lowest risk of MetS. Thus, we set the low-risk group as the reference group. The missing data rate in this study was 1.1%, with the FBG levels and HOMA-IR having the most missing data (5.1%). We used full-information maximum likelihood estimation to minimize the bias caused by missing values58. We used R 4.2.0 and Mplus 7.3 to carry out the analyses mentioned above.
Results
Number of latent clusters
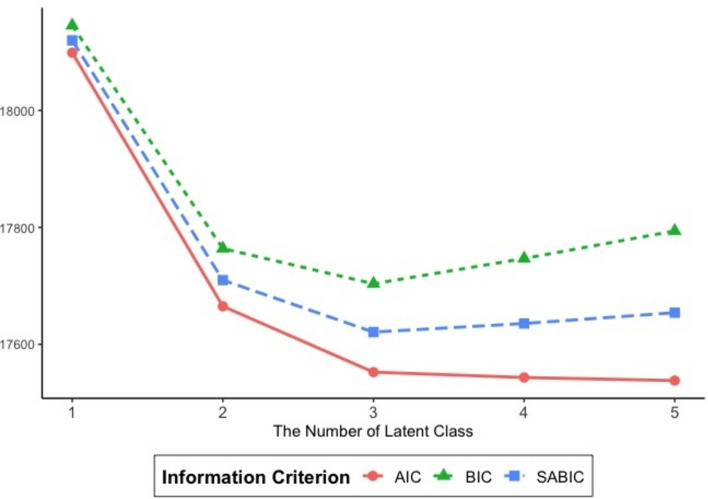
Table 1 lists the statistical criteria used to evaluate model fit, which yielded conflicting outcomes. Comparing the criteria as the number of latent classes increased from 1 to 5, the AIC was lowest when there were 5 latent classes, while the BIC and SABIC were lowest when there were 3 latent classes (Fig. 1). Furthermore, the first insignificant LMR LRT and BLRT appears for 4 and 5 class respectively, indicating that no more than 4 latent classes are required for the entire sample. The 4-class model also had the highest entropy value, which is ideal when close to 1. Researchers are encouraged to examine and report entropy, but not to solely rely on the value for determining the final class solution52. While there is no definitive consensus on the best criteria for determining the optimal number of latent clusters, it is accepted that: (a) the BIC is one of the most reliable measures and should be included50,52, and (b) the final model should be selected based on theoretical interpretability. We used substantive reasons to guide our decision. First, the BIC was the lowest for this model, indicating a better fit and parsimony49,50,52. Second, in similar prior studies16,22, the configuration of MetS components by classes was most similar when using three classes. Therefore, the three-class model was deemed the best fit for this analysis.
Table 1.
Comparison of different latent classes based on model selection statistics.
| Number of latent classes | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Information criterion | AIC | 18,098.83 | 17,664.99 | 17,552.36 | 17,543.22 | 17,538.02 |
| BIC | 18,145.36 | 17,763.85 | 17,703.56 | 17,746.76 | 17,793.90 | |
| SABIC | 18,119.94 | 17,709.84 | 17,620.96 | 17,635.56 | 17,654.10 | |
| Model comparison | LMR LRT | 445.51*** | 128.80*** | 26.76 | 22.88 | |
| BLRT | −9041.42*** | −8815.49*** | −8750.18*** | −8736.60 | ||
| Entropy | 0.454 | 0.675 | 0.774 | 0.742 | ||
| Latent class proportions (%) | 1 | 40.9 | 5.6 | 5.7 | 2.8 | |
| 2 | 59.1 | 35.2 | 10.9 | 3.4 | ||
| 3 | 59.2 | 56.9 | 5.7 | |||
| 4 | 26.5 | 35.9 | ||||
| 5 | 52.2 | |||||
AIC Akaike information criterion, BIC Bayesian information criterion, SABIC sample-adjusted Bayesian information criterion, BLRT bootstrap adjusted likelihood ratio test, LMR-LRT Lo-Mendell-Rubin adjusted likelihood ratio test.
*p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 1.
Information criterion based on the number of latent classes. AIC Akaike information criterion, BIC Bayesian information criterion, SABIC sample size-adjusted BIC.
Distribution of MetS components among the participants
The participants exhibited a mean WC of 84.41 ± 9.39 cm and a mean FBG level of 85.70 ± 18.08 mg/dL. SBP averaged 126.61 ± 19.84 mmHg, while DBP averaged 81.66 ± 11.59 mmHg. The HDL-C levels had a mean of 45.04 ± 10.07 mg/dL, and the TG levels had a mean of 162.00 ± 95.72 mg/dL. Regarding medication use, 5.8% (143) of participants were taking anti-diabetic medications, 19.2% (476) were using anti-hypertensive medications, and 0.5% (12) were taking lipid-lowering medications (Table 2).
Table 2.
Distribution of metabolic syndrome components among the participants (N = 2479).
| Components (variables) | N (%) or M ± SD | |
|---|---|---|
| High WC | (cm) | 84.41 ± 9.39 |
| High FBG | (mg/dL) | 85.70 ± 18.08 |
| High DBP/SBP | (mmHg) | 81.66 ± 11.59/126.61 ± 19.84 |
| Low HDL-C | (mg/dL) | 45.04 ± 10.07 |
| High TG | (mg/dL) | 162.00 ± 95.72 |
| Taking anti-diabetic medication | No | 2333 (94.1) |
| Yes | 143 (5.8) | |
| Taking anti-hypertensive medication | No | 1991 (80.3) |
| Yes | 476 (19.2) | |
| Taking lipid lowering medication | No | 2467 (99.5) |
| Yes | 12 (0.5) |
DBP diastolic blood pressure, FBG fasting blood glucose, HDL-C high-density lipoprotein cholesterol, M mean, SBP systolic blood pressure, SD standard deviation, TG triglycerides, WC waist circumference.
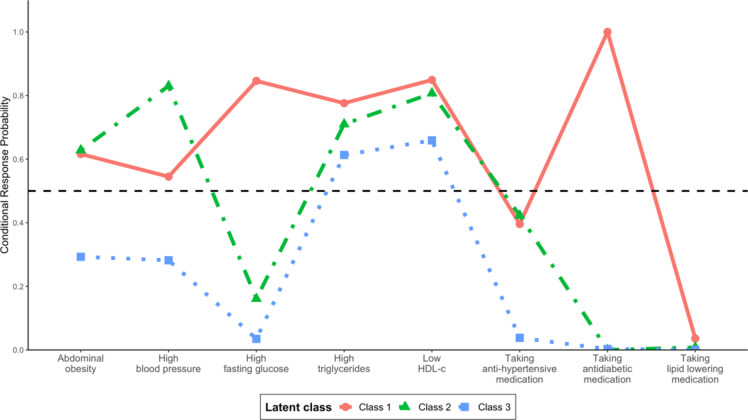
Probability of MetS components in each latent class
Table 3 and Fig. 2 present the distribution and characteristics of each class based on the conditional probabilities for each component. The first class (diabetic) comprised 5.6% of participants and was the most likely to take anti-diabetic medication (100.0%), followed by a decreasing likelihood of low HDL-C levels (84.9%), high FBG (84.6%), high TG (77.6%), high WC (61.6%), and high BP (54.5%). The second class (hypertension) comprised 35.2% of participants. Individuals in this class were most likely to have high BP (83%), followed by a decreased likelihood of having low HDL-C (80.7%), high TG (71%), and high WC (62.8%). The largest class (low-risk) included 59.2% of participants who were most likely to have low HDL-C levels (65.9%), followed by a declining likelihood of having high TG levels (61.3%).
Table 3.
Response probability of metabolic syndrome components for each latent class.
| Components (variables) | Latent classes | |||
|---|---|---|---|---|
| Diabetic (class 1) | Hypertensive (class 2) | Low-risk (class 3) | ||
| Prevalence | N (%) | 142 (5.6) | 803 (35.2) | 1534 (59.2) |
| High WC | No | 0.38 | 0.37 | 0.71 |
| Yes | 0.62 | 0.63 | 0.29 | |
| High FBG | No | 0.15 | 0.84 | 0.97 |
| Yes | 0.85 | 0.16 | 0.03 | |
| Taking anti-diabetic medication | No | 0.00 | 1.00 | 1.00 |
| Yes | 1.00 | 0.00 | 0.00 | |
| High BP | No | 0.45 | 0.17 | 0.72 |
| Yes | 0.55 | 0.83 | 0.28 | |
| Taking anti-hypertensive medication | No | 0.60 | 0.58 | 0.96 |
| Yes | 0.40 | 0.42 | 0.04 | |
| Low HDL-C | No | 0.15 | 0.19 | 0.34 |
| Yes | 0.85 | 0.81 | 0.66 | |
| High TG | No | 0.22 | 0.29 | 0.39 |
| Yes | 0.78 | 0.71 | 0.61 | |
| Taking lipid lowering medication | No | 0.96 | 0.99 | 1.00 |
| Yes | 0.04 | 0.01 | 0.00 | |
BP blood pressure, FBG fasting blood glucose, HDL-C high-density lipoprotein cholesterol, TG triglycerides, WC waist circumference.
Values in bold indicate response probabilities higher than 0.5.
Fig. 2.
Response probability of metabolic syndrome components in each latent class.
Characteristics associated with MetS class membership
Table 4 shows the results of the conditional model, incorporating covariates and employing a 3-step bias correction method. Setting the low-risk group as the reference, older age (Relative risk ratio [RRR] = 1.12, p < 0.001), a family history of chronic disease (RRR = 4.01, p < 0.001), a higher HOMA-IR (RRR = 4.76, p < 0.001), a higher WBC count (B = 1.19, p = 0.007), experience with hysterectomy (RRR = 2.59, p = 0.003), being a non-drinker (moderate: RRR = 0.48, p = 0.041; heavy: RRR = 0.06, p < 0.001), increased physical activity (RRR = 1.01, p = 0.006), and excessive sleep (RRR = 1.89, p = 0.009) led to greater odds of being in the diabetic group than the low-risk group.
Table 4.
Characteristics associated with metabolic syndrome class membership (ref. low-risk group).
| Variables | Category | Diabetic | Hypertensive | ||||
|---|---|---|---|---|---|---|---|
| B | RRR | p | B | RRR | p | ||
| Age | 0.11 | 1.12 | < .001 | 0.13 | 1.14 | < .001 | |
| Marital status | (Ref. single/separated) | ||||||
| Married | −0.20 | 0.82 | .467 | 0.07 | 1.07 | .795 | |
| Monthly income | (Ref. < ₩ 2 million) | ||||||
| ≥ ₩ 2 million | −0.45 | 0.34 | .185 | −0.78 | 0.46 | .017 | |
| Type of profession | (Ref. unemployed) | ||||||
| Manual laborer | −0.44 | 0.64 | .088 | 0.04 | 1.04 | .876 | |
| Non-manual laborer | −0.68 | 0.50 | .094 | −0.50 | 0.61 | .163 | |
| Education level | (Ref. high school and below) | ||||||
| College and above | −1.09 | 0.34 | .135 | −2.33 | 0.10 | .067 | |
| Family history | (Ref. no) | ||||||
| Yes | 1.39 | 4.01 | < .001 | 1.25 | 3.49 | < .001 | |
| HOMA-IR | 1.56 | 4.76 | < .001 | 1.47 | 4.37 | < .001 | |
| CRP | (Ref. normal) | ||||||
| Abnormal | 0.60 | 1.82 | .370 | 0.61 | 1.84 | .353 | |
| WBC | (Thous/Ul) | 0.18 | 1.19 | .007 | 0.20 | 1.22 | .003 |
| Age at menarche | (Ref. under 13 years old) | ||||||
| 13 years or older | −0.10 | 0.91 | .669 | 0.06 | 1.06 | .781 | |
| Gravidity (# births) | (Ref. none) | ||||||
| 1 to 5 | −1.57 | 0.21 | .339 | −0.62 | 0.54 | .781 | |
| 6 and over | 0.27 | 1.30 | .303 | −0.25 | 0.78 | .615 | |
| Age at first delivery | −0.08 | 0.92 | .053 | −0.03 | 0.97 | .401 | |
| Experience with hysterectomy | (Ref. no) | ||||||
| Yes | 0.95 | 2.59 | .003 | 0.40 | 1.48 | .247 | |
| Smoking | (Ref. non-smoker) | ||||||
| Former smoker | −0.30 | 0.74 | .811 | 0.55 | 1.73 | .424 | |
| Current smoker | −1.86 | 0.16 | .163 | 0.09 | 1.09 | .883 | |
| Alcohol consumption | (Ref. non-drinker) | ||||||
| Former drinker | −1.94 | 0.14 | .107 | −0.08 | 0.93 | .923 | |
| Moderate drinker | −0.72 | 0.48 | .041 | 0.00 | 1.00 | .992 | |
| Heavy drinker | −2.81 | 0.06 | < .001 | 1.49 | 4.45 | .094 | |
| Physical activity | (kcal/per week) | 0.01 | 1.01 | .006 | 0.01 | 1.01 | .006 |
| Stress | −0.01 | 0.99 | .623 | −0.02 | 0.99 | .414 | |
| Unrecommended dietary habits | 0.32 | 01.38 | .201 | −0.08 | 0.92 | .703 | |
| Sleep duration | (Ref. adequate) | ||||||
| Insufficient | 0.07 | 1.07 | .835 | 0.01 | 1.01 | .984 | |
| Excessive | 0.64 | 1.89 | .009 | 0.47 | 1.59 | .045 | |
CRP C-reactive protein, HOMA-IR homeostatic model assessment for insulin resistance, WBC white blood cell, RRR relative risk ratio.
Those who were older (RRR = 1.14, p < 0.001) had an average monthly income of less than 2 million won (RRR = 0.46, p = 0.017), a family history of chronic disease (RRR = 3.49, p < 0.001), a higher HOMA-IR (RRR = 4.37, p < 0.001), a higher WBC count (RRR = 1.22, p = 0.003), increased physical activity (RRR = 1.01, p = 0.006), and excessive sleep (RRR = 1.59, p = 0.043) were more likely to be in the hypertensive group than the low-risk group.
Risk of CVD by MetS class
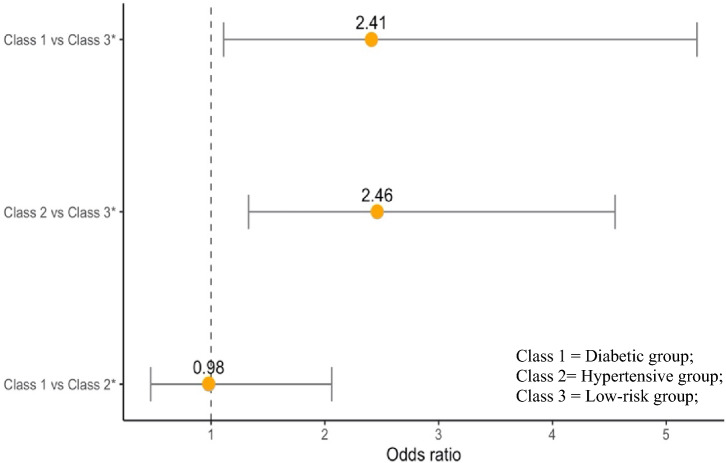
Of the 2479 participants,121 participants (4.9%) developed CVD. We calculated the probability of developing CVD based on the MetS classes while accounting for classification errors. Table 5 displays the risk of CVD according to the MetS class. CVD was significantly more likely to occur in the diabetic group (odds ratio [OR] = 2.46, p = 0.027) and hypertensive group (OR = 2.41, p = 0.004) than in the low-risk group. However, there was no significant difference in the risk of CVD between the diabetic and hypertensive groups (OR = 0.98, p = 0.959) (Fig. 3).
Table 5.
Comparing the risk of cardiovascular disease by metabolic syndrome latent classes.
| Comparison | χ2 | OR [95% CI] | p | |
|---|---|---|---|---|
| Class 1 | Class 3* | 8.30 | 2.41 [1.11, 5.27] | .004 |
| Class 2 | Class 3* | 4.89 | 2.46 [1.33, 4.55] | .027 |
| Class 1 | Class 2* | 0.00 | 0.98 [0.47, 2.06] | .959 |
CI confidence interval, OR odds ratio.
Class 1 = diabetic group, Class 2 = hypertensive group, Class 3 = low-risk group.
*Reference group.
Fig. 3.
Odds ratio for cardiovascular disease by metabolic syndrome latent class.
Discussion
We identified the clustering patterns of MetS components in post-menopausal women in South Korea and explored the characteristics associated with MetS class membership. Moreover, we examined the hazard probability of CVD according to three MetS classes. Our findings highlight the need for intervention programs targeting MetS in post-menopausal women and underscore the importance of CVD prevention initiatives.
We identified three MetS classes: diabetes (5.6%), hypertension (35.2%), and low-risk (59.2%). Studies16,18–21 that identified similar MetS clustering patterns reported varying numbers of classes, ranging from two to four. These discrepancies likely stem from differences in the study populations, MetS definitions, and the factors considered. Although our study provides valuable insights into effective strategies for preventing MetS in post-menopausal women, further research is required to validate whether the characteristics of the derived classes hold in diverse populations.
Unlike previous studies, we did not identify a latent class of healthy participants who did not meet the MetS criteria. Among the 1524 participants in the low-risk group, only 100 had no components that fit the MetS diagnostic criteria. The differentiation observed may be linked to menopause-related hormonal shifts, such as reduced estrogen and heightened progesterone, which lead to changes in fat distribution, muscle loss, and a rise in metabolic risk factors59.
The diabetic group, comprising only 5.6% of participants, is of particular note because every individual in this group was actively prescribed anti-diabetic medications. Ahanchi, et al.22 also recognized a congruent clustering pattern based on the use of anti-diabetic medications. The diabetic group showed clear metabolic changes associated with insulin resistance, which leads to conditions such as hyperglycemia and diabetes, concurrently resulting in suboptimal absorption of free fatty acids and consequently fostering increased TG production within hepatocytes5,60. Furthermore, the accumulation of surplus TG impairs lipoprotein lipase activity, hindering the hydrolysis of TG and impeding the production of active HDL-C5. The emergence of hyperinsulinemia, an adaptive response to insulin resistance, contributes to the development of hypertension by intensifying salt retention and elevating cytosolic Ca2+ levels in the kidneys9. Consequently, the diabetic group served as a clear representation of the metabolic changes associated with insulin resistance.
The hypertensive group (comprising 35.2% of participants) exhibited a higher prevalence of hypertension, which previous studies identified as an independent factor for MetS20. Menopause—marked by decreased estrogen levels and increased arterial stiffness, sodium sensitivity, and fat accumulation—leads to higher blood pressure in middle-aged women61. Women should be made aware of the risk of developing post-menopausal hyperglycemia or hypertension and encouraged to make lifestyle changes well before menopause.
The low-risk group, representing 59.2% of participants, exhibited notably elevated occurrences of low HDL-C (65.9%) and high TG (61.3%). These observations are associated with decreased estrogen levels during menopause, leading to unfavourable changes in HDL-C subtypes, specifically a decrease in HDL-2 and an increase in HDL-3, along with elevated TG levels. Furthermore, a substantial percentage of individuals with diabetes and hypertension displayed coexisting dyslipidemia, which is consistent with the high prevalence of low HDL-C and high TG levels across all groups. Post-menopausal women are especially susceptible to dyslipidemia due to hormonal changes. However, awareness and treatment rates for dyslipidemia lag behind those for other chronic illnesses. It is imperative to emphasize early dyslipidemia prevention in middle-aged women through community-based health services and regular screenings to prevent CVD. Implementing a mandatory lipid screening system or providing accessible lipid testing devices for middle-aged women could improve the awareness, treatment, and control of dyslipidemia.
Among general characteristics, age, monthly income, and family history distinguished the low-risk group from the diabetic and hypertensive groups. Biological aging lowers glucose tolerance and insulin secretion, which can lead to atherosclerosis; however, this can be mitigated by addressing obesity and promoting a healthy lifestyle62. It is also essential to proactively identify patients with a family history of chronic disease and consider those with such a history as potential targets for MetS management interventions—even if they are currently healthy. Monthly income distinguished low-risk individuals from the hypertensive group, which is consistent with studies that demonstrated an inverse linear relationship between household income and the prevalence of hypertension63,64. These findings imply that there may be socioeconomic disparities in access to primary prevention interventions among middle-aged women.
Among the biological characteristics, HOMA-IR and WBC counts were correlated with MetS classes, indicating a link between insulin resistance, inflammation, and MetS. Oda and Kawai65 suggested that cytokine secretion from mast cells mediates systemic inflammatory responses and contributes to MetS development by stimulating the synthesis of inflammatory markers. Given that HOMA-IR and WBC count are fairly easy to measure, these markers could serve as cost-effective indicators for early MetS diagnosis.
Sleep duration, a health-related behavioral characteristic, distinguished the low-risk group from the other two groups. Studies have shown varying effects of sleep duration on MetS, particularly in women, although the findings regarding the relationship between sleep duration and MetS have been inconsistent45,46,66. Prolonged sleep can have adverse health outcomes including sleep fragmentation, increased fatigue due to poor sleep quality, reduced exposure to sunlight, and physical inactivity. Women are more susceptible to sleep disruption than men owing to fluctuations in estrogen and estradiol levels during menopause, which are often accompanied by hypersomnia. Incorporating sleep duration assessments into MetS management could help identify individuals at high risk of developing MetS and determine the need for sleep-related interventions.
Among fertility-related characteristics, experience with hysterectomy differentiated the low-risk and diabetic groups. A study67 showed that women with a history of hysterectomy had higher abdominal fat tissue volumes and weights than those without such a history. Including fertility-related questions in women’s health questionnaires could help identify individuals at risk of diabetes and guide preventive interventions. Compared to men, women experience more severe outcomes of MetS39, and post-menopausal women show an increase in prevalence owing to hormonal changes and pregnancy experiences61. Specifically, fertility-related characteristics such as age of menarche, gravidity, age of first delivery, and hysterectomy status have been reported as risk factors for MetS in previous studies 34–38,67,68. In this study, only hysterectomy status influenced MetS patterns, aligning with findings that other fertility-related characteristics showed no significant correlation with MetS after adjusting for confounders like health behaviors39.
Alcohol consumption was used to distinguish between the low-risk and diabetic groups, with heavy drinkers being more likely to belong to the low-risk group. While a meta-analysis40 indicated a lower prevalence of MetS among drinkers, especially those with moderate alcohol intake, we found that heavy drinkers had the lowest likelihood of being in the diabetic group.
Physical activity also served as a distinguishing factor between the low-risk and other groups; those who were physically active were more likely to be in the diabetic or hypertensive groups. This could be owing to reverse causation, as those at a higher risk of these conditions might engage more often in health management behaviors, leading to increased physical activity, which is commonly the initial recommended lifestyle change for managing diabetes and hypertension68.
We compared the rates at which individuals in the three MetS classes experienced cardiovascular events. The diabetic and hypertensive groups were considerably more likely to experience a cardiovascular event than the low-risk group; however, there was no statistically significant difference between the diabetic and hypertensive groups.
Riahi et al.16 identified three distinct groups for both genders and found considerable differences in CVD risk between the healthy and MetS groups but not between the healthy and low-risk groups. This comparison suggests that MetS risk factor combinations vary based on the characteristics of the population, implying that a MetS management manual should consider both the specific traits of individuals and combinations of their risk factors.
We found that dyslipidemia, marked by low HDL-C and high TG levels, was common across all classes, denoting an increased CVD risk when coupled with other factors such as diabetes and hypertension. However, neither low HDL-C nor high TG levels alone increased this risk considerably, underlining the role of early dyslipidemia in reducing CVD risk24. Additionally, as the prevalence of dyslipidemia is notably higher in post-menopausal women, who face a greater CVD risk from dyslipidemia than men20, lifestyle interventions and frequent screenings for the prevention of concurrent manifestation of dyslipidemia, diabetes, and hypertension after menopause could help lower the incidence of CVD.
It is important to recognize this study’s strengths and limitations. Utilizing the most comprehensive cohort dataset in the Republic of Korea enhanced the representativeness of our results and allowed for the identification of MetS clustering patterns and related factors in an understudied population. However, the limitations of LCA should be acknowledged, including its challenge in detecting rare latent classes in small sample sizes and its data-driven approach. Moreover, relying on baseline survey data for MetS onset identification and related factors, while minimizing missing data, hinders the examination of pre- and post-menopausal transitions and changes in MetS classes over time. Caution is warranted when estimating temporal relationships and when carefully considering potential reverse causality. Additionally, there exist a potential recall or social desirability bias from using self-reported questionnaires. Future research should supplement these with objective measures like electronic medical records. Moreover as this study utilized secondary data, variables such as abortion and breastfeeding that were not included in the dataset could not be analyzed, making it difficult to control for residual confounding. It is crucial to consider these limitations when interpreting the results. Future research should address these constraints to deepen our understanding of the complex relationships between MetS, its components, and related health risks.
Conclusion
We utilized LCA to delineate MetS clustering patterns in post-menopausal women, categorizing them into diabetic, hypertensive, and low-risk groups based on a range of characteristics, including general, biological, fertility-related, and health-related behaviors. We employed a bias-adjusted 3-step approach to ascertain features linked to class membership. This study highlights the need for specialized MetS management programs in healthcare that cater to post-menopausal women. By comparing CVD risk across MetS classes and identifying high-risk groups, this study provides a framework for targeted screenings. These findings suggest that programs designed to reflect the characteristics and CVD risk factors in post-menopausal women can play a vital role in helping middle-aged women recognize health issues and establish actionable goals to enhance their self-management abilities.
Supplementary Information
Abbreviations
- AIC
Akaike information criterion
- BLRT
Bootstrap-adjusted likelihood ratio test
- LMR LRT
Lo-Mendell-Rubin-adjusted likelihood ratio test
- SABIC
Sample size-adjusted Bayesian information criterion
- BIC
Bayesian information criterion
- BP
Blood pressure
- CI
Confidence interval
- CRP
C-reactive protein
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- FBG
Fasting blood glucose
- HDL-C
High-density lipoprotein cholesterol
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- KoGES
Korean Genome Epidemiology Study
- LCA
Latent class analysis
- M
Mean
- MetS
Metabolic syndrome
- OR
Odds ratio
- RRR
Relative risk ratio
- SBP
Systolic blood pressure
- SD
Standard deviation
- TG
Triglycerides
- WBC
White blood cell
- WC
Waist circumference
Author contributions
GSK supervised the research process and developed the concept and design of the study. YJC acquired the participants and/or data, analysed the data, and prepared the original draft. GSK, YJC, SHC, KHL, CGP, and MSS reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
Data availability
The data supporting the findings of this study are available from the Korean Genome Epidemiology Study (KoGES). Restrictions apply to the availability of the data, which were used under a licence for this study. Any additional information required to reanalyze the data reported in this paper is available from KoGES epidemiological data online sharing system (https://nih.go.kr/ko/main/contents.do?menuNo=300566) with permission.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72403-1.
References
- 1.Reaven, G. M. Role of insulin resistance in human disease. Diabetes37, 1595–1607. 10.1016/s0899-9007(96)00380-2 (1988). [DOI] [PubMed] [Google Scholar]
- 2.Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation112, 2735–2752. 10.1016/s0084-3741(08)70316-0 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Huh, J. H., Kang, D. R., Kim, J. Y. & Koh, K. K. Metabolic syndrome fact sheet 2021: Executive report. Cardiometab Syndr. J.1, 125–134. 10.51789/cmsj.2021.1.e15 (2021). [Google Scholar]
- 4.Scholze, J. et al. Epidemiological and economic burden of metabolic syndrome and its consequences in patients with hypertension in Germany, Spain and Italy; A prevalence-based model. BMC Public Health10, 1–12. 10.1186/1471-2458-10-529 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckel, R. H., Alberti, K., Grundy, S. M. & Zimmet, P. Z. The metabolic syndrome. Lancet375, 181–183. 10.1016/s0140-6736(09)61794-3 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Esteghamati, A., Zandieh, A., Khalilzadeh, O., Meysamie, A. & Ashraf, H. Clustering of metabolic syndrome components in a Middle Eastern diabetic and non-diabetic population. Diabetol. Metab. Syndr.2, 1–8. 10.1186/1758-5996-2-36 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson, T. F., Funkhouser, E. & Roseman, J. Factor analysis of metabolic syndrome components in the Coronary Artery Risk Development in Young Adults (CARDIA) study: Examination of factors by race-sex groups and across time. Ann. Epidemiol.20, 194–200. 10.1016/j.annepidem.2009.11.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford, E. S. Factor analysis and defining the metabolic syndrome. Ethn. Dis.13, 429–437 (2003). [PubMed] [Google Scholar]
- 9.Gray, R. S. et al. Risk factor clustering in the insulin resistance syndrome: The Strong Heart Study. Am. J. Epidemiol.148, 869–878. 10.1093/oxfordjournals.aje.a009712 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Hanley, A. J. et al. Factor analysis of metabolic syndrome using directly measured insulin sensitivity: The Insulin Resistance Atherosclerosis Study. Diabetes51, 2642–2647. 10.2337/diabetes.51.8.2642 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Lehto, S., Rönnemaa, T., Pyörälä, K. & Laakso, M. Cardiovascular risk factors clustering with endogenous hyperinsulinaemia predict death from coronary heart disease in patients with type II diabetes. Diabetologia43, 148–155. 10.1007/s001250050023 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Maison, P., Byrne, C. D., Hales, C. N., Day, N. E. & Wareham, N. J. Do different dimensions of the metabolic syndrome change together over time?: Evidence supporting obesity as the central feature. Diabetes Care24, 1758–1763. 10.2337/diacare.24.10.1758 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Meigs, J. B. et al. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham offspring studies. Diabetes52, 2160–2167. 10.2337/diabetes.52.8.2160 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Sakkinen, P. A., Wahl, P., Cushman, M., Lewis, M. R. & Tracy, R. P. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am. J. Epidemiol.152, 897–907. 10.1093/aje/152.10.897 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Wang, J.-J. et al. The metabolic syndrome defined by factor analysis and incident type 2 diabetes in a Chinese population with high postprandial glucose. Diabetes Care27, 2429–2437. 10.2337/diacare.27.10.2429 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Riahi, S. M. et al. Patterns of clustering of the metabolic syndrome components and its association with coronary heart disease in the Multi-Ethnic Study of Atherosclerosis (MESA): A latent class analysis. Int. J. Cardiol.271, 13–18. 10.1016/j.ijcard.2018.05.080 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Lanza, S. T. & Rhoades, B. L. Latent class analysis: an alternative perspective on subgroup analysis in prevention and treatment. Prev. Sci.14, 157–168. 10.1007/s11121-011-0201-1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldhoon-Hainerová, I. et al. Glucose homeostasis and insulin resistance: Prevalence, gender differences and predictors in adolescents. Diabetol. Metab. Syndr.6, 1–9. 10.1016/j.appet.2014.12.041 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han, M. S. Metabolic syndrome emerging form menopause. J. Korean Soc. Menopause10.6118/jksm.2011.17.3.127 (2011). [Google Scholar]
- 20.Regitz-Zagrosek, V., Lehmkuhl, E. & Mahmoodzadeh, S. Gender aspects of the role of the metabolic syndrome as a risk factor for cardiovascular disease. Gend. Med.4, S162–S177. 10.1016/s1550-8579(07)80056-8 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Abbasi-Ghahramanloo, A., Soltani, S., Gholami, A., Erfani, M. & Yosaee, S. Clustering and combining pattern of metabolic syndrome components among Iranian population with latent class analysis. Med. J. Islam. Repub. Iran30, 445. 10.1590/1516-3180.2013.1314326 (2016). [PMC free article] [PubMed] [Google Scholar]
- 22.Ahanchi, N. S. et al. Application of latent class analysis to identify metabolic syndrome components patterns in adults: Tehran lipid and glucose study. Sci. Rep.9, 1–8. 10.1038/s41598-018-38095-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyko, E. J. et al. Latent class analysis of the metabolic syndrome. Diabetes Res. Clin. Pract.89, 88–93. https://linkinghub.elsevier.com/retrieve/pii/S0168822710000896 (2010). [DOI] [PMC free article] [PubMed]
- 24.Arguelles, W. et al. Characterization of metabolic syndrome among diverse Hispanics/Latinos living in the United States: Latent class analysis from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Int. J. Cardiol.184, 373–379. 10.1016/j.ijcard.2015.02.100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pradhan, A. D. Sex differences in the metabolic syndrome: Implications for cardiovascular health in women. Clin. Chem.60, 44–52. 10.1373/clinchem.2013.202549 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Dekker, J. M. et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation112, 666–673. 10.1161/circulationaha.104.516948 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Gami, A. S. et al. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol49, 403–414 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Lin, T.-Y. et al. Dynamics of detailed components of metabolic syndrome associated with the risk of cardiovascular disease and death. Sci. Rep.11, 1–10. 10.1038/s41598-021-83118-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, Y., Han, B.-G. & the KoGES Group. Cohort profile: The Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol.46, e20–e20 10.1093/ije/dyv316 (2017). [DOI] [PMC free article] [PubMed]
- 30.Matthews, D. R. et al. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia28, 412–419. 10.1007/bf00280883 (1985). [DOI] [PubMed] [Google Scholar]
- 31.Nehring, S. M., Goyal, A., Bansal, P. & Patel, B. (StatPearls [Internet], 2023).
- 32.Jung, H. et al. Relationship between age at menarche and metabolic diseases in Korean postmenopausal women: The Korea National Health and Nutrition Examination Survey 2016–2018. PLOS ONE18, e0280929. 10.1371/journal.pone.0280929 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, M. J., Lee, I. S., Shin, E. K., Joung, H. & Cho, S. I. The timing of sexual maturation secular trends of menarchial age in Korean adolescents. Korean J. Pediatr.29, 610–616. 10.3345/kjp.2006.49.6.610 (2006). [Google Scholar]
- 34.Mousavi, E., Gharipour, M., Tavassoli, A., Sadri, G. H. & Sarrafzadegan, N. Multiparity and risk of metabolic syndrome: Isfahan Healthy Heart Program. Metab. Syndr. Relat. Disord.7, 519–524. 10.1089/met.2008.0076 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Lao, X. et al. Parity and the metabolic syndrome in older Chinese women: The Guangzhou Biobank Cohort Study. Clin. Endocrinol.65, 460–469. 10.1111/j.1365-2265.2006.02615.x (2006). [DOI] [PubMed] [Google Scholar]
- 36.Gunderson, E. P. et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: The CARDIA study. Am. J. Obstet. Gynecol.201, 177.e171-177.e179. 10.1016/j.ajog.2009.03.031 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen, A., Pieper, C. F., Brown, A. J. & Bastian, L. A. Number of children and risk of metabolic syndrome in women. J. Womens Health15, 763–773. 10.1089/jwh.2006.15.763 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Akter, S. et al. Higher gravidity and parity are associated with increased prevalence of metabolic syndrome among rural Bangladeshi women. PLOS ONE8, e68319. 10.1371/journal.pone.0068319 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, Y. et al. Higher parity and risk of metabolic syndrome in Korean postmenopausal women: Korea National Health and Nutrition Examination Survey 2010–2012. J. Obstet. Gynaecol. Res.44, 2045–2052. 10.1111/jog.13766 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Alkerwi, A. A. et al. Alcohol consumption and the prevalence of metabolic syndrome: A meta-analysis of observational studies. Atherosclerosis204, 624–635 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Bae, Y.-J. Relationship among practicing healthy diet and metabolic syndrome indicators in adults—From the Korea National Health and Nutrition Examination Survey, 2013–2014. J. Nutr. Health49, 459–470. 10.4163/jnh.2016.49.6.459 (2016). [Google Scholar]
- 42.Ministry of Health and Welfare. Let’s Do This the Healthy Life. (Korea Institute for Health and Social Affairs, 1999).
- 43.Hirshkowitz, M. et al. National Sleep Foundation’s updated sleep duration recommendations. Sleep Health1, 233–243. 10.1016/j.sleh.2015.10.004 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Yoon, H.-S. et al. Associations of sleep duration with metabolic syndrome and its components in adult Koreans: From the health examinees study. Sleep Biol. Rhythms14, 361–368. 10.1007/s41105-016-0065-7 (2016). [Google Scholar]
- 45.Xi, B., He, D., Zhang, M., Xue, J. & Zhou, D. Short sleep duration predicts risk of metabolic syndrome: A systematic review and meta-analysis. Sleep Med. Rev.18, 293–297. 10.1016/j.smrv.2013.06.001 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Kim, C. E. et al. Association between sleep duration and metabolic syndrome: A cross-sectional study. BMC Public Health18, 1–8. 10.1186/s12889-018-5557-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arora, T. et al. Self-reported long total sleep duration is associated with metabolic syndrome: The Guangzhou Biobank Cohort Study. Diabetes Care34, 2317–2319. 10.2337/dc11-0647 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim, G. S. et al. Patterns and predictors of fall injury transitions among Korean older adult fallers: A 2-year longitudinal study. Sci. Rep.12, 22188. 10.1038/s41598-022-26665-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asparouhov, T. & Muthén, B. Auxiliary variables in mixture modeling: Three-step approaches using Mplus. Struct. Equ Model. Multidiscip. J.21, 329–341. 10.1080/10705511.2014.915181 (2014). [Google Scholar]
- 50.Nylund, K. L., Asparouhov, T. & Muthén, B. O. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct. Equ. Model. A Multidiscip. J.14, 535–569. 10.1080/10705510701575396 (2007). [Google Scholar]
- 51.Bolck, A., Croon, M. & Hagenaars, J. Estimating latent structure models with categorical variables: One-step versus three-step estimators. Polit. Anal.12, 3–27. 10.1093/pan/mph001 (2004). [Google Scholar]
- 52.Weller, B. E., Bowen, N. K. & Faubert, S. J. Latent class analysis: A guide to best practice. J. Black Psychol.46, 287–311. 10.1177/0095798420930 (2020). [Google Scholar]
- 53.Lythgoe, D. T., Garcia-Fiñana, M. & Cox, T. F. Latent class modeling with a time-to-event distal outcome: A comparison of one, two and three-step approaches. Struct. Equ. Model. Multidiscip. J.26, 51–65. 10.1080/10705511.2018.1495081 (2019). [Google Scholar]
- 54.Clark, S. L. & Muthén, B. Relating Latent Class Analysis Results to Variables Not Included in the Analysis. https://www.statmodel.com/download/relatinglca.pdf (2009).
- 55.Lo, Y., Mendell, N. R. & Rubin, D. B. Testing the number of components in a normal mixture. Biometrika88, 767–778. 10.1093/biomet/88.3.767 (2001). [Google Scholar]
- 56.El-Habil, A. M. An application on multinomial logistic regression model. Pak. J. Stat. Oper. Res.10.18187/pjsor.v8i2.234 (2012). [Google Scholar]
- 57.Singer, J. D. & Willett, J. B. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. (Oxford University Press, 2003).
- 58.Lee, T. H. & Shi, D. A comparison of full information maximum likelihood and multiple imputation in structural equation modeling with missing data. Psychol. Methods26, 466–485. 10.1037/met0000381 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Min, S. H., Docherty, S. L., Im, E.-O. & Yang, Q. Health behavior profiles among midlife women: Identifying at-risk subgroups for metabolic syndrome using latent class analysis. Ann. Behav. Med.56, 1–13. 10.1093/abm/kaac003 (2022). [DOI] [PubMed] [Google Scholar]
- 60.Kim, M. K. & Park, J. H. Metabolic syndrome. J. Korean Med. Assoc.55, 1005–1013 (2012). [Google Scholar]
- 61.Pu, D., Tan, R., Yu, Q. & Wu, J. Metabolic syndrome in menopause and associated factors: A meta-analysis. Climacteric20, 583–591. 10.1080/13697137.2017.1386649 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Hong, E. G. Changes in glucose metabolism with aging. J. Korean Diabetes20, 215–219. 10.4093/jkd.2019.20.4.215 (2019). [Google Scholar]
- 63.Anstey, D. E., Christian, J. & Shimbo, D. Income inequality and hypertension control. J. Am. Heart Assoc.8, e013636. 10.1161/jaha.119.013636 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplan, M. S., Huguet, N., Feeny, D. H. & McFarland, B. H. Self-reported hypertension prevalence and income among older adults in Canada and the United States. Soc. Sci. Med.70, 844–849. 10.1016/j.socscimed.2009.11.019 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Oda, E. & Kawai, R. Comparison between high-sensitivity C-reactive protein (hs-CRP) and white blood cell count (WBC) as an inflammatory component of metabolic syndrome in Japanese. Intern. Med.49, 117–124. 10.2169/internalmedicine.49.2670 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Iftikhar, I. H. et al. Sleep duration and metabolic syndrome: An updated dose–risk meta analysis. Ann. Am. Thorac. Soc.12, 1364–1372. 10.1101/2020.08.30.20184747 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirchengast, S., Gruber, D., Sator, M. & Huber, J. Hysterectomy is associated with postmenopausal body composition characteristics. J. Biosoc. Sci.32, 37–46. 10.1017/s0021932000000377 (2000). [PubMed] [Google Scholar]
- 68.Shim, J. Y. et al. Prevention and treatment of metabolic syndrome in Korean adults. Korean J. Fam. Pract.5, 375–420 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the Korean Genome Epidemiology Study (KoGES). Restrictions apply to the availability of the data, which were used under a licence for this study. Any additional information required to reanalyze the data reported in this paper is available from KoGES epidemiological data online sharing system (https://nih.go.kr/ko/main/contents.do?menuNo=300566) with permission.