Abstract
Fructose 2,6-bisphosphate and several glycolytic intermediates were measured in two rat muscles, extensor digitorum longus and gastrocnemius, which were electrically stimulated in situ. Both the duration and the frequency of stimulation were varied to obtain different rates of glycolysis. There was no relationship between fructose 2,6-bisphosphate content and the increase in tissue lactate in contracting muscle. However, in gastrocnemius stimulated at low frequencies (less than or equal to 5 Hz), there was a 2-fold increase in fructose 2,6-bisphosphate at 10s, followed by a return to basal values, whereas lactate increased only after 1 min of contraction. The concentrations of hexose 6-phosphates, fructose 1,6-bisphosphate and triose phosphates were all increased during the 3 min stimulation. During tetanus (frequencies greater than or equal to 10 Hz) fructose 2,6-bisphosphate was not increased, whereas glycolysis was maximally stimulated and resulted in an accumulation of tissue lactate, mostly from glycogen. The concentrations of hexose 6-phosphate increased continuously during the 1 min tetanus, whereas fructose 1,6-bisphosphate was increased at 10s and then decreased progressively. It therefore appears that fructose 2,6-bisphosphate does not play a role in the stimulation of glycolysis during tetanus; it may, however, be involved in the control of glycolysis when the muscles are stimulated at low frequencies for short periods of time.
Full text
PDF

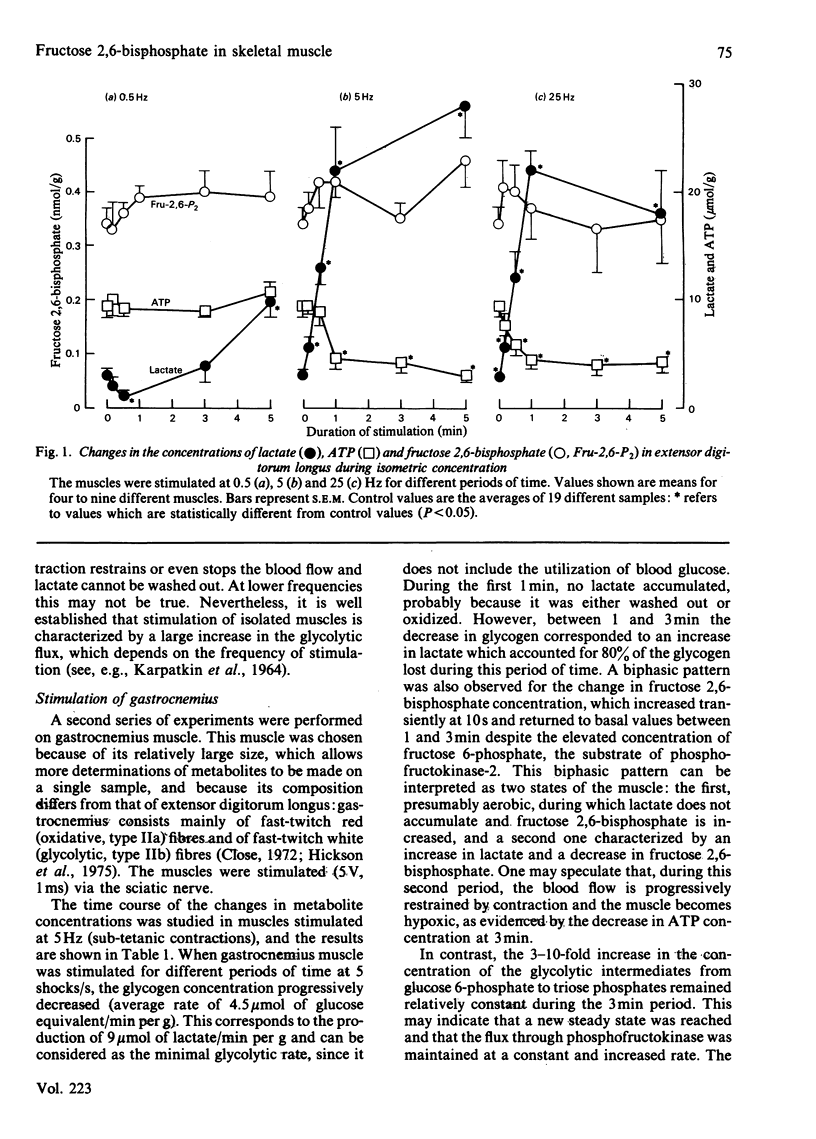
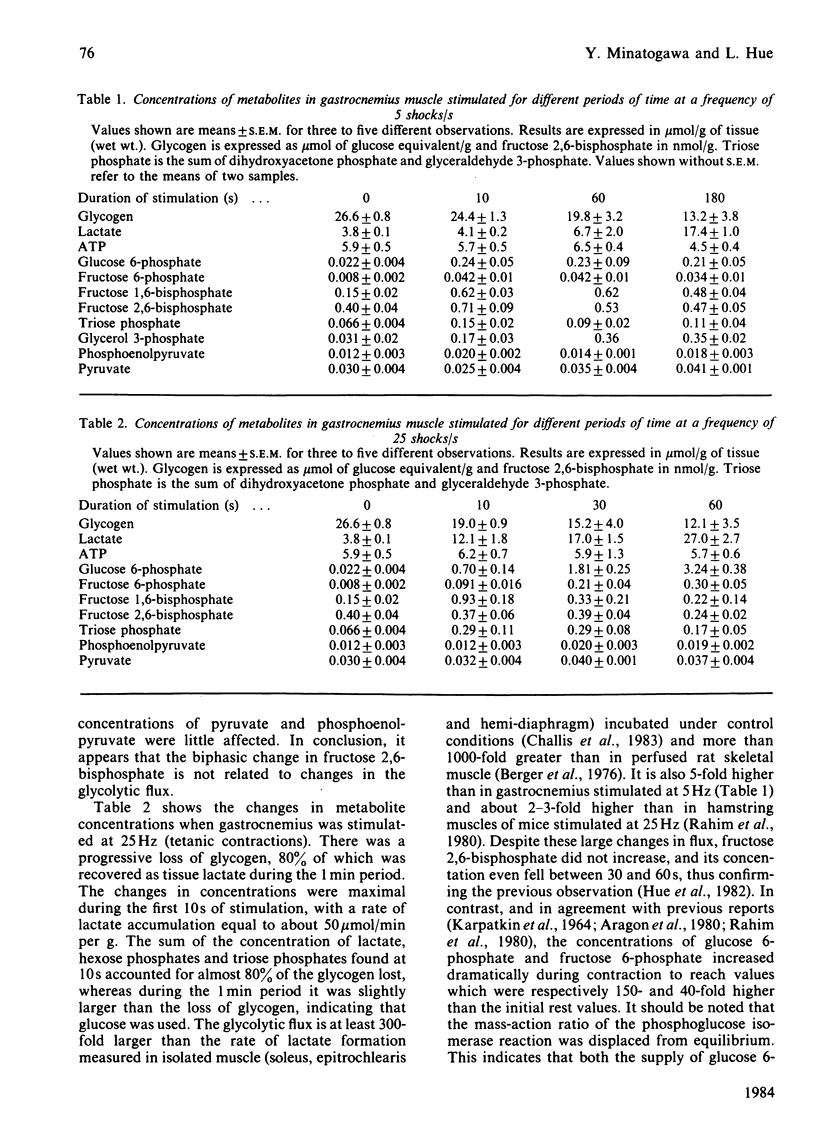
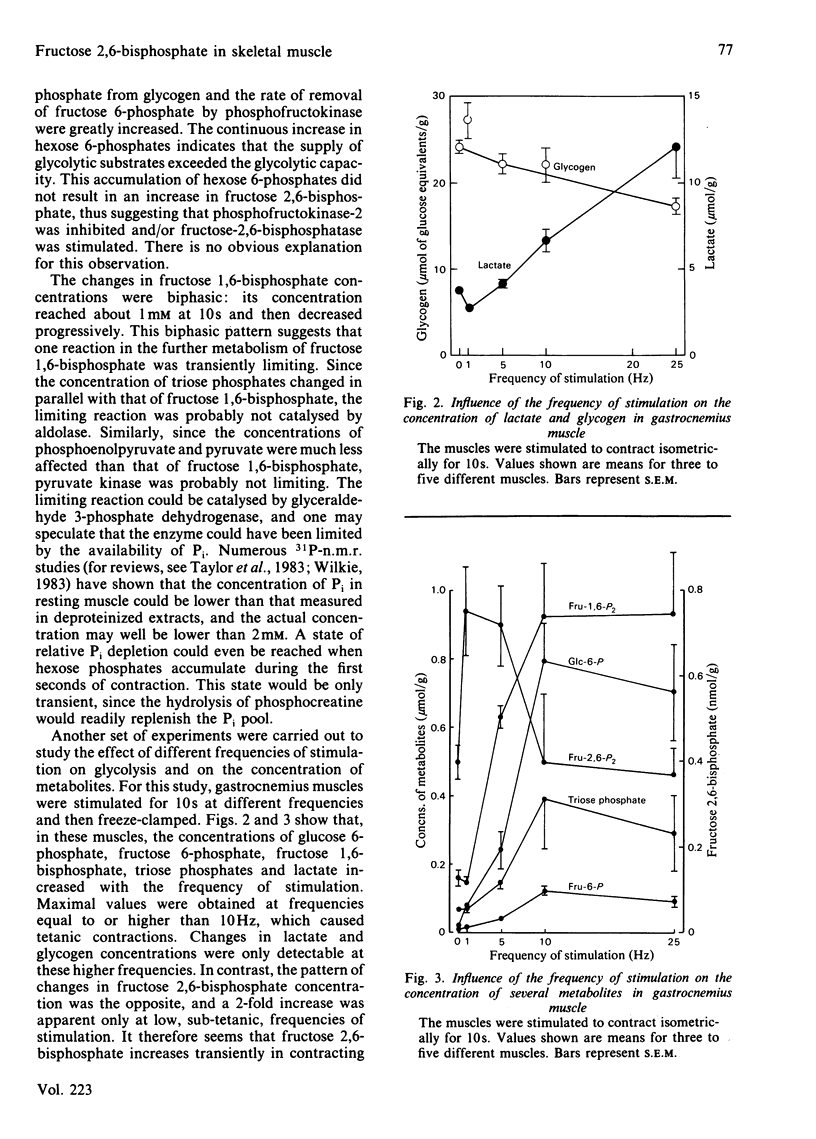


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aragón J. J., Tornheim K., Lowenstein J. M. On a possible role of IMP in the regulation of phosphorylase activity in skeletal muscle. FEBS Lett. 1980 Aug 25;117 (Suppl):K56–K64. doi: 10.1016/0014-5793(80)80570-9. [DOI] [PubMed] [Google Scholar]
- Berger M., Hagg S. A., Goodman M. N., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Effects of starvation, diabetes, fatty acids, acetoacetate, insulin and exercise on glucose uptake and disposition. Biochem J. 1976 Aug 15;158(2):191–202. doi: 10.1042/bj1580191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challiss R. A., Espinal J., Newsholme E. A. Insulin sensitivity of rates of glycolysis and glycogen synthesis in soleus, stripped soleus, epitrochlearis, and hemi-diaphragm muscles isolated from sedentary rats. Biosci Rep. 1983 Jul;3(7):675–679. doi: 10.1007/BF01172878. [DOI] [PubMed] [Google Scholar]
- Clarke F. M., Masters C. J. On the association of glycolytic enzymes with structural proteins of skeletal muscle. Biochim Biophys Acta. 1975 Jan 13;381(1):37–46. doi: 10.1016/0304-4165(75)90187-7. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- DANFORTH W. H., HELMREICH E. REGULATION OF GLYCOLYSIS IN MUSCLE. I. THE CONVERSION OF PHOSPHORYLASE BETA TO PHOSPHORYLASE ALPHA IN FROG SARTORIUS MUSCLE. J Biol Chem. 1964 Oct;239:3133–3138. [PubMed] [Google Scholar]
- Foe L. G., Latshaw S. P., Kemp R. G. Binding of hexose bisphosphates to muscle phosphofructokinase. Biochemistry. 1983 Sep 13;22(19):4601–4606. doi: 10.1021/bi00288a039. [DOI] [PubMed] [Google Scholar]
- Gadian D. G., Radda G. K., Brown T. R., Chance E. M., Dawson M. J., Wilkie D. R. The activity of creatine kinase in frog skeletal muscle studied by saturation-transfer nuclear magnetic resonance. Biochem J. 1981 Jan 15;194(1):215–228. doi: 10.1042/bj1940215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heylen A., Van Schaftingen E., Hers H. G. The stimulation of phosphofructokinase from human erythrocytes by fructose 2,6-bisphosphate. FEBS Lett. 1982 Jun 21;143(1):141–143. doi: 10.1016/0014-5793(82)80291-3. [DOI] [PubMed] [Google Scholar]
- Hickson R. C., Heusner W. W., Van Huss W. D., Taylor J. F., Carrow R. E. Effects of an anabolic steroid and sprint training on selected histochemical and morphological observations in rat skeletal muscle types. Eur J Appl Physiol Occup Physiol. 1976 Sep 23;35(4):251–259. doi: 10.1007/BF00423284. [DOI] [PubMed] [Google Scholar]
- Hue L., Blackmore P. F., Shikama H., Robinson-Steiner A., Exton J. H. Regulation of fructose-2,6-bisphosphate content in rat hepatocytes, perfused hearts, and perfused hindlimbs. J Biol Chem. 1982 Apr 25;257(8):4308–4313. [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L. Role of fructose 2,6-bisphosphate in the stimulation of glycolysis by anoxia in isolated hepatocytes. Biochem J. 1982 Aug 15;206(2):359–365. doi: 10.1042/bj2060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARPATKIN S., HELMREICH E., CORI C. F. REGULATION OF GLYCOLYSIS IN MUSCLE. II. EFFECT OF STIMULATION AND EPINEPHRINE IN ISOLATED FROG SARTORIUS MUSCLE. J Biol Chem. 1964 Oct;239:3139–3145. [PubMed] [Google Scholar]
- Kitajima S., Uyeda K. A binding study of the interaction of beta-D-fructose 2,6-bisphosphate with phosphofructokinase and fructose-1,6-bisphosphatase. J Biol Chem. 1983 Jun 25;258(12):7352–7357. [PubMed] [Google Scholar]
- Mayr G. W., Heilmeyer L. M., Jr Phosphofructokinase is a calmodulin binding protein. FEBS Lett. 1983 Aug 8;159(1-2):51–57. doi: 10.1016/0014-5793(83)80415-3. [DOI] [PubMed] [Google Scholar]
- Mommaerts W. F., Vegh K., Homsher E. Activation of phosphorylase in frog muscle as determined by contractile activity. J Gen Physiol. 1975 Nov;66(5):657–669. doi: 10.1085/jgp.66.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim Z. H., Perrett D., Lutaya G., Griffiths J. R. Metabolic adaptation in phosphorylase kinase deficiency. Changes in metabolite concentrations during tetanic stimulation of mouse leg muscles. Biochem J. 1980 Jan 15;186(1):331–341. doi: 10.1042/bj1860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter E. A., Ruderman N. B., Gavras H., Belur E. R., Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Am J Physiol. 1982 Jan;242(1):E25–E32. doi: 10.1152/ajpendo.1982.242.1.E25. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Bore P. J., Styles P., Gadian D. G., Radda G. K. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983 Jul;1(1):77–94. [PubMed] [Google Scholar]
- Tornheim K., Lowenstein J. M. Control of phosphofructokinase from rat skeletal muscle. Effects of fructose diphosphate, AMP, ATP, and citrate. J Biol Chem. 1976 Dec 10;251(23):7322–7328. [PubMed] [Google Scholar]
- Van Schaftingen E., Jett M. F., Hue L., Hers H. G. Control of liver 6-phosphofructokinase by fructose 2,6-bisphosphate and other effectors. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3483–3486. doi: 10.1073/pnas.78.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Wilkie D. R. The control of glycolysis in living muscle studied by nuclear magnetic resonance and other techniques. Biochem Soc Trans. 1983 Jun;11(3):244–246. doi: 10.1042/bst0110244. [DOI] [PubMed] [Google Scholar]


