Abstract
Endothelial inflammation plays a crucial role in vascular-related diseases, a leading cause of global mortality. Among various cellular players, endothelial progenitor cells (EPCs) emerge as non-differentiated endothelial cells circulating in the bloodstream. Recent evidence highlights the transformative role of EPCs in shifting from an inflammatory/immunosuppressive crisis to an anti-inflammatory/immunomodulatory response. Despite the importance of these functions, the regulatory mechanisms governing EPC activities and their physiological significance in vascular regenerative medicine remain elusive. Surprisingly, the current literature lacks a comprehensive review of EPCs’ effects on inflammatory processes. This narrative review aims to fill this gap by exploring the cutting-edge role of EPCs against inflammation, from molecular intricacies to broader medical perspectives. By examining how EPCs modulate inflammatory responses, we aim to unravel their anti-inflammatory significance in vascular regenerative medicine, deepening insights into EPCs’ molecular mechanisms and guiding future therapeutic strategies targeting vascular-related diseases.
Subject terms: Stem-cell research, Haematopoietic stem cells
Introduction
Recent studies have shown that vascular regeneration is not merely the process of securing endothelial cells (ECs) to form vascular structures. Instead, it is a composite process that involves collaboration with hematopoietic lineage cells, aimed at creating regenerative and anti-inflammatory microenvironments for organ recovery (Fig. 1)1,2. Hematopoietic cells can induce new blood vessel formation by producing and secreting pro-angiogenic factors during the inflammation process1. The cells of this system are usually divided into two main types: innate (myeloid) and adaptive (lymphoid) cells. Innate immune cells include tissue resident cells, such as macrophages and mast cells, and circulating immune cells, such as monocytes/macrophages and neutrophils, that are key members of angiogenic mediators3. Myeloid lineage macrophages that accumulate in ischemic tissues play a dual role in promoting vascular regeneration mechanisms: functioning as either regenerative or anti-inflammatory macrophages, and concurrently preventing tissue fibrosis. Similarly, lymphoid lineage helper T cells localize to tissues as Th2 cells and regulatory T cells. These cells exhibit anti-immunity and anti-inflammatory functions, influenced by environmental factors from M2 macrophages and others, which contribute to tissue repair through shared regenerative and anti-fibrotic actions3.
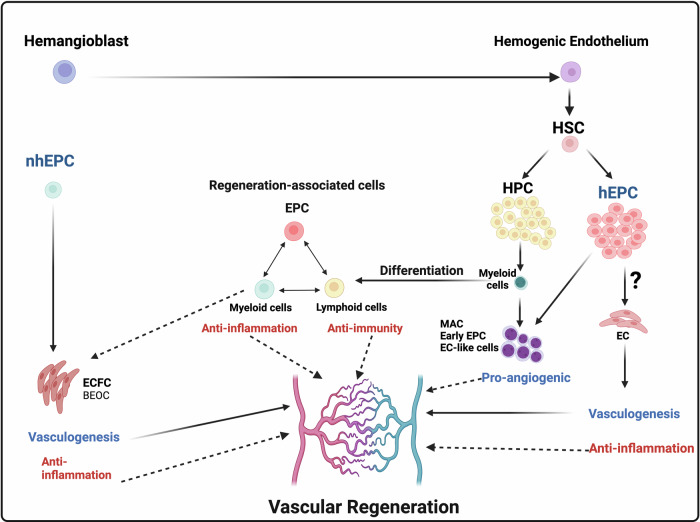
Fig. 1. Collaborative functions of EPCs and blood cells for vascular regeneration.
This figure illustrates the hierarchical differentiation of endothelial progenitor cells (EPCs) and collaborative interactions in promoting vascular regeneration. It highlights the roles of EPCs in endothelial repair and their coordination with various blood cell types to facilitate the restoration of vascular function. Noted that the differentiation capacity of hEPCs into ECs remains a challenged topic of controversy, prompting the need for further investigations in this area.
ECs have long been recognized for their dual role in maintaining blood vessels and modulating immunity through immune cell trafficking, antigen presentation, and cytokine expression in tissues4. Emerging evidence remarks on a tissue-specific and vessel type-specific immunomodulatory role for distinct subtypes of ECs, known as ‘immunomodulatory ECs’, including highly CD34 expressing ECs and endothelial progenitor cells (EPCs)5,6. Building on this understanding, this review will specifically delve into the mechanism employed by EPCs to orchestrate a harmonious interplay between endothelial and hematopoietic lineage cells for tissue regeneration, creating an anti-inflammatory microenvironment essential for optimal tissue repair.
EPC phenotype
EPCs, first identified in 1997, play a crucial role in maintaining vascular homeostasis by serving as a circulating pool of progenitors capable of replacing impaired endothelium7. Various studies8–11 have organized the conceptual framework of EPC-mediated EC supply for vasculogenesis in humans, which is categorizes into circulating EPCs and resident EPCs, contributing to the dynamic regulation of vascular health and repair. Circulating EPCs in vivo consist of hematopoietic lineage cell-derived EPCs (hEPCs), originated from bone marrow (BM)12, and non-hematopoietic lineage cell-derived EPCs (non-hEPCs), also known as endothelial colony-forming cells (ECFCs), which are believed to mobilize from resident EPCs into the organ blood vessels9,11 (Table 1). While both cell types secrete cytokines and growth factors with immunomodulatory effects, ECFCs demonstrate a greater capacity for direct endothelial replacement and vascular repair11,13–15, whereas hEPCs primarily exert their effects through paracrine signaling and immune cell modulation rather than endothelial differentiation16. Taken together, hematopoietic and non-hEPCs exhibit distinct but complementary mechanisms of action in modulating inflammatory responses and promoting tissue repair. Further research is warranted to elucidate the precise mechanisms underlying their immunomodulatory activities and to develop novel strategies for harnessing their therapeutic benefits. It is worth mentioning that, utilizing fluorescence in situ hybridization and cell-tracing analyses, it has been demonstrated that hEPCs lack the capacity to differentiate into ECs11,14,15. Thus, the differentiation capacity of hEPCs into ECs remains a challenged topic of controversy, prompting the need for further investigations in this area (Fig. 1).
Table 1.
Referenced phenotypes of EPCs
| EPC phenotype | Status | Positive Phenotyping markers | Negative phenotyping markers | Note | |
|---|---|---|---|---|---|
| nhEPC | ECFCs | Ex vivo | CD31/CD34/CD144 | CD45/CD133/ CD14/CD41a | Late-outgrowth EPC |
| hEPC | CD34+ or CD133+ | In vivo | CD34/CD133 /CD45 | CD14 | Immature hEPC subset |
| Cultured EPCs | Ex vivo | CD31/CD45 | CD41a/CD235a | Early-outgrowth EPC, including MACs | |
| Monocytic EPCs | In vivo | CD14/CD45 | CD133/CD34-/dim | Circulating monocyte subset |
EPCs Endothelial progenitor cells, ECFCs Endothelial colony-forming cells, hEPCs hematopoietic lineage cell-derived cells, nhEPCs non-hEPC, MACs myeloid angiogenic cells
Similarity and difference between hematopoietic and non-hematopoietic lineage cell-derived EPCs in anti-inflammatory/immunomodulatory activities
Given to the fact that EPCs possess anti-inflammatory and immunomodulatory properties, they are considered potential candidates for therapeutic interventions in various diseases. Here, we discuss the similarities and differences between hEPCs and non-hEPCs or ECFCs in their anti-inflammatory and immunomodulatory activities. While both linages exhibit anti-inflammatory and immunomodulatory activities, there are notable differences in their origin, phenotype, and functional properties (Table 2):
Table 2.
Anti-inflammatory/Immunomodulatory Activities of hEPCs vs. non-hEPCs or ECFCs
| Functions | hEPCs | non-hEPCs or ECFCs |
|---|---|---|
| Cytokine Secretion & Paracrine effects: | Secreting various cytokines and growth factors with immunomodulatory properties in greater amount, such as IL-10, TGF-β, and VEGF17. | Secreting various angiogenic and anti-inflammatory factors, including HGF, PGE2, and Ang-118–20. |
| Endothelial Stabilization: | Contributing to endothelial integrity and function by enhancing the expression of endothelial cell adhesion molecules and inhibiting leukocyte adhesion and transmigration. Also, composing myeloid cells such as M2 macrophages for strong anti-inflammatory effect21,22. | Possessing a remarkable ability to integrate into existing vascular networks and contributing to endothelial repair and regeneration for vascular homeostasis and anti- inflammation at the site of injury13,22. |
| Induction of Regulatory T Cells (Tregs): | Promoting the expansion and activation of regulatory T cells for a crucial role in immune tolerance and suppression of excessive inflammatory responses24. | Having a less effect on Treg cells expansion. |
For paracrine effects, hEPCs secrete various cytokines and growth factors with immunomodulatory properties in greater amount, such as IL-10, TGF-β, and VEGF, which suppress inflammatory responses and promote tissue healing17. Similar to hEPCs, ECFCs secrete various angiogenic and anti-inflammatory factors, including hepatocyte growth factor (HGF), PGE2, and angiopoietin-1 (Ang-1), which modulate immune responses and promote tissue healing18–20.
However, for function of endothelial stabilization, hEPCs contribute to endothelial integrity and function by enhancing the expression of endothelial cell adhesion molecules and inhibiting leukocyte adhesion and transmigration, thereby reducing inflammation at the vascular interface. Moreover, hEPCs compose myeloid cells as well such as M2 macrophages, the latter has strong anti-inflammatory effect21,22. In addition, ECFCs possess a remarkable ability to integrate into existing vascular networks and contribute to endothelial repair and regeneration. By directly replacing damaged endothelial cells, ECFCs restore vascular homeostasis and alleviate inflammation at the site of injury13,22.
Finding the optimal generation of ECFCs from CD34+ cells requires the presence of hematopoietic cells secreting angiogenic cytokines23. Vascular regeneration is not simply a matter of securing endothelial cells from non-hEPCs to form vascular structures in situ; it is also a composite process. This process involves collaborating with hEPCs to manage regenerative microenvironments for organs.
The induction of Tregs is also a difference between hEPCs and non-hEPCs. hEPCs promote the expansion and activation of Tregs, which play a crucial role in immune tolerance and suppression of excessive inflammatory responses24, while ECFCs have less effect on the expansion of Tregs.
Concurrent mechanism of angiogenesis, immunosuppression and anti-inflammation
Various evidence in cancer biology indicates that angiogenesis and immunosuppression frequently occur simultaneously in response to diverse stimuli25,26. This notion is corroborated by reports indicating that an ever-growing array of hematopoietic cell types can promote both angiogenesis and immunosuppression, which include M2 alternatively activated macrophages, tumor-associated macrophages27, Tie2-expressing monocytes28, and myeloid-derived suppressor cells. This dual role not only facilitates angiogenesis, but also creates an immune and inflammatory suppressive environment, fostering tumor expansion and progression in cancer tissues25,29,30. In this process, various collaborative factors capable of promoting both immunomodulation and angiogenesis in cancer, such as vascular endothelial growth factors (VEGF), prostaglandin E2 (PGE2), transforming growth factor-β (TGF-β), Interleukin 10 (IL-10), Interleukin 4 (IL-4), Interleukin 6 (IL-6), macrophage colony-stimulating factor (M-CSF), granulocyte-colony stimulating factor (G-CSF), adenosine, etc., have been investigated for years and reported to be expressed by appropriately stimulated hematopoietic lineage cells25,26. This collaborative property is inherently applicable to EPCs in regenerative tissues. EPCs secrete multiple factors which contribute to angiogenesis, anti-inflammation and immunosuppression, such as VEGF, PGE2, TGF-β, IL-10, IL-6, and G-CSF18,31.
Recently, regenerative conditioning of naive peripheral blood mononuclear cells has not only expands EPC numbers in culture, but has also transformed the hematopoietic phenotype from pro-inflammatory (M1 monocytes/macrophages, T helper1 (Th1) cells, mature dendritic cells (DCs), natural killer cells (NKs), and B-cells, etc.) to anti-inflammatory and regenerative cells such as definitive EPCs, M2 macrophages, Th2 cells, regulatory T cells (Tregs), regulatory B cells, and immature DCs21,32,33. The regenerative signal stimuli has reprogrammed hematopoietic lineage cells to represent “regeneration-associated cells” acquiring immunosuppressive, anti-inflammatory, and angiogenic properties, which were proven in in vivo experiments in different species (Fig. 1)32–35. While the collaborative function of EPCs and hematopoietic cells remains partially understood, numerous independent studies have provided substantial evidence highlighting the anti-inflammatory properties of EPCs, which underscores their role in creating a regenerative microenvironment in tissues through the harmonization of EPCs and hematopoietic cells4,5. Interestingly, ECFCs express higher levels of TNFR2. According to Naserian et al., inhibiting TNF/TNFR2 signaling hampers EPC immunomodulatory functions, underscoring the importance of the TNF/TNFR2 immune checkpoint axis in EPC immunoregulation such as the production of different anti-inflammatory cytokines (IL-10, TGF-β, and HLA-G). These findings highlight that EPCs leverage the TNF/TNFR2 axis to evade immune rejection by T cells and facilitate vasculogenesis process. Future studies may unveil the potential of a TNFR2 agonist administration to enhance EPC immunoregulatory function, thereby advancing the effectiveness of EPC therapy4. Landhoff et al. investigated the immune privilege of EPC-derived ECs in comparison to mature ECs. Their results indicate that EPC-derived ECs exhibit down-regulation of MHC I, vascular cell adhesion molecule-1 (VCAM-1; a marker for activated endothelium), and IFN-γR2. Additionally, they show strong phosphorylation of STAT1 in contrast to mature ECs5. Notably, MHC I activation triggers recipient cytotoxic CD8+ T lymphocytes (CTL), humoral immune response through alloantibody, as well as complement-mediated lysis, contributing to graft destruction36,37. The study demonstrates the immune-privileged state of EPC-derived ECs attributed to their remarkably low alloimmunogenicity and robust resistance to pre-formed alloresponses, both in vitro and in vivo, making these cells excellent candidates for establishing and storing allogeneic EC for transplantation.
Angiogenic factors from EPCs for anti-inflammatory properties
Numerous angiogenic growth factors secreted by EPCs are commonly associated with the inflammatory response and are listed as follows (Figs. 2, 3; Table 3):
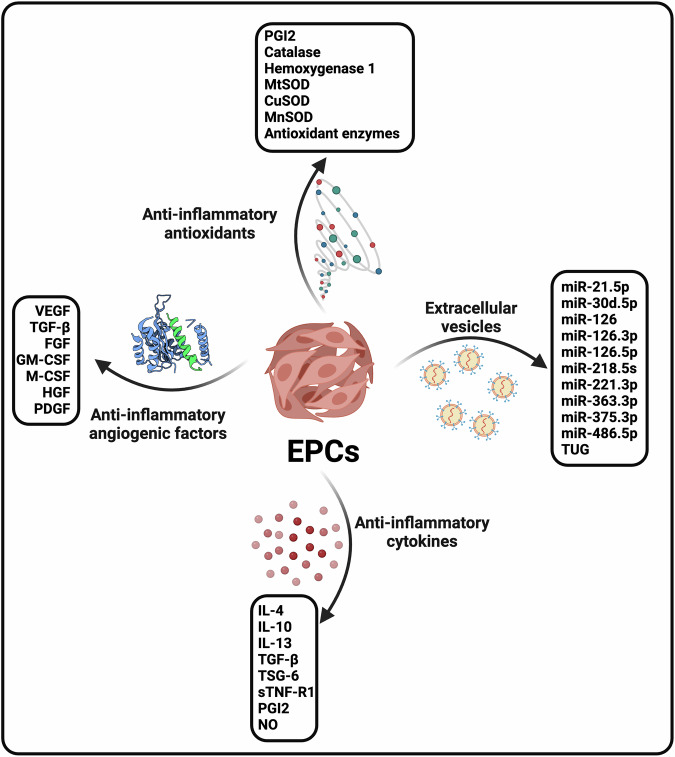
Fig. 2. Anti-inflammatory weapons of EPCs.
This figure depicts the anti-inflammatory mechanisms employed by endothelial progenitor cells (EPCs) and outlines the diverse strategies utilized by EPCs to mitigate inflammation, including the suppression of pro-inflammatory cytokines, the inhibition of leukocyte adhesion and migration, and the modulation of immune cell activity.
Fig. 3. Increased and decreased inflammation-related factors by EPCs.
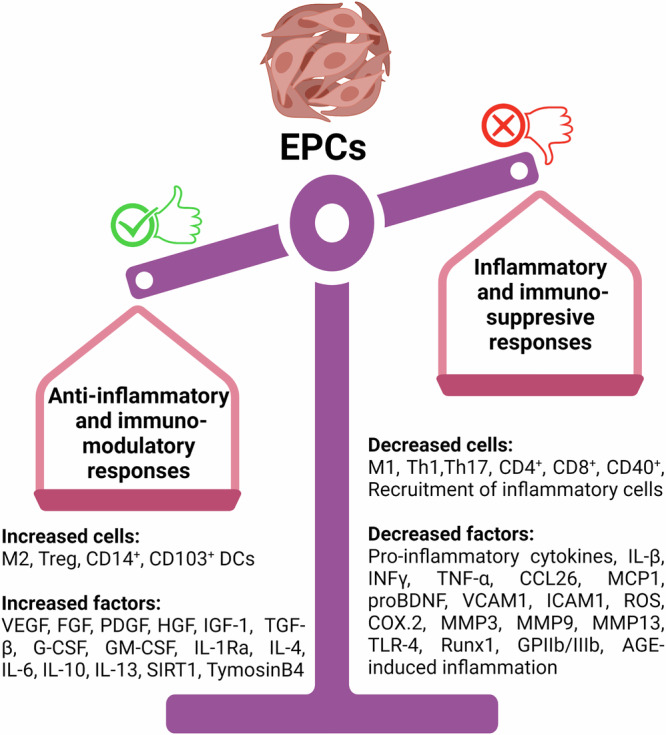
In this figure, we present an overview of the inflammation-related factors that are either increased or decreased by endothelial progenitor cells (EPCs), highlighting the dynamic regulation of inflammatory mediators by EPCs, including the upregulation of anti-inflammatory factors and the downregulation of pro-inflammatory molecules.
Table 3.
Angiogenic factors relating to anti-inflammatory and immunoregulatory of EPCs
| Factor | EPC phenotype | Effect on inflammation and immunity | Involved pathway | Refs. |
|---|---|---|---|---|
| VEGF | CD34+/CD133+ | ↓ inflammation | ↓ TLR4-NF-κB and PI3K-Akt | 39,40,43 |
| TGF-β | CD34+/CD133+ |
↓pro-inflammatory cytokine ↑ M2 and Treg ↓Th17 |
Unknown | 45,48 |
| FGF | ECFCs |
↓IL-1, TNF-α, IL-6, and MCP-1 ↓CD40 |
ERK1/2 NF-κB2 |
51,52 |
| G-CSF/GM-CSF | CD34+/CD133+ |
↑ CD103+ DCs ↑M2, Th2 and Treg ↓TNF-α and IL-1β ↓T cell recruitment |
Unknown Epigenetic modifications |
53,54 |
| HGF | ECFCs |
↑ IL-1Ra ↓TNF-α, IL-6, and IL-1β ↑CD14+ and IL-10 ↓CD4+ ↑ Th2 |
NF-κB ERK1/2 |
20,57,61 |
| PDGF | ECFCs |
↑IL-4, IL-10, and IL-13 ↓MMP-3, MMP-13, COX-2, IL-1, IL-6, and TNF- α ↑ M2 and Treg |
NF-κB p65 translocation PI3K/Akt-MEK/ERK |
63,64 |
VEGF
Various studies claim that EPCs secrete VEGF in conditioned media. In mammals, VEGFs consist of five members, namely VEGFs A–D and placenta growth factor38. Amongst these growth factors, VEGFR-3 and its ligand VEGF-C have been proposed to potently antagonize the inflammatory response via suppression of the TLR4-NF-κB and PI3K-Akt pathways, which represents a “self-control” mechanism during innate immunity39,40. According to a recent study, VEGF-C therapy improved cardiac lymphangiogenesis and the post-MI acute inflammatory response by directing immune cells to the mediastinal lymph nodes41. Single-cell transcriptomic profiling showed that ECFCs overexpressed VEGF-C42. Additionally, EPCs decrease inflammatory status and exert reno-protective effects against glomerulonephritis through VEGF43. Building upon these findings, it must be acknowledged that VEGF-C secreted by transplanted EPCs is at least one of the critical factors that mediate the protective effects of EPCs against inflammatory responses.
TGF-β
Another EPC angiogenic factor with significant anti-inflammatory properties is TGF-β44. This factor stimulates M2 macrophage and Foxp3+ Treg polarization, attenuates pro-inflammatory cytokine/chemokine expression, and modulates the synthesis of matrix remodeling genes24. These characteristics make TGF-β as one of EPCs’ potent anti-inflammatory weapons45–47. Besides, TGF-β directly activates the Treg polarization and suppresses Th17, exerting its anti-immunity properties48.
FGF
Fibroblast growth factor (FGF) is another EPC angiogenic factor with considerable anti-inflammatory qualities and is well-known as an immunosuppressive cytokine49,50. Through the ERK1/2 signaling pathway, FGF dramatically reduces the levels of inflammatory factors such as IL-1, tumor necrosis factor (TNF-α), IL-6, and Monocyte chemoattractant Protein-1 (MCP-1)51. Moreover, FGF downregulates CD40 expression, a key player in inflammation, through the NF-κB2 pathway and counteracts CD40-mediated inflammation52.
G-CSF/GM-CSF
As angiogenic agents of EPCs, the anti-inflammatory effects of GM-CSF and G-CSF have been well documented. According to these findings, G-CSF induces a shift towards Th2 and Treg lymphocytes, as well as M2 macrophages53. Furthermore, GM-CSF reduces pro-inflammatory cytokines (TNF-α and IL-1β) and prevents T cell migration to the CNS via epigenetic regulation54. Additionally, GM-CSF promotes CD103+ DCs, which in turn induce Foxp3+ Treg polarization and T cell-mediated tolerance, exerting immunomodulatory effects55,56.
HGF
According to recent studies, HGF exerts potent anti-inflammatory effects by increasing expression of IL-1Ra20 and decreasing the mRNA levels of TNF-α, IL-6, and IL-1β57. Also, HGF inhibits macrophage-mediated pro-inflammatory cytokines, leading to the suppression of chronic endothelium inflammation58. Mechanistically, HGF suppresses the inflammatory arm of the NF-κB pathway59 and activates the anti-inflammatory arm of this signaling60. Moreover, HGF induces IL-10 production by stimulating immunomodulatory CD14+ monocytes through the ERK1/2 pathway, inhibits activated CD4+ cell proliferation, and modulates the T cell cytokine profile from a Th1 to Th2 profile61. These data shed light on one of the mechanisms behind the strong anti-inflammatory and immunomodulatory properties of EPCs.
PDGF
Platelet-derived growth factor (PDGF) is another EPC angiogenic factor that possesses significant anti-inflammatory functions62. PDGF upregulates the anti-inflammatory cytokines (IL-4, IL-10, and IL-13) while downregulating the pro-inflammatory cytokines (MMP-3, MMP-13, COX-2, IL-1, IL-6, and TNF- α) by reducing NF-κB p65 translocation63. Additionally, studies have documented that EPCs promote the nerve regenerative ability of MSCs through PDGF-BB/PDGFR-β signaling and its downstream PI3K/Akt-MEK/ERK pathways64. Moreover, PDGF weakens T-cell proliferation and induces Treg and M2 activation65,66, emphasizing the crucial role of PDGF in orchestrating the inflammatory and immunological response.
Anti-inflammatory factors from EPCs
Hereby, we aimed to identify factors secreted by EPCs that contribute not only to angiogenesis but also to anti-inflammation. Both ex vivo EPCs (early EPCs and ECFCs) and in vivo EPCs (isolated as CD34+ from peripheral blood (PB) or BM) have been shown to secrete a significant amount of cytokines with anti-inflammatory properties32,67–69. From a mechanistic viewpoint, these factors exert their anti-inflammatory properties through the JAK/STAT signaling pathway, synthesis of the suppressor of cytokine signaling proteins, and modification of NF-κB activity in immune cells70. This section will concentrate on the anti-inflammatory factors released by EPCs, as illustrated in Figs. 2 and 3 and summarized in Table 4:
Table 4.
Anti-inflammatory and anti-immunity cytokines of EPCs
| Factor | EPC phenotype | Effect on inflammation | Involved pathway | Refs. |
|---|---|---|---|---|
| IL-10 | CD34+/CD133+ |
↑ M2 and Treg ↑ anti-inflammatory response |
STAT-3 HuR/matrix metalloproteinase-9 |
71,75 |
| TGF-β1 | CD34+/CD133+ |
↑ Treg ↓Th17 ↑ anti-inflammatory response |
Modification of inflammatory genes (Foxf1, IκB, and Brahma) | 73,141 |
| IL-4 | CD34+/CD133+ |
↑Th2 and M2a ↑ anti-inflammatory response |
STAT6 PPARγ |
74–76 |
| IL-13 | CD34+ |
↑Th2 and M2a ↑ anti-inflammatory response |
STAT6 PPARγ |
74 |
| TSG-6 | CD34+ | ↑ M2 and Treg |
TLR/NF-κB SOCS3/STAT3 |
77,78 |
| sTNF-R1 | CD34+/CD133+ |
↓Th17 ↓TNF-α functions ↑ anti-inflammatory response ↑ M2 and ↓inflammatory cell infiltration |
NFκB | 81–83 |
| NO and PGI | ECFCs | ↓platelets translocation, glycoprotein IIb/IIIa activation, aggregation, and adhesion to collagen | COX-2 and NO synthase | 69,84 |
IL-10 is a potent anti-inflammatory immunosuppressive cytokine with a broad range of effects both directly and indirectly on innate and adaptive immunity through the induction of M2 macrophages and Treg polarization71. In models of acute myocardial infarction (MI), IL-10 has been shown to suppress in vivo inflammatory responses, contributing to improved myocardial recovery. These beneficial effects of IL-10 are mediated by the suppression of HuR/matrix metalloproteinase-9 (MMP-9) and the enhancement of angiogenesis and anti-inflammatory responses via STAT-3 activation72.
Although TGF-β1 is a pleiotropic cytokine involved in a wide range of vital processes, including embryonic development, cellular maturation and differentiation, wound healing, and immune regulation, it also plays a significant role in the anti-inflammatory response by encouraging the proliferation of Tregs and inhibiting the differentiation of Th cells73.
IL-4 is a well-known anti-inflammatory agent that encourages polarization of Th2 and M2a macrophages, thereby increasing its anti-inflammatory potential through indirect angiogenesis74,75. Furthermore, IL-4 increases fatty acid uptake and oxidation, as well as mitochondrial biogenesis, via STAT6. This regulates programs controlled by PPARγ, which plays a complex role in limiting pro-inflammatory gene expression by LPS in M1 macrophages. Consequently, IL-4 controls the initiation, magnitude, and duration of inflammation76.
IL-13 is closely related to IL-4 and shares many biological properties with it, including the ability to polarize macrophages to the M2a phenotype. When combined with IL-4, IL-13 synergistically polarizes macrophages to an anti-inflammatory phenotype74.
TNF-α-induced protein-6 (TSG-6) binds to the resident CD44 macrophages, leading to decrease in TLR/NF-κB signaling and the attenuation of the early phase of the inflammatory response77. Additionally, TSG-6 attenuates inflammatory pathways via inducing M2 macrophage polarization through the SOCS3/STAT3 pathway78. Abd-allah et al.79 reported that transplantation of CD34+ progenitors significantly upregulated levels of the TSG-6 gene, as an anti-inflammatory factor, in a rat experimental model of acute lung injury79. It is worth noting that TSG-6 is secreted in response to TNF-α and IL-1β and its overexpression is noteworthy due to its known anti-inflammatory effects80.
Soluble TNF-receptor 1 (sTNF-R1) is one of the proteolytically shed soluble extracellular domains of TNF-R1, well-known for its ability to neutralize TNF-α functions and inhibit Th17 cell polarization, thereby exerting remarkable immunomodulatory and anti-inflammatory effects81. Besides, Yagi et al.82 demonstrated that sTNF-R1 released from MSCs reduces systemic inflammation by decreasing inflammatory cell infiltration and increasing CD163+ cells, a marker of M2 macrophages82. Bouchentouf et al.83 showed that culturing EPCs solely with insulin-like growth factor 1 (IGF-1), instead of the combination of IGF-1, VEGF, EGF, and FGF, remarkably increased the secretion of cytokine by early EPCs, including sTNF-R1. The released sTNF-R1 functioned as an anti-inflammatory cytokine by neutralizing TNF-α, ultimately restoring cardiac function after MI, implying another anti-inflammatory and immunoregulatory mechanism for EPC therapy in regenerative tissues83.
NO and prostaglandin I (PGI), upregulated by early EPCs in activated platelets, inhibit platelet translocation, glycoprotein IIb/IIIa activation, aggregation, and adhesion to collagen through upregulation of cyclooxygenase-2 and inducible nitric oxide (NO) synthase. Moreover, early EPCs as well as ECFCs produce a significant amount of NO to preserve vascular function and mitigate the inflammatory response69,84. These findings contribute to our understanding of EPC biology and highlight their potential roles in regulating platelet function and inflammation85,86.
Anti-oxidant factors from EPCs
Further scientific documents have reported that EPCs exert their anti-inflammatory functions by means of several antioxidants, as below (Figs. 2 and 3). According to these reports, CD34/VEGFR-2+ EPCs exhibit anti-thrombotic and anti-inflammatory functions by down- expressing pro-inflammatory cytokines and over-expressing PGI2, leading to platelet inactivation85,87. Of note, PGI2 regulates both the innate and adaptive immunity and exerts anti-inflammatory/immunomodulatory effects. Formerly, Dernbach et al.88 demonstrated that early EPCs tolerate a certain degree of oxidative stress by expressing antioxidant enzymes such as mitochondrial superoxide dismutase (mSOD) and hemoxygenase-188. Urbich et al.89 used shotgun proteomics to identify the hemoglobin scavenger receptor CD163, as well as antioxidant enzymes such as mSOD and hemoxygenase-1 in early EPC-conditioned media, which function as anti-inflammatory properties against cocultured ECs89. Similarly, early EPC-conditioned media elevated the expression level of antioxidant enzymes in HUVECs, such as catalase, copper/zinc SOD (Cu/ZnSOD), and manganese SOD (MnSOD), implying another anti-inflammatory defense of EPCs in regenerative tissues90.
Anti-inflammatory miRNAs from EPCs
MicroRNAs (miRNAs) are small non-coding RNA molecules known for their ability to regulate gene expression by binding to specific mRNA targets and inhibiting their translation into proteins91. Growing evidence suggests that the EPC-derived miRNAs, primarily delivered through extracellular vesicles (EVs), play important roles in the regulation of inflammatory processes (Figs. 2 and 3). Furthermore, dysregulation of miRNA expression has been implicated in the development of inflammatory diseases (Table 5). One mechanism through which miRNAs exert anti-inflammatory effects is by targeting mRNAs encoding pro-inflammatory proteins. For example, miR-126 has been shown to regulate the expression of several pro-inflammatory cytokines, including TNF-α and IL-6, by binding to their mRNA targets and inhibiting their translation92. Another mechanism by which miRNAs exert anti-inflammatory effects is by targeting mRNAs that encode negative regulators of inflammation. For example, miR-146a/b which is induced by inflammatory stimuli in EPCs, acts as an anti-inflammatory miRNA by targeting several key genes involved in the inflammatory response. Mechanistically, it has been shown to target the mRNA encoding IRAK1, a protein that activates the pro-inflammatory transcription factor NF-κB. By inhibiting IRAK1 expression, miR-146a can reduce NF-κB activity and thereby dampen inflammatory signaling93. In a mouse model of atherosclerosis, overexpression of miR-146a in EPCs has been shown to reduce inflammation94. Furthermore, miR-10a, another EPC-derived miRNA, has been shown to suppress the expression of the pro-inflammatory cytokine IL-6 in macrophages. miR-10a targets the transcription factor Bcl-6, which is known to regulate the expression of IL-6. In addition, miR-10a has been shown to inhibit the activation of the NF-κB pathway, which is a key regulator of inflammation95. Zheng and colleagues demonstrated that miR-10a levels in septic patients were significantly lower than those in infectious patients and healthy controls. Notably, infectious patients also exhibited reduced levels of miR-10a compared with healthy controls95. Moreover, suppression of miR-10a and miR-21 in aged EPCs increased Hmga2 expression, rejuvenated EPCs, resulting in decreased senescence-associated β–galactosidase expression, increased self-renewal potential, decreased p16Ink4a/p19Arf expression, and improved EPC angiogenesis in vitro and in vivo96. EPCs derived EVs carrying TUG1 was proved to ameliorate sepsis-induced organ damage in the murine model via macrophage M2 polarization. Mechanistically, EPCs derived EVs transmitted TUG1 to promote M2 macrophage polarization through the impairment of miR-9-5p-dependent SIRT1 inhibition97. More recently, Yuan et al.98 revealed that miR-222-3p, obtained from EPC-EVs, polarized macrophages to their anti-inflammatory subset via the SOCS3/JAK2/STAT3 signaling pathway. This polarization promoted functional repair after spinal cord injury in the mouse model, highlighting the role of EPC-EVs in modulating macrophage polarization. Overall, the anti-inflammatory effects of EPC-derived miRNAs are mediated by their ability to regulate the expression of key inflammatory genes and pathways, making them promising targets for the development of new anti-inflammatory therapies.
Table 5.
Anti-inflammatory miRNAs in EPC-EVs
| Anti-inflammatory factor | EPC phenotype | Mechanism of action | Refs. |
|---|---|---|---|
| miR -126 | CD34+/CD133+ | ↓IL-6, IFNγ, TNF-α, L-10, VCAM1 and MCP-1 | 92 |
| miR-218-5p and miR-363-3p | CD34+/CD133+ |
↓ chemokine CCL26 by targeting p120‐catenin subfamilies ↓ IL-6, ICAM-1, and IL-1β by suppression of the NOX4-dependent p38 MAPK pathway and targeting JMY-related apoptosis |
103 |
| miR-221-3p | CD34+/CD133+ |
↑VEGF, CD31, and Ki67 ↓ AGE-induced cell hypertrophy, apoptosis, the inflammatory response |
107 |
| miR-222-3p | CD34+/CD133+ | ↑ M2 polarization | 98 |
| miR-30d-5p | Cultured EPCs | ↑ M2 polarization | 108 |
| miR-486-5p | ECFCs |
↓inflammatory cell recruitment ↑Akt phosphorylation |
111 |
| miR-21-5p | Cultured EPCs | ↓RUNX1 and TLR-4, resulting in anti-inflammatory feedback | 112 |
| TUG1 | Cultured EPCs |
↑SIRT1 ↑ M2polarization ↓Inflammatory cytokines |
97 |
| miR-126-3p and 5p | ECFCs |
↑ SPRED1, PI3K/Akt/GSK3β and RAF/ERK1/2 axis ↓ HMGB1, VCAM1, and VEGFα |
109,110,117 |
Anti-inflammatory extracellular vesicles from EPCs
The therapeutic application of EPCs and their derivatives, such as EPC-derived EVs (EPC-EVs), plays vital role in endothelium hemostasis and has been widely evaluated for treating several diseases, especially vascular-related disorders, primarily mediated by the transfer of miRs99–101. Hence, we offered the EPC-EVs therapeutics for inflammation-related diseases (Table 5):
Cardiovascular disease (CVDs)
More recently, a convincing study investigated the effects of EPC-EV transplantation on cardiac function in type 2 diabetes mellitus (T2DM). Venkat et al. reported that the CD133+ EPC-EVs treatment induced an increase in miR-126 and a decrease in MCP-1 and VCAM1, leading to decreased cardiac inflammation and oxidative stress after stroke in T2DM mice92. Also, ECFC-EVs mitigate atherosclerosis, inflammation response, and atherosclerosis-related endothelial dysfunction in the experimental mouse models of DM102. Ki et al. also reported that CD34+/CD133+ EPC-EVs containing miR-218-5p and miR-363-3p exhibit cardioprotective effects on MI damage by targeting JMY-related apoptosis and mesenchymal-endothelial transition103. In a similar study, Zhuo et al. found that deletion of Rab27a, as one of the major genes involved in EV biogenesis, weakened the therapeutic functionality of EPCs in MI conditions. Mechanistically, Rab27a deletion inhibits the PI3K/cyclinD1/Akt/FoxO3a pathway and reduces EV secretion in CD34+/VEGFR-2+ EPCs104. In turn, it is worth noting that suppression of PI3K/Akt induces inflammatory cascades105,106. Therefore, it could be inferred that the knockout of EPC-derived EVs initiates an inflammatory process, providing another rationale for the anti-inflammatory properties observed in intact EPCs for CVDs.
Skin problems
Regarding the anti-inflammatory functions of EPC-EVs, studies have reported that miR-221-3p of CD34/CD133+ EPC-EVs improved skin regeneration in diabetic mice through upregulation of VEGF, CD31, and Ki67. Additionally, miR-221-3p countered AGE-induced cell hypertrophy, apoptosis, inflammatory responses, and vascular dysfunction, showcasing its potential in mitigating diabetic complications107. Moreover, miR-30d-5p derived from cultured EPCs-EVs exhibited notable effects on promoting anti-inflammatory responses and M2 polarization, which stimulated the proliferation of glucose-impaired human keloid fibroblasts108. These findings not only contribute to our understanding of EPC-EV functions, but also open new avenues for innovative approaches in diabetes treatment.
LPS-induced lung injury
Studies on the anti-inflammatory functions of EPC-EVs on lung injuries unravel that administration of ECFC-EVs prominently suppresses LPS-induced inflammation of lung tissue, alveolar edema, neutrophil recruitment, and inflammatory cytokines in the bronchoalveolar lavage fluid. From a mechanistic viewpoint, these EVs contain plentiful miR-126 molecules, which target PI3K regulatory subunit 2 and suppress the inflammation-related HMGB1 and the permeability factor VEGFα109. Also, miR-126 activates RAF/ERK signaling through SPRED1 overexpression, which is responsible for recovering pulmonary inflammation and the damaged lung tissue110, proposing a great anti-inflammatory property of ECFC-EVs.
Kidney injury
In the context of kidney-related inflammation, Medica et al. demonstrated that ECFC-EVs preserve human glomerular ECs and podocytes from inflammation-mediated damage43. ECFC-EVs containing miR-486-5p remarkably decrease ischemic kidney damage through decreasing inflammatory cell recruitment and inducing Akt phosphorylation to maintain ischemic tissue111. Additionally, miR-21-5p of cultured EPC-EVs reduces serum inflammatory factors, sepsis-induced kidney injury, and improves renal function by downregulating RUNX1 and toll-like receptor 4, resulting in anti-inflammatory feedback and endothelial protection of kidney tissues in the model of sepsis-induced AKI112,113.
Sepsis-related inflammation
Ma et al. recently found that cultured EPCs-EVs transport taurine upregulated gene 1 (TUG1) into macrophages, increasing SIRT1 expression by binding to miR-9-5p, resulting in promoted M2 polarization and an anti-inflammatory response97. TUG1, an anti-inflammatory long noncoding RNA, inhibits sepsis-induced inflammation and lung injury by suppressing inflammatory agents secretion114,115. It is worth reminding ourselves that SIRT1 improves the production of M2 macrophages and anti-inflammatory cytokines116. In light of the previous evidence, Zhou et al. have shown that ECFC-EVs significantly alleviate sepsis-related inflammation via the transfer of miR-126-3p and miR-126-5p to recipient ECs, which inhibit HMGB1 (an inflammation inducer) and VCAM1 expression117.
Targeting blood cell conversion into anti-inflammatory phenotypes
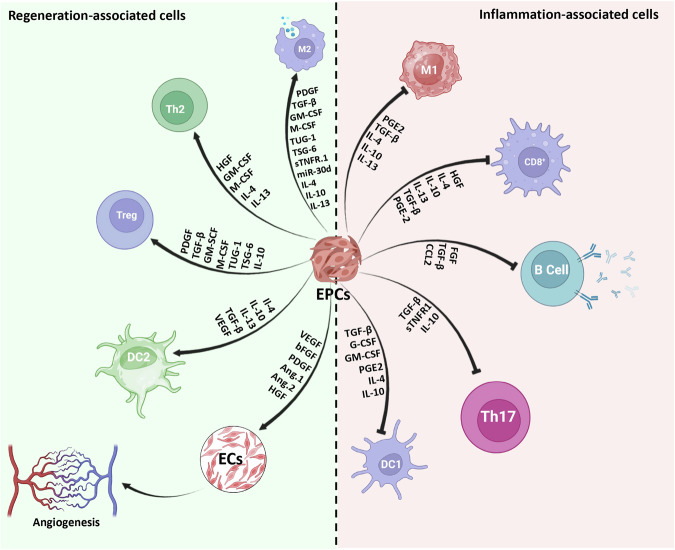
As mentioned above, cultured EPCs induce the conversion of macrophages and T cells into M2 and Tregs, respectively, by means of several factors like IL-10, TSG-6, TUG-1, and so forth97,118–120 (Fig. 4). Despite the fact that EPCs are the prominent cell source for angiogenic and vasculogenic goals, the number of these cells in PB and BM is less than 0.1% and 0.01%, respectively121. In 2014, Masuda et al. developed a new culture medium for MNCs, named the Quality and Quantity culture system (QQ-culture system), which selectively expands the functional EPC population without prior isolation of EPCs from healthy volunteers32. MNCs cultured in the QQ-culture system (QQ-MNCs) have greater therapeutic potency in regenerative applications than MNCs obtained from conventional culture.122. The preconditioning of MNCs induces conversion of the populations of inflammatory M1 macrophages to anti-inflammatory M2 macrophages and shifts T cell phenotypes toward Treg and Th2. Meanwhile, the fraction of cells associated with tissue injuries, such as immunogenic DCs and CD3+/CD8+ T cells, are decreased in the MNCs preconditioning culture system123. These cellular population changes lead to a shift from an inflammatory condition into an anti-inflammatory microenvironment (Table 6).
Fig. 4. Anti-inflammatory and immunomodulatory mechanisms of EPCs.
This figure elucidates the anti-inflammatory and immunomodulatory mechanisms employed by endothelial progenitor cells (EPCs). It illustrates how EPCs exert their therapeutic effects through the suppression of inflammation, the modulation of immune responses, and the promotion of tissue repair and regeneration.
Table 6.
Phenotype conversion of blood cells induced by EPCs
| Target cell | Intermediary factor | Effects |
|---|---|---|
| Macrophages | PDGF, TGF-β, GM-CSF, M-CSF, TUG1, TSG-6, sTNF.R1, IL-4, IL-10, IL-13, miR30d |
↑ M2a/2b/2c polarization ↓M1 polarization |
| T cells | PDGF, TGF-β, HGF, GM-CSF, M-CSF, sTNF.R1, TUG1, IL-4, IL-10, IL-13 |
↑ Th2 and Treg polarization ↓T1 polarization, Th17 differentiation, and cytotoxic T cell maturation |
| B cells | FGF, CCL2 |
↑ regulatory ↓ synthesis of Ab |
| DCs | GM-CSF, M-CSF, PGE2 |
↑ CD103+ DCs ↓ maturation of immunogenic DCs |
| NKs | TGF-β, IL-6 | ↓ maturation of NKs |
EPC therapy for inflammatory disease
Preclinical trials
Several preclinical investigations have been performed regarding the anti-inflammatory effects of EPCs in several diseases, as outlined in Table 7:
Table 7.
Preclinical studies related to anti-inflammatory effects of EPCs
| Authors | EPC phenotype | Dosage | Method | Experimental model | Condition | Effects | Refs. |
|---|---|---|---|---|---|---|---|
| Bai et al. | ECFC | 10 μg/100 g (exosomes weight/mice weight) every three days for two weeks. | Intravenous | Mice | Atherosclerosis | ↓ inflammatory response | 102 |
| Zhou et al. | ECFC | EPC-derived exosomes | intratracheal | Mice | Acute lung injury | ↓cytokine and protein concentrations | 109 |
| Wang et al. | Cultured EPCs | 1 ml EPC-conditioned media | intraperitoneal | Rat | Spinal cord injuries | ↓ M1 macrophage and IL- 6 | 118 |
| Shoeibi et al. | ECFC | 4 × 106 EPCs | Intravenous | Rabbit | Atherosclerosis | ↓ infiltration of inflammatory elements | 124 |
| Hinkel et al. | CD34+/CD133+ | 5 × 106 EPCs or 15 mg EPC-derived thymosin B4 | Intravenous | Pig | Acute myocardial infarction | ↓ post-ischemic inflammatory responses, causing cardioprotection | 125 |
| Schuh et al. | CD34+/CD133+ | 1 × 106 EPCs | Intramyocardial | Rat | Chronic myocardial infarction | ↓ macrophage/neutrophil infiltration | 126 |
| Mena et al. | CD34+/CD133+ | 1 × 105 EPCs | Intravenous | Node mice | Hind limb ischemia | ↓ Inflammatory reactions | 130 |
| Penack et al. | CD34+/CD133+ | 1 × 106 EPCs | Intravenous | Murine | GVHD |
↑ anti-inflammatory effects and mobilization, ↓ monocyte infiltration |
132 |
| Cho et al. | CD34+/CD133+ | 3 × 106 EPCs | Intravenous | Rabbit | Balloon-mediated arterial injury | ↓ inflammatory cells recruitment | 133 |
| Mao et al. | Cultured EPCs | 5 × 106 EPCs | Intravenous | Rat | Acute lung injury | ↓TNF-α, IL-1β, neutrophil elastase, myeloperoxidase, and MMP-9, | 134 |
| Rafat et al. | CD34+/CD133+ | 1 × 106 EPCs | Intravenous | Mice | Acute respiratory distress syndrome | ↓ infiltration of inflammatory cells and restenosis | 135 |
| Guo et al. | CD34+/CD133+ | 3 × 106 EPCs | Intravenous | Rat | Glomerulonephritis | ↓ inflammatory response | 136 |
| Huang et al. | CD34+/CD133+ | 1 × 106 EPCs | Intra-arterial | Rat | Chronic kidney disease | ↓TNF-α and MMP-9 | 138 |
| Kim et al. | ECFC | 6 × 106 EPCs | Intracardial | Balb/c nude mice | Hind limb ischemia | ↓instant blood-mediated inflammatory reactions | 139 |
| Moubarik et al. | ECFC | 4 × 106 EPCs | Intravenous | Rat | tMCAO | ↑ IGF-1, ↓ proBDNF, | 140 |
| Acosta et al. | CD34+/CD133+ | 3 × 105 EPCs | Intracerebral | Mice | Inflammation-associated stroke vasculome | modulation of inflammation-related genes, including Brahma, NF-κB inhibitors, ITIH-5, and Foxf1 | 141 |
| Zhang et al. | CD34+/CD133+ | 5 × 106 EPCs | Intravenous | Rat | Intracerebral hemorrhage | ↓proinflammatory factors (IFN-γ, IL-6, and TNFα) and ↑ anti-inflammatory agents (TGFβ1 and IL-10) | 142 |
CVDs
Scientific reports have investigated the anti-inflammatory functions of EPCs in animal models of CVDs. Shoeibi et al. demonstrated that 4 × 106 ECFC transplantation decreases the infiltration of inflammatory cells into the atherosclerotic plaques in the experimental rabbit model of atherosclerosis124. Other study showed that embryonic EPCs secreted thymosin B4, which exerts cardioprotective properties via regulating post-ischemic inflammatory responses in the AMI pig experimental model125. Additionally, intramyocardial injection of 1 × 106 CD34/CD133+ EPCs reduced macrophage/neutrophil infiltration into the infarct zone, resulting in cardioprotective effects in a chronic setting126. Furthermore, VEGF and FGF levels are increased in target tissue after CD34/CD133+ EPC administration, proposing enhanced reendothelialization and suppressing inflammatory responses127,128.
Hind limb ischemia
Regarding the EPC therapy of ischemic hind limb, it has been indicated that the CD34+ EPC injection incites wound healing through angiogenesis enhancement and inflammation suppression in murine models129. Furthermore, Mena et al. showed that transplantation of 1 × 105 CD34/CD133+ EPCs increased vasculature and decreased inflammatory reactions in the nude mouse model of hind limb ischemia130. Similarly, immunohistochemistry studies revealed that groups treated with 5 × 104 CD34+ positive EPCs displayed less fibrosis and reduced inflammation area131. Together, these reports show the anti-inflammatory roles of EPCs in wound healing.
Graft-versus-host disease (GVHD)
When it comes to EPC therapy for GVHD, Penack et al. documented that mobilizing EPCs from BM into the bloodstream is an approach to ameliorate the inflammatory response in the murine models of GVHD132. Similarly, Cho et al. clarified that GM-CSF-mobilized CD34/CD133+ EPCs reduced monocyte infiltration and inflammatory responses, leading to improved reendothelialization and endothelium regeneration133.
Lung disease
In terms of EPC therapy in lung regeneration, Mao et al. illustrated that cultured EPCs mitigate lung injury by suppressing the secretion of inflammatory mediators in the rat model of acute lung injury134. Also, it has been illustrated that 1 × 106 transplantations of CD34/CD133+ EPCs suppress vascular inflammatory cell infiltration and restenosis, leading to reendothelialization in a mouse model of acute respiratory distress syndrome135.
Kidney disease
Regarding EPC treatment in kidney-related disorders, CD34/CD133+ EPC injection mitigates disease development by suppressing inflammatory response in the glomerulonephritis models136. In an experimental ischemia/reperfusion kidney injury model, Liang et al. found that transplantation of CD34/CD133+ EPCs significantly reduced the production of reactive oxygen species and inflammatory chemokines137. Besides, Huang et al. demonstrated that 1 × 106 CD34/CD133+ EPCs injection significantly suppressed the expression of TNF-α and MMP-9 after chronic kidney disease (CKD) induction, preventing the CKD progress through augmentation of angiogenesis and an anti-inflammatory response138. Additionally, Kim et al. demonstrated that the transplantation of 6 × 106 ECFC into the infra-kidney region of Balb/c nude mice suppressed the immediate blood-mediated inflammatory response139. Taken together, these findings provide evidence for the use of EPCs in the treatment of kidney-related disorders.
Neurologic-related disease
In this context, Moubarik et al. proposed that the intravenous injection of 4 × 106 ECFC attenuated neurological injury by upregulation of IGF-1, a neural protective cytokine, and downregulating of precursor brain-derived neurotrophic factor (proBDNF), a pro-inflammatory factor, which led to improved EPC functionality in an experimental rat model of transient middle cerebral artery occlusion140. Similarly, Acosta et al. have reported that injection of CD34/CD133+ EPCs decreased both the inflammation-related stroke volume and the immunoreactions by modulating inflammation-related genes (Brahma, NF-κB inhibitors, ITIH-5, and Foxf1)141. Moreover, treatment with CD34/CD133+ EPCs upgraded the neurological functions via triggering an anti-inflammatory response in the rat model of intracerebral hemorrhage142, supporting the use of EPCs in the anti-inflammatory treatment of neurologic-related disorders.
Ovarian senescence
In an experimental mouse model, Kim et al. reported that intravenously administered CD34/KDR+ hEPCs (twice, 5 × 104 cells, 4 days interval) reduced pro-inflammatory cytokines, endoplasmic reticulum stress via PERK and IRE1, and increased anti-inflammatory cytokines and blastocyst numbers, demonstrating hEPCs’ protective role against ovarian senescence143. Based on these findings, it could be decided that EPCs play a fundamental role in target tissue regeneration by activating anti-inflammatory response.
Clinical trials
While clinical trials investigating cell-based approaches to CVDs have shown promising results, it is important to note that as of now, no cell-based therapy has obtained regulatory approval for any cardiovascular indication144. In this section, we are addressing some anti-inflammatory-related effects of EPC transplantation in several clinical trials. The promising efficacy of EPC transplantation in several diseases, particularly in vascular medicine, has been well documented in the scientific literature145–147. However, the mechanistic perspective of the anti-inflammatory properties of EPC therapy is poorly understood in clinical trial settings. In this regard, Klomp et al. reported that CD34+ EPC-capturing stents meaningfully regress intimal hyperplasia in an 18-month monitoring period (Table 8)148. Supportively, a 28-day follow-up showed that CD34+ EPC capturing stents significantly reduce neointimal area and inflammatory response149,150. This EPC capture technology is engineered to attract circulating CD34+ EPCs, mitigate inflammatory signals, and contribute to vascular reparation151. Moreover, Yau et al. reported that progenitors inhibit the synthesis of inflammatory cytokines during left ventricular assist device implantation in a randomized phase 2 clinical trial152. Also, Steinhoff et al. convincingly demonstrated that intramyocardial administration of CD133+ decrease inflammatory cytokines, such as IL-6, IP10 and NT-proBNP, leading to improvement of cardiac function in a phase III clinical trial of MI153. Supporting evidence comes from Zhang et al., who showed that autologous CD133+ EPC therapy for diabetic peripheral arterial disease significantly reduced levels of IL-6 and reduced amputation rate after 4 weeks, reflecting a stronger anti-inflammatory guideline of EPCs154. Regarding the above-mentioned QQ-culturing of MNCs, Tanaka et al. investigated the safety and efficacy of QQ-MNC therapy for chronic non-healing ischemic extremity wounds in a prospective clinical study. They showed that transplanting 2 × 107 QQ-MNCs increased the anti-inflammatory response, wound closure, vascular perfusion, skin perfusion pressure, and decreased pain intensity in all patients, indicating the feasibility and safety of MNC-QQ therapy in clinical trial settings155. As the therapy involves transplanting highly vasculogenic cells obtained from a small blood sample, it may be an effective and highly vasculogenic strategy for regenerative medicine.
Table 8.
Clinical studies related to anti-inflammatory effects of EPCs
| Authors | Dosage | Method | Condition | Follow-up period | Effects | Refs. |
|---|---|---|---|---|---|---|
| Klomp et al. | - | EPC capturing stents | Percutaneous coronary intervention | 18 months | ↓ Intimal hyperplasia | 148 |
| Aoki et al. | - | EPC capturing stents | Angina or silent ischemia. | 28 days | ↓Neointimal area and inflammatory response | 150 |
| Saito et al. | - | EPC capturing stents | Ischaemic coronary disease | 12 months | ↓Inflammatory and prothrombotic signals | 151 |
| Yau et al. |
15 × 106 Mesenchymal Precursor Cells |
Intramyocardial | Advanced heart failure | 12 months | ↓ Synthesis of inflammatory cytokines, septicity, hemorrhage and thrombosis | 152 |
| Steinhoff et al. | 0.5–5 × 106 CD133/VEGFR-2 EPCs | intramyocardially | Myocardial infarction | 24 months | ↓ inflammatory cytokines, such as IL-6, IP10, and NT-proBNP | 153 |
| Zhang et al. | 10 × 106 CD133+ EPCs | intra-arterial | Peripheral arterial disease | 4 weeks | ↓ inflammatory cytokines, such as IL-6 and amputation rate | 154 |
In summary, delving into the anti-inflammatory weaponry of EPCs is imperative for a comprehensive understanding of their pivotal role in both direct and indirect tissue regeneration. Notably, prior clinical trials evaluating inflammatory responses post-EPC transplantation have been limited, leaving a gap in our comprehension of their potential to mitigate immune reactions. Building upon our current knowledge, it becomes evident that EPCs possess remarkable anti-inflammatory functions, positioning them as key players in the realm of EPC therapy for future clinical applications. Recognizing the transformative impact of EPCs on shifting from inflammatory crises to anti-inflammatory and immunomodulatory responses underscores their potential as game-changers in advancing vascular regenerative medicine. This newfound comprehension not only deepens our insights into the intricate molecular aspects of EPC function but also unlocks promising avenues for the development of therapeutic strategies targeting vascular-related diseases.
Acknowledgements
The authors wish to thank the personnel of the Shonan Research Institute of Innovative Medicine and the Center for Cell therapy & Regenerative Medicine of Shonan Kamakura General Hospital for kindest guidance and help. This study has no funding.
Author contributions
T.A. designed the study and put forward the conception; M.H. literature review, wrote the manuscript and prepared figures; S.K., A.A.S., and T.A. contributed to manuscript writing and put forward the conception. All authors reviewed and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chavakis, T., Mitroulis, I. & Hajishengallis, G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat. Immunol.20, 802–811 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salybekov, A. A., Hassanpour, M., Kobayashi, S. & Asahara, T. Therapeutic application of regeneration-associated cells: a novel source of regenerative medicine. Stem Cell. Res. Ther.14, 191 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke, J. P. Inflammation and its role in regeneration and repair: a caution for novel anti-inflammatory therapies. Circ. Res.124, 1166–1168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naserian, S. et al. The TNF/TNFR2 signaling pathway is a key regulatory factor in endothelial progenitor cell immunosuppressive effect. Cell Commun. Signal.18, 1–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladhoff, J., Fleischer, B., Hara, Y., Volk, H.-D. & Seifert, M. Immune privilege of endothelial cells differentiated from endothelial progenitor cells. Cardiovasc. Res.88, 121–129 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Arakelian, L. et al. Endothelial CD34 expression and regulation of immune cell response in-vitro. Sci. Rep.13, 13512 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science275, 964–966 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Timmermans, F. et al. Endothelial progenitor cells: identity defined? J. Cell. Mol. Med.13, 87–102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asahara, T., Kawamoto, A. & Masuda, H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem cells29, 1650–1655 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Hebbel, R. P. Blood endothelial cells: utility from ambiguity. J. Clin. Investig.127, 1613–1615 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medina, R. J. et al. Endothelial progenitors: a consensus statement on nomenclature. Stem cells Transl. Med.6, 1316–1320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asahara, T. et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res.85, 221–228 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Minami, Y. et al. Angiogenic potential of early and late outgrowth endothelial progenitor cells is dependent on the time of emergence. Int. J. Cardiol.186, 305–314 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Fujisawa, T. et al. Endothelial progenitor cells do not originate from the bone marrow. Circulation140, 1524–1526 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin, Y. et al. Origin, prospective identification, and function of circulating endothelial colony-forming cells in mice and humans. JCI insight8, e164781 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng, C.-Y. & Cheung, C. Origins and functional differences of blood endothelial cells. Semin. Cell Dev. Biol.155, 23–29 (2024). [DOI] [PubMed] [Google Scholar]
- 17.Medina, R. J. et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med. Genom.3, 1–13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alwjwaj, M., Kadir, R. R. A. & Bayraktutan, U. The secretome of endothelial progenitor cells: a potential therapeutic strategy for ischemic stroke. Neural Regen. Res.16, 1483 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Y. et al. Upregulated miR-206 aggravates deep vein thrombosis by regulating GJA1-mediated autophagy of endothelial progenitor cells. Cardiovasc. Ther.2022, 9966306 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molnar, C. et al. Anti-inflammatory effects of hepatocyte growth factor: induction of interleukin-1 receptor antagonist. Eur. Cytokin Netw.15, 303–311 (2004). [PubMed]
- 21.Everaert, B. R. et al. Identification of macrophage genotype and key biological pathways in circulating angiogenic cell transcriptome. Stem Cells Int.2019, 9545261 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hur, J. et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler. Thromb. Vasc. Biol.24, 288–293 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Lee, J. H., Lee, S. H., Yoo, S. Y., Asahara, T. & Kwon, S. M. CD34 hybrid cells promote endothelial colony-forming cell bioactivity and therapeutic potential for ischemic diseases. Arterioscler. Thromb. Vasc. Biol.33, 1622–1634 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Schwartzenberg, S. et al. Association between circulating early endothelial progenitors and CD4 + CD25+ regulatory T-cells: a possible cross-talk between immunity and angiogenesis. Am. J. Immunol.1, 143–147 (2005). [Google Scholar]
- 25.Motz, G. T. & Coukos, G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat. Rev. Immunol.11, 702–711 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Rivera, L. B. & Bergers, G. Intertwined regulation of angiogenesis and immunity by myeloid cells. Trends Immunol.36, 240–249 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagemann, T. et al. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J. Immunol.176, 5023–5032 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Venneri, M. A. et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood J. Am. Soc. Hematol.109, 5276–5285 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol.9, 162–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chambers, S. E., O’Neill, C. L., O’Doherty, T. M., Medina, R. J. & Stitt, A. W. The role of immune-related myeloid cells in angiogenesis. Immunobiology218, 1370–1375 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Yan, F., Liu, X., Ding, H. & Zhang, W. Paracrine mechanisms of endothelial progenitor cells in vascular repair. Acta Histochem.124, 151833 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Masuda, H. et al. Vasculogenic conditioning of peripheral blood mononuclear cells promotes endothelial progenitor cell expansion and phenotype transition of anti‐inflammatory macrophage and T lymphocyte to cells with regenerative potential. J. Am. Heart Assoc.3, e000743 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salybekov, A. A. et al. Regeneration-associated cells improve recovery from myocardial infarction through enhanced vasculogenesis, anti-inflammation, and cardiomyogenesis. PloS ONE13, e0203244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka, R. et al. Quality-control culture system restores diabetic endothelial progenitor cell vasculogenesis and accelerates wound closure. Diabetes62, 3207–3217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kashiyama, N. et al. Vasculogenically conditioned peripheral blood mononuclear cells inhibit mouse immune response to induced pluripotent stem cell-derived allogeneic cardiac grafts. PloS ONE14, e0217076 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merola, J. et al. Progenitor-derived human endothelial cells evade alloimmunity by CRISPR/Cas9-mediated complete ablation of MHC expression. JCI Insight4, e129739 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreisel, D. et al. Non-hematopoietic allograft cells directly activate CD8 + T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat. Med.8, 233–239 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Holmes, K., Roberts, O. L., Thomas, A. M. & Cross, M. J. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell. Signal.19, 2003–2012 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Hu, W.-H. et al. The binding of kaempferol-3-O-rutinoside to vascular endothelial growth factor potentiates anti-inflammatory efficiencies in lipopolysaccharide-treated mouse macrophage RAW264. 7 cells. Phytomedicine80, 153400 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Zhang, Y. et al. Activation of vascular endothelial growth factor receptor-3 in macrophages restrains TLR4-NF-κB signaling and protects against endotoxin shock. Immunity40, 501–514 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Vieira, J. M. et al. The cardiac lymphatic system stimulates resolution of inflammation following myocardial infarction. J. Clin. Investig.128, 3402–3412 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdelgawad, M. E., Desterke, C., Uzan, G. & Naserian, S. Single-cell transcriptomic profiling and characterization of endothelial progenitor cells: new approach for finding novel markers. Stem Cell Res. Ther.12, 1–16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medica, D. et al. Extracellular vesicles derived from endothelial progenitor cells protect human glomerular endothelial cells and podocytes from complement-and cytokine-mediated injury. Cells10, 1675 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiao, X. et al. Redirecting TGF-β signaling through the β-Catenin/Foxo complex prevents kidney fibrosis. J. Am. Soc. Nephrol.29, 557–570 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, J. et al. The role of endogenous Smad7 in regulating macrophage phenotype following myocardial infarction. FASEB J.36, e22400 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawakubo, A. et al. Origin of M2 Mϕ and its macrophage polarization by TGF-β in a mice intervertebral injury model. Int. J. Immunopathol. Pharmacol.36, 03946320221103792 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandolfo, M. T. et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int.76, 717–729 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Huang, T. et al. Regenerating myofiber directs Tregs and Th17 responses in inflamed muscle through the intrinsic TGF-β signaling mediated IL-6 production. Am. J. Physiol. Endocrinol. Metab.323, E92–E106 (2022). [DOI] [PubMed]
- 49.Mallis, P. et al. Mesenchymal stromal cell delivery as a potential therapeutic strategy against COVID-19: Promising evidence from in vitro results. World J. Biol. Chem.13, 47 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vašíček, J. et al. Secretome analysis of rabbit and human mesenchymal stem and endothelial progenitor cells: a comparative study. Int. J. Mol. Sci.22, 12283 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, N. et al. Fibroblast growth factor 21 exerts its anti‐inflammatory effects on multiple cell types of adipose tissue in obesity. Obesity27, 399–408 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Fujihara, C. et al. Fibroblast growth factor‐2 inhibits CD40‐mediated periodontal inflammation. J. Cell. Physiol.234, 7149–7160 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Wen, Q. et al. G-CSF-induced macrophage polarization and mobilization may prevent acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant.54, 1419–1433 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Lotfi, N. et al. Roles of GM-CSF in the pathogenesis of autoimmune diseases: an update. Front. Immunol.10, 1265 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang, Y.-N. et al. Granulocyte colony-stimulating factor alleviates bacterial-induced neuronal apoptotic damage in the neonatal rat brain through epigenetic histone modification. Oxid. Med. Cell. Longev.2018, 9797146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coombes, J. L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β–and retinoic acid–dependent mechanism. J. Exp. Med.204, 1757–1764 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rong, S.-l et al. Anti-inflammatory activities of hepatocyte growth factor in post-ischemic heart failure. Acta Pharmacol. Sin.39, 1613–1621 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kusunoki, H., Taniyama, Y., Otsu, R., Rakugi, H. & Morishita, R. Anti-inflammatory effects of hepatocyte growth factor on the vicious cycle of macrophages and adipocytes. Hypertens. Res.37, 500–506 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Gong, R., Rifai, A. & Dworkin, L. D. Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: targeting the inflamed vascular endothelium. J. Am. Soc. Nephrol.17, 2464–2473 (2006). [DOI] [PubMed] [Google Scholar]
- 60.da Silva, C. G. et al. Hepatocyte growth factor preferentially activates the anti‐inflammatory arm of NF‐κB signaling to induce A20 and protect renal proximal tubular epithelial cells from inflammation. J. Cell. Physiol.227, 1382–1390 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen, P. M. et al. Induction of immunomodulatory monocytes by human mesenchymal stem cell‐derived hepatocyte growth factor through ERK1/2. J. Leukoc. Biol.96, 295–303 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Wyler von Ballmoos, M. et al. Endothelial progenitor cells induce a phenotype shift in differentiated endothelial cells towards PDGF/PDGFRβ axis-mediated angiogenesis. PLoS One5, e14107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang, S. et al. Long-term local PDGF delivery using porous microspheres modified with heparin for tendon healing of rotator cuff tendinitis in a rabbit model. Carbohydr. Polym.209, 372–381 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Fang, J. et al. Endothelial progenitor cells promote viability and nerve regenerative ability of mesenchymal stem cells through PDGF-BB/PDGFR-β signaling. Aging12, 106 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang, F. et al. Bone marrow mesenchymal stem cells induce M2 microglia polarization through PDGF-AA/MANF signaling. World J. Stem Cells12, 633 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang, J.-M. et al. Platelet-derived growth factor-BB protects mesenchymal stem cells (MSCs) derived from immune thrombocytopenia patients against apoptosis and senescence and maintains MSC-mediated immunosuppression. Stem Cells Transl. Med.5, 1631–1643 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Majka, M. et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34+ cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner: Presented at the 41st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 3-7, 1999, and published in abstract form in Blood. 1999; 94 (suppl 1): 465a. Blood J. Am. Soc. Hematol.97, 3075–3085 (2001). [DOI] [PubMed] [Google Scholar]
- 68.Jarajapu, Y. P. et al. Vasoreparative dysfunction of CD34+ cells in diabetic individuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms. PLoS ONE9, e93965 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng, C.-C. et al. Distinct angiogenesis roles and surface markers of early and late endothelial progenitor cells revealed by functional group analyses. BMC Genom.14, 1–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morris, R., Kershaw, N. J. & Babon, J. J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci.27, 1984–2009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mosser, D. M. & Zhang, X. Interleukin‐10: new perspectives on an old cytokine. Immunol. Rev.226, 205–218 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krishnamurthy, P. et al. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ. Res.104, e9–e18 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mantel, P.-Y. & Schmidt-Weber, C. B. Transforming growth factor-beta: recent advances on its role in immune tolerance. Suppr. Regul. Immune Responses, 677, 303–338 (2010). [DOI] [PubMed]
- 74.Junttila, I. S. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol.9, 888 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zullo, J. A. et al. The secretome of hydrogel-coembedded endothelial progenitor cells and mesenchymal stem cells instructs macrophage polarization in endotoxemia. Stem cells Transl. Med.4, 852–861 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gordon, S. & Martinez, F. O. Alternative activation of macrophages: mechanism and functions. Immunity32, 593–604 (2010). [DOI] [PubMed] [Google Scholar]
- 77.Choi, H., Lee, R. H., Bazhanov, N., Oh, J. Y. & Prockop, D. J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood J. Am. Soc. Hematol.118, 330–338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song, W.-J. et al. TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates DSS-induced colitis by inducing M2 macrophage polarization in mice. Sci. Rep.7, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abd-Allah, S. H., Shalaby, S. M., Abd-Elbary, E., Saleh, A. A. & El-Magd, M. A. Human peripheral blood CD34+ cells attenuate oleic acid–induced acute lung injury in rats. Cytotherapy17, 443–453 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Day, A. J. & Milner, C. M. TSG-6: a multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol.78, 60–83 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Ke, F. et al. Soluble tumor necrosis factor receptor 1 released by skin-derived mesenchymal stem cells is critical for inhibiting Th17 cell differentiation. Stem cells Transl. Med.5, 301–313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yagi, H. et al. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol. Ther.18, 1857–1864 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouchentouf, M. et al. A novel and simplified method of culture of human blood-derived early endothelial progenitor cells for the treatment of ischemic vascular disease. Cell Transplant.20, 1431–1443 (2011). [DOI] [PubMed] [Google Scholar]
- 84.Carneiro, G. D. et al. Administration of endothelial progenitor cells accelerates the resolution of arterial thrombus in mice. Cytotherapy21, 444–459 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Abou-Saleh, H. et al. Endothelial progenitor cells bind and inhibit platelet function and thrombus formation. Circulation120, 2230–2239 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bou Khzam, L. et al. Early outgrowth cells versus endothelial colony forming cells functions in platelet aggregation. J. Transl. Med.13, 1–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cesari, F. et al. NT-proBNP and the anti-inflammatory cytokines are correlated with endothelial progenitor cells’ response to cardiac surgery. Atherosclerosis199, 138–146 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Dernbach, E. et al. Antioxidative stress–associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood104, 3591–3597 (2004). [DOI] [PubMed] [Google Scholar]
- 89.Urbich, C. et al. Proteomic characterization of human early pro-angiogenic cells. J. Mol. Cell. Cardiol.50, 333–336 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Yang, Z. et al. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis211, 103–109 (2010). [DOI] [PubMed] [Google Scholar]
- 91.Statello, L., Guo, C.-J., Chen, L.-L. & Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol.22, 96–118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Venkat, P. et al. CD133+ exosome treatment improves cardiac function after stroke in type 2 diabetic mice. Transl. stroke Res.12, 112–124 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olivieri, F. et al. Anti-inflammatory effect of ubiquinol-10 on young and senescent endothelial cells via miR-146a modulation. Free Radic. Biol. Med.63, 410–420 (2013). [DOI] [PubMed] [Google Scholar]
- 94.Su, Z.-F. et al. Regulatory effects of miR-146a/b on the function of endothelial progenitor cells in acute ischemic stroke in mice. Kaohsiung J. Med. Sci.33, 369–378 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Zheng, G. et al. miR-10a in peripheral blood mononuclear cells is a biomarker for sepsis and has anti-inflammatory function. Mediat. Inflamm.2020, 4370983 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu, S. et al. MicroRNA-10A* and MicroRNA-21 modulate endothelial progenitor cell senescence via suppressing high-mobility group A2. Circ. Res.112, 152–164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma, W. et al. Functional delivery of lncRNA TUG1 by endothelial progenitor cells derived extracellular vesicles confers anti-inflammatory macrophage polarization in sepsis via impairing miR-9-5p-targeted SIRT1 inhibition. Cell Death Dis.12, 1056 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuan, F. et al. Endothelial progenitor cell-derived exosomes promote anti-inflammatory macrophages via SOCS3/JAK2/STAT3 axis and improve the outcome of spinal cord injury. J. Neuroinflamm.20, 156 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ke, X. et al. Human endothelial progenitor cell-derived exosomes increase proliferation and angiogenesis in cardiac fibroblasts by promoting the mesenchymal–endothelial transition and reducing high mobility group box 1 protein B1 expression. DNA Cell Biol.36, 1018–1028 (2017). [DOI] [PubMed] [Google Scholar]
- 100.Terriaca, S. et al. Endothelial progenitor cell-derived extracellular vesicles: potential therapeutic application in tissue repair and regeneration. Int. J. Mol. Sci.22, 6375 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salybekov, A. A., Kunikeyev, A. D., Kobayashi, S. & Asahara, T. Latest advances in endothelial progenitor cell-derived extracellular vesicles translation to the clinic. Front. Cardiovasc. Med.8, 734562 (2021). [DOI] [PMC free article] [PubMed]
- 102.Bai, S. et al. Endothelial progenitor cell–derived exosomes ameliorate endothelial dysfunction in a mouse model of diabetes. Biomed. Pharmacother.131, 110756 (2020). [DOI] [PubMed] [Google Scholar]
- 103.Ke, X. et al. Exosomal miR-218-5p/miR-363-3p from endothelial progenitor cells ameliorate myocardial infarction by targeting the P53/JMY signaling pathway. Oxid. Med. Cell. Longev. 2021, 5529430 (2021). [DOI] [PMC free article] [PubMed]
- 104.Zhou, W. et al. Rab27a deletion impairs the therapeutic potential of endothelial progenitor cells for myocardial infarction. Mol. Cell. Biochem.476, 797–807 (2021). [DOI] [PubMed] [Google Scholar]
- 105.Schabbauer, G., Tencati, M., Pedersen, B., Pawlinski, R. & Mackman, N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler. Thromb. Vasc. Biol.24, 1963–1969 (2004). [DOI] [PubMed] [Google Scholar]
- 106.Zhao, L., Yang, X. R. & Han, X. MicroRNA‑146b induces the PI3K/Akt/NF‑κB signaling pathway to reduce vascular inflammation and apoptosis in myocardial infarction by targeting PTEN. Exp. Ther. Med.17, 1171–1181 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu, J. et al. miRNA-221-3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice. Diab. Metab. Syndr. Obes.13, 1259 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xiong, W. et al. Endothelial progenitor cell (EPCs)-derived exosomal miR-30d-5p inhibits the inflammatory response of high glucose-impaired fibroblasts by affecting the M1/M2 polarization of macrophages. Rev. Romana de. Med. de. Lab.30, 435–451 (2022). [Google Scholar]
- 109.Zhou, Y. et al. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit. Care23, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu, X., Liu, Z., Hu, L., Gu, W. & Zhu, L. Exosomes derived from endothelial progenitor cells ameliorate acute lung injury by transferring miR-126. Exp. Cell Res.370, 13–23 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Viñas, J. L. et al. Transfer of microRNA-486-5p from human endothelial colony forming cell–derived exosomes reduces ischemic kidney injury. Kidney Int.90, 1238–1250 (2016). [DOI] [PubMed] [Google Scholar]
- 112.Zhang, Y. et al. Endothelial progenitor cells-derived exosomal microRNA-21-5p alleviates sepsis-induced acute kidney injury by inhibiting RUNX1 expression. Cell Death Dis.12, 1–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luo, M.-C. et al. Runt-related transcription factor 1 (RUNX1) binds to p50 in macrophages and enhances TLR4-triggered inflammation and septic shock. J. Biol. Chem.291, 22011–22020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qiu, N., Xu, X. & He, Y. LncRNA TUG1 alleviates sepsis-induced acute lung injury by targeting miR-34b-5p/GAB1. BMC Pulm. Med.20, 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Han, J. et al. lncRNA TUG1 regulates ulcerative colitis through miR-142-5p/SOCS1 axis. Microb. Pathog.143, 104139 (2020). [DOI] [PubMed] [Google Scholar]
- 116.Schug, T. T. et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol. Cell. Biol.30, 4712–4721 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou, Y. et al. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol. Ther.26, 1375–1384 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang, T., Fang, X. & Yin, Z.-S. Endothelial progenitor cell-conditioned medium promotes angiogenesis and is neuroprotective after spinal cord injury. Neural Regen. Res.13, 887 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu, Y. et al. Mesenchymal stem cells enhance microglia M2 polarization and attenuate neuroinflammation through TSG-6. Brain Res.1724, 146422 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Jung, M. et al. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res. Cardiol.112, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Urbich, C. & Dimmeler, S. Endothelial progenitor cells: characterization and role in vascular biology. Circ. Res.95, 343–353 (2004). [DOI] [PubMed] [Google Scholar]
- 122.Kado, M. et al. Human peripheral blood mononuclear cells enriched in endothelial progenitor cells via quality and quantity controlled culture accelerate vascularization and wound healing in a porcine wound model. Cell Transplant.27, 1068–1079 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sekine, K. et al. Transplantation of fibroblast sheets with blood mononuclear cell culture exerts cardioprotective effects by enhancing anti-inflammation and vasculogenic potential in rat experimental autoimmune myocarditis model. Biology11, 106 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shoeibi, S., Mahdipour, E., Mohammadi, S., Moohebati, M. & Ghayour-Mobarhan, M. Treatment of atherosclerosis through transplantation of endothelial progenitor cells overexpressing dimethylarginine dimethylaminohydrolase (DDAH) in rabbits. Int. J. Cardiol.331, 189–198 (2021). [DOI] [PubMed] [Google Scholar]
- 125.Hinkel, R. et al. Thymosin β4 is an essential paracrine factor of embryonic endothelial progenitor cell–mediated cardioprotection. Circulation117, 2232–2240 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schuh, A. et al. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res. Cardiol.103, 69–77 (2008). [DOI] [PubMed] [Google Scholar]
- 127.Loiola, R. A. et al. Secretome of endothelial progenitor cells from stroke patients promotes endothelial barrier tightness and protects against hypoxia-induced vascular leakage. Stem Cell Res. Ther.12, 1–23 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 128.Zhang, H.-F. et al. Enhancement of cardiac lymphangiogenesis by transplantation of CD34 + VEGFR-3+ endothelial progenitor cells and sustained release of VEGF-C. Basic Res. Cardiol.114, 1–17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee, J. H. et al. Specific disruption of Lnk in murine endothelial progenitor cells promotes dermal wound healing via enhanced vasculogenesis, activation of myofibroblasts, and suppression of inflammatory cell recruitment. Stem cell Res. Ther.7, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mena, H. A. et al. Acidic preconditioning of endothelial colony-forming cells (ECFC) promote vasculogenesis under proinflammatory and high glucose conditions in vitro and in vivo. Stem Cell Res. Ther.9, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lim, S. et al. Predicting in vivo therapeutic efficacy of bioorthogonally labeled endothelial progenitor cells in hind limb ischemia models via non-invasive fluorescence molecular tomography. Biomaterials266, 120472 (2021). [DOI] [PubMed] [Google Scholar]
- 132.Penack, O. et al. Depletion of vascular endothelial progenitor cells inhibits inflammation. Blood112, 694 (2008). [Google Scholar]
- 133.Cho, H.-J. et al. Mobilized endothelial progenitor cells by granulocyte-macrophage colony-stimulating factor accelerate reendothelialization and reduce vascular inflammation after intravascular radiation. Circulation108, 2918–2925 (2003). [DOI] [PubMed] [Google Scholar]
- 134.Mao, M., Hao, L., Wang, Y. & Liu, Q.-Q. Transplantation of endothelial progenitor cells attenuates lipopolysaccharide-induced lung injury via inhibiting the inflammatory secretion of neutrophils in rats. Am. J. Med. Sci.357, 49–56 (2019). [DOI] [PubMed] [Google Scholar]
- 135.Rafat, N. et al. Bone marrow-derived progenitor cells attenuate inflammation in lipopolysaccharide-induced acute respiratory distress syndrome. BMC Res. Notes7, 1–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guo, W., Feng, J.-M., Yao, L., Sun, L. & Zhu, G.-Q. Transplantation of endothelial progenitor cells in treating rats with IgA nephropathy. BMC Nephrol.15, 1–13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liang, C.-J. et al. Endothelial progenitor cells derived from Wharton’s jelly of human umbilical cord attenuate ischemic acute kidney injury by increasing vascularization and decreasing apoptosis, inflammation, and fibrosis. Cell Transplant.24, 1363–1377 (2015). [DOI] [PubMed] [Google Scholar]
- 138.Huang, T.-H. et al. Peripheral blood-derived endothelial progenitor cell therapy prevented deterioration of chronic kidney disease in rats. Am. J. Transl. Res.7, 804 (2015). [PMC free article] [PubMed] [Google Scholar]
- 139.Kim, J. H. et al. Endothelial colony-forming cell coating of pig islets prevents xenogeneic instant blood-mediated inflammatory reaction. Cell Transplant.20, 1805–1815 (2011). [DOI] [PubMed] [Google Scholar]
- 140.Moubarik, C. et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev. Rep.7, 208–220 (2011). [DOI] [PubMed] [Google Scholar]
- 141.Acosta, S. A., Lee, J. Y., Nguyen, H., Kaneko, Y. & Borlongan, C. V. Endothelial progenitor cells modulate inflammation-associated stroke vasculome. Stem cell Rev. Rep.15, 256–275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang, R. et al. The therapeutic value of bone marrow-derived endothelial progenitor cell transplantation after intracerebral hemorrhage in rats. Front. Neurol.8, 174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim, G. A. et al. Intravenous human endothelial progenitor cell administration into aged mice enhances embryo development and oocyte quality by reducing inflammation, endoplasmic reticulum stress and apoptosis. J. Vet. Med. Sci.80, 1905–1913 (2018). [DOI] [PMC free article] [PubMed]
- 144.Fanaroff, A. C. et al. A path forward for regenerative medicine: navigating regulatory challenges: summary of findings from the cardiac safety research consortium/texas heart institute international symposium on cardiovascular regenerative medicine. Circ. Res.123, 495–505 (2018). [DOI] [PubMed] [Google Scholar]
- 145.Sukmawati, D. & Tanaka, R. Introduction to next generation of endothelial progenitor cell therapy: a promise in vascular medicine. Am. J. Transl. Res.7, 411 (2015). [PMC free article] [PubMed] [Google Scholar]
- 146.Prasad, M. et al. Promise of autologous CD34+ stem/progenitor cell therapy for treatment of cardiovascular disease. Cardiovasc. Res.116, 1424–1433 (2020). [DOI] [PubMed] [Google Scholar]
- 147.Rai, B., Shukla, J., Henry, T. D. & Quesada, O. Angiogenic CD34 stem cell therapy in coronary microvascular repair—a systematic review. Cells10, 1137 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Klomp, M., Beijk, M. A., Tijssen, J. G. & de Winter, R. J. Significant intimal hyperplasia regression between 6 and 18 months following Genous™ endothelial progenitor cell capturing stent placement. Int. J. Cardiol.147, 289–291 (2011). [DOI] [PubMed] [Google Scholar]
- 149.Kutryk, M. J. & Kuliszewski, M. A. In vivo endothelial progenitor seeding of stented arterial segments and vascular grafts. Circulation108, IV–573 (2003). [Google Scholar]
- 150.Aoki, J. et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) registry. J. Am. Coll. Cardiol.45, 1574–1579 (2005). [DOI] [PubMed] [Google Scholar]
- 151.Saito, S. et al. Japan-United States of America Harmonized Assessment by Randomized Multicentre Study of OrbusNEich’s Combo StEnt (Japan-USA HARMONEE) study: primary results of the pivotal registration study of combined endothelial progenitor cell capture and drug-eluting stent in patients with ischaemic coronary disease and non-ST-elevation acute coronary syndrome. Eur. Heart J.39, 2460–2468 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yau, T. M. et al. Intramyocardial injection of mesenchymal precursor cells and successful temporary weaning from left ventricular assist device support in patients with advanced heart failure: a randomized clinical trial. Jama321, 1176–1186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Steinhoff, G. et al. Cardiac function improvement and bone marrow response–: outcome analysis of the randomized perfect phase iii clinical trial of intramyocardial cd133+ application after myocardial infarction. EBioMedicine22, 208–224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang, X. et al. Transcatheter arterial infusion of autologous CD133+ cells for diabetic peripheral artery disease. Stem Cells Int.2016, 6925357 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tanaka, R. et al. Phase I/IIa feasibility trial of autologous quality-and quantity-cultured peripheral blood mononuclear cell therapy for non-healing extremity ulcers. Stem Cells Transl. Med.11, 146–158 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]