Abstract
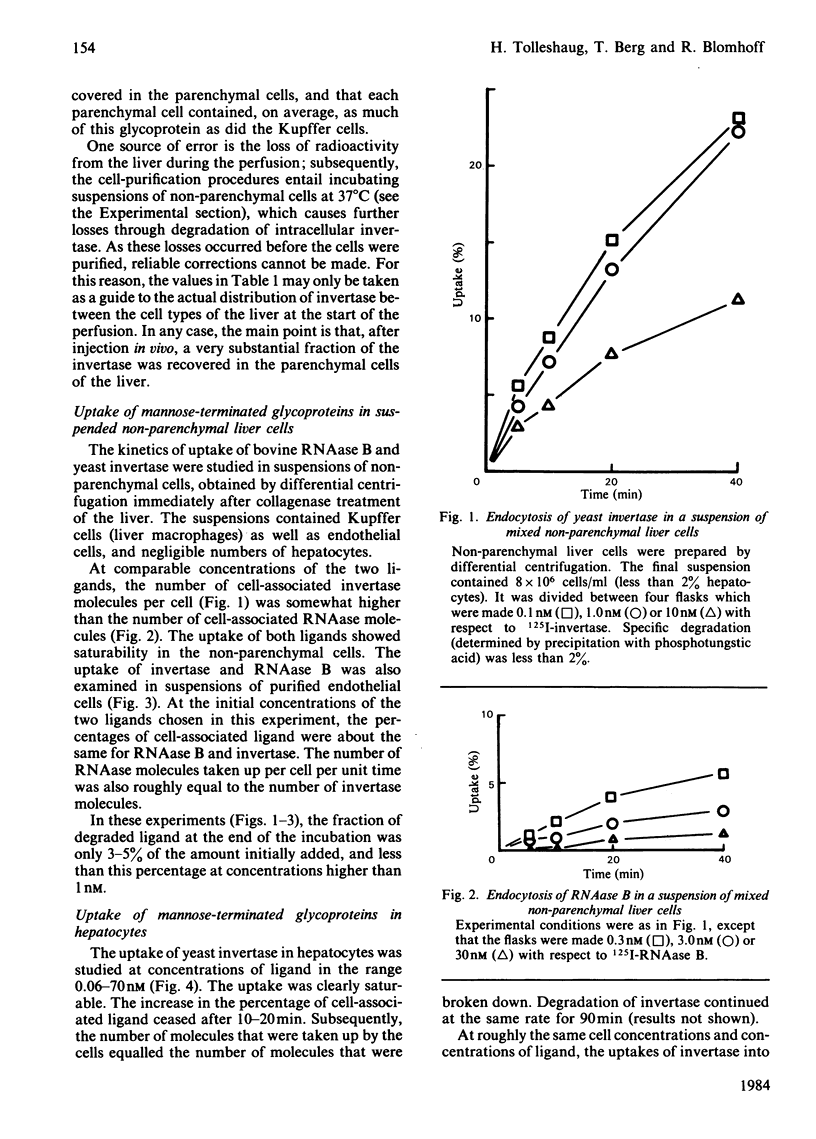
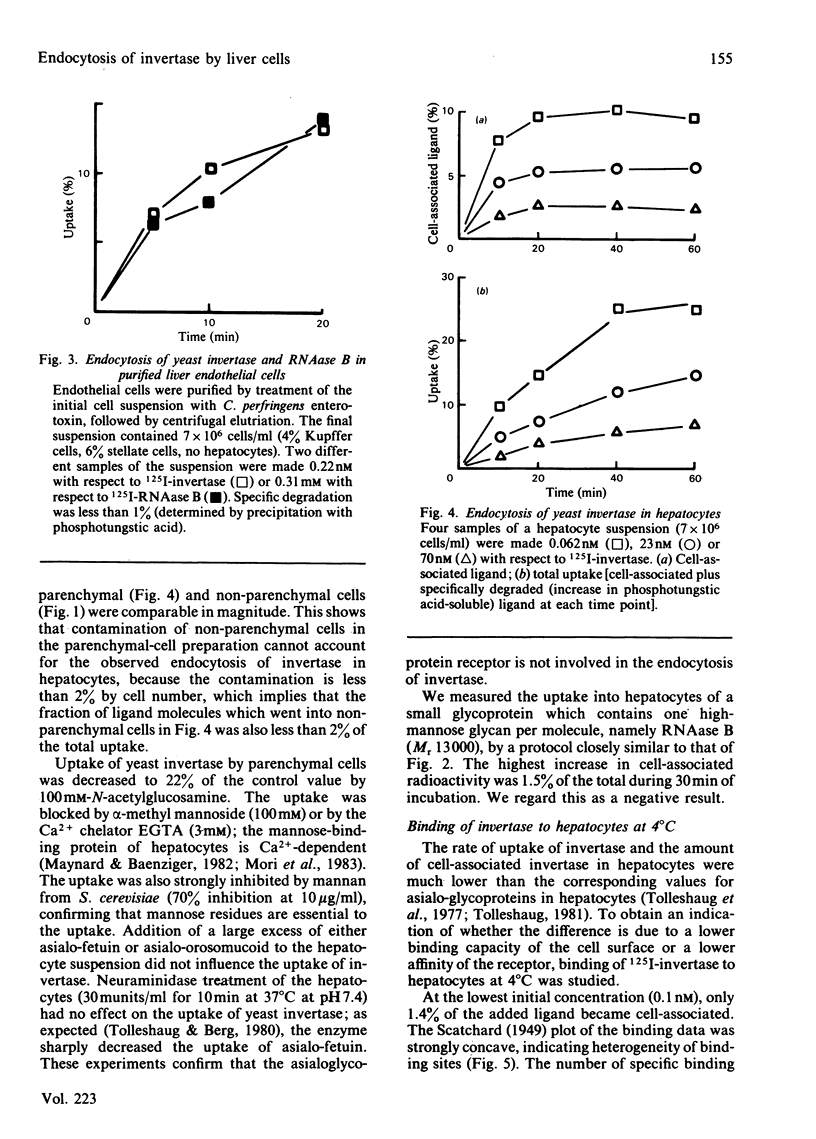
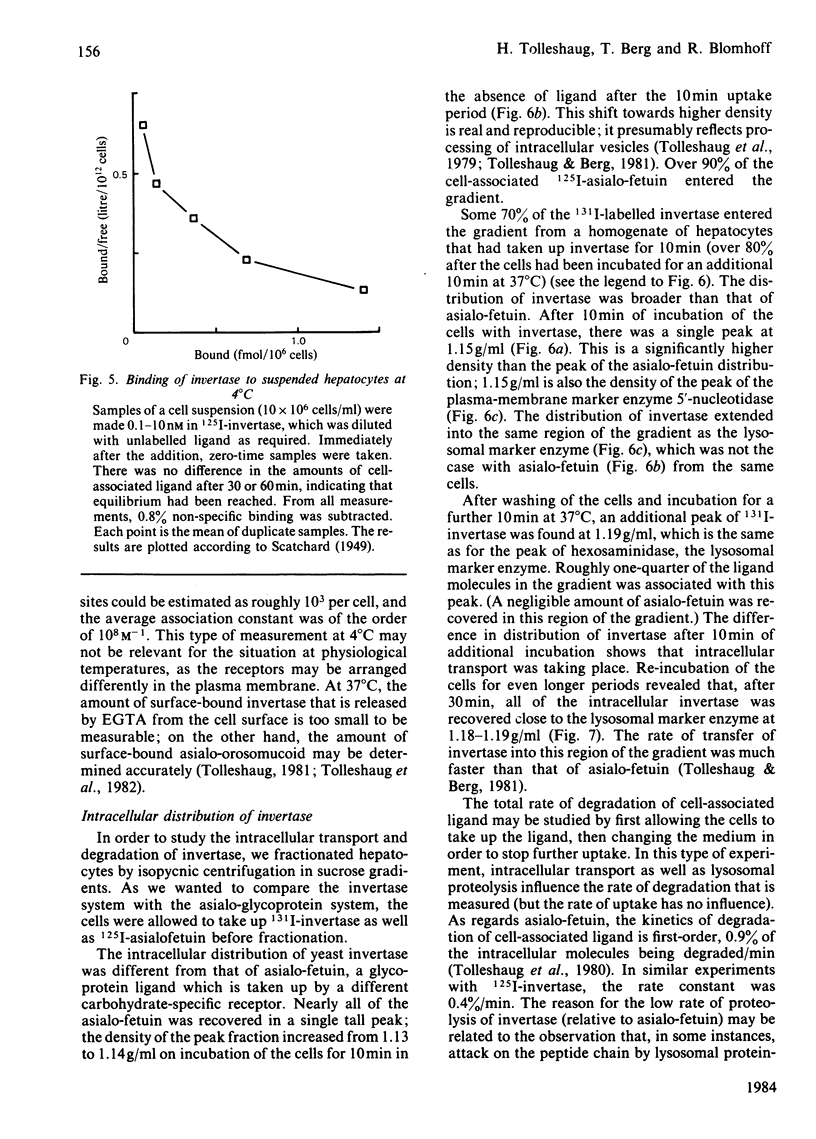
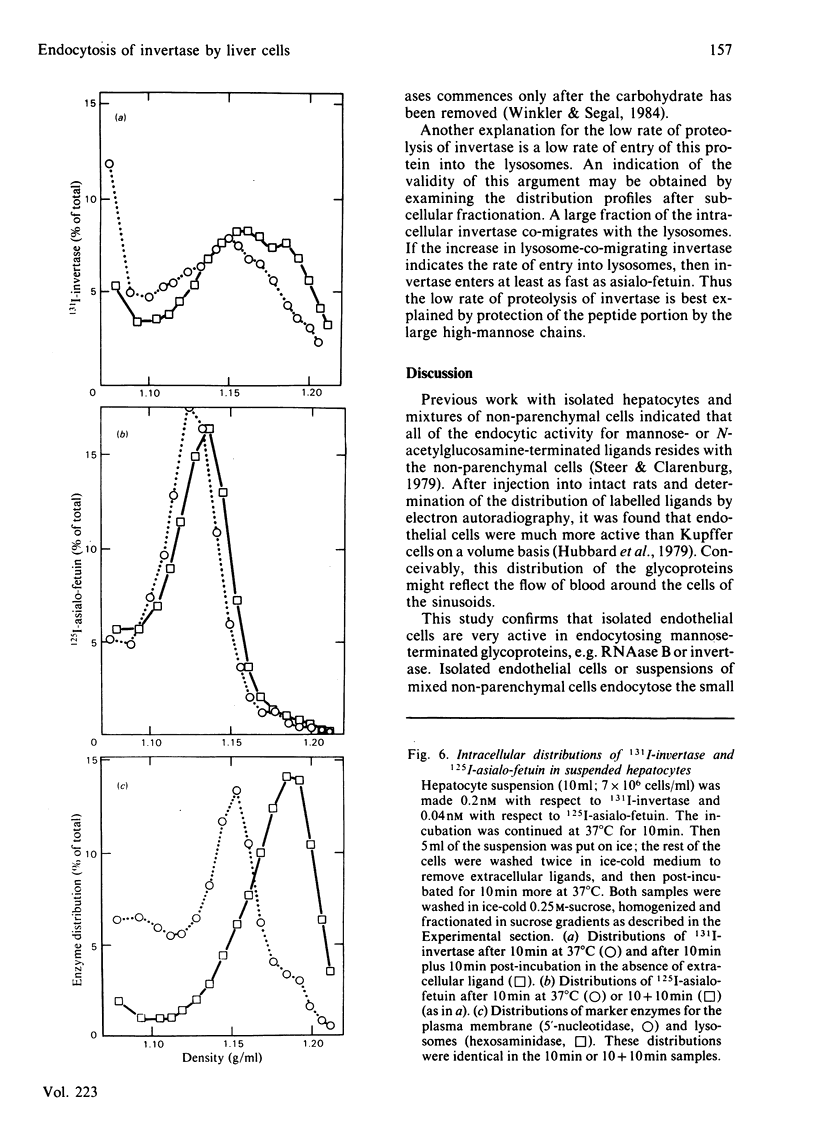
Even though most of the hepatic binding capacity for mannose-terminated glycoproteins has previously been shown to reside in the hepatocytes (not in the non-parenchymal cells), detailed evidence for the specific uptake of mannose-terminated ligands has been lacking. In the present studies, yeast invertase, a large glycoprotein (Mr 270 000) containing about 50% mannose, was shown to be taken up into hepatocytes by receptor-mediated endocytosis. The uptake was saturable and could be specifically inhibited by mannosides or by a Ca2+ chelator. The asialo-glycoprotein receptor was not involved. The low-Mr (13 000) ligand ribonuclease B, which contains a single high-mannose glycan, was not taken up by hepatocytes; however, it was taken up as fast as invertase by non-parenchymal liver cells. After injection of 131I-invertase into a rat in vivo, about one-half of the labelled protein was recovered in the hepatocytes. On a per-cell basis, each endothelial cell contained 3-4 times as much radioactivity as did the hepatocytes. On fractionation of hepatocytes in sucrose gradients, invertase showed a different intracellular distribution from that of asialo-fetuin, in that invertase moved much faster into that region of the gradient where the lysosomes were recovered. This indicates that invertase and asialo-fetuin are not transported intracellularly by identical mechanisms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg T., Boman D. Distribution of lysosomal enzymes between parenchymal and Kupffer cells of rat liver. Biochim Biophys Acta. 1973 Oct 10;321(2):585–596. doi: 10.1016/0005-2744(73)90201-5. [DOI] [PubMed] [Google Scholar]
- Berg T., Tolleshaug H. The effects of ammonium ions and chloroquine on uptake and degradation of 125I-labeled asialo-fetuin in isolated rat hepatocytes. Biochem Pharmacol. 1980 Mar 15;29(6):917–925. doi: 10.1016/0006-2952(80)90222-1. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomhoff R., Holte K., Naess L., Berg T. Newly administered [3H]retinol is transferred from hepatocytes to stellate cells in liver for storage. Exp Cell Res. 1984 Jan;150(1):186–193. doi: 10.1016/0014-4827(84)90713-4. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Tolleshaug H., Berg T. Binding of calcium ions to the isolated asialo-glycoprotein receptor. Implications for receptor function in suspended hepatocytes. J Biol Chem. 1982 Jul 10;257(13):7456–7459. [PubMed] [Google Scholar]
- Granum P. E., Skjelkvåle R. Chemical modification and characterization of enterotoxin from clostridium perfringens type A. Acta Pathol Microbiol Scand B. 1977 Feb;85B(1):89–94. doi: 10.1111/j.1699-0463.1977.tb01678.x. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Wilson G., Ashwell G., Stukenbrok H. An electron microscope autoradiographic study of the carbohydrate recognition systems in rat liver. I. Distribution of 125I-ligands among the liver cell types. J Cell Biol. 1979 Oct;83(1):47–64. doi: 10.1083/jcb.83.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehle L. Biosynthesis of the core region of yeast mannoproteins. Formation of a glucosylated dolichol-bound oligosaccharide precursor, its transfer to protein and subsequent modification. Eur J Biochem. 1980 Aug;109(2):589–601. doi: 10.1111/j.1432-1033.1980.tb04832.x. [DOI] [PubMed] [Google Scholar]
- Maynard Y., Baenziger J. U. Characterization of a mannose and N-acetylglucosamine-specific lectin present in rat hepatocytes. J Biol Chem. 1982 Apr 10;257(7):3788–3794. [PubMed] [Google Scholar]
- Maynard Y., Baenziger J. U. Oligosaccharide specific endocytosis by isolated rat hepatic reticuloendothelial cells. J Biol Chem. 1981 Aug 10;256(15):8063–8068. [PubMed] [Google Scholar]
- Mori K., Kawasaki T., Yamashina I. Identification of the mannan-binding protein from rat livers as a hepatocyte protein distinct from the mannan receptor on sinusoidal cells. Arch Biochem Biophys. 1983 Apr 15;222(2):542–552. doi: 10.1016/0003-9861(83)90552-0. [DOI] [PubMed] [Google Scholar]
- Nilsson M., Berg T. Uptake and degradation of formaldehyde-treated 125I-labelled human serum albumin in rat liver cells in vivo and in vitro. Biochim Biophys Acta. 1977 Mar 29;497(1):171–182. doi: 10.1016/0304-4165(77)90150-7. [DOI] [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. Subcellular distribution of a mammalian hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1976 Dec 10;251(23):7539–7544. [PubMed] [Google Scholar]
- Redshaw M. R., Lynch S. S. An improved method for the preparation of iodinated antigens for radioimmunoassay. J Endocrinol. 1974 Mar;60(3):527–528. doi: 10.1677/joe.0.0600527. [DOI] [PubMed] [Google Scholar]
- Sakaguchi G., Uemura T., Riemann H. P. Simplified method for purification of Clostridium perfringens type A enterotoxin. Appl Microbiol. 1973 Nov;26(5):762–767. doi: 10.1128/am.26.5.762-767.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Rup D., Lodish H. F. Difficulties in the quantification of asialoglycoprotein receptors on the rat hepatocyte. J Biol Chem. 1980 Oct 10;255(19):9033–9036. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980 Jan;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Steer C. J., Clarenburg R. Unique distribution of glycoprotein receptors on parenchymal and sinusoidal cells of rat liver. J Biol Chem. 1979 Jun 10;254(11):4457–4461. [PubMed] [Google Scholar]
- Tolleshaug H., Berg T. Chloroquine reduces the number of asialo-glycoprotein receptors in the hepatocyte plasma membrane. Biochem Pharmacol. 1979 Oct 1;28(19):2919–2922. doi: 10.1016/0006-2952(79)90586-0. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Frölich W., Norum K. R. Intracellular localization and degradation of asialofetuin in isolated rat hepatocytes. Biochim Biophys Acta. 1979 Jun 1;585(1):71–84. doi: 10.1016/0304-4165(79)90326-x. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Holte K. Effects of local anesthetics and related compounds on the endocytosis and catabolism of asialo-glycoproteins in isolated hepatocytes. Biochim Biophys Acta. 1982 Jan 12;714(1):114–121. doi: 10.1016/0304-4165(82)90132-5. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Holte K. Kinetics of internalization and degradation of asialo-glycoproteins in isolated rat hepatocytes. Eur J Cell Biol. 1980 Dec;23(1):104–109. [PubMed] [Google Scholar]
- Tolleshaug H., Berg T. Investigation of the uptake of asialo-glycoproteins by isolated rat hepatocytes. Implications for its biological significance. Hoppe Seylers Z Physiol Chem. 1980 Aug;361(8):1155–1164. doi: 10.1515/bchm2.1980.361.2.1155. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Nilsson M., Norum K. R. Uptake and degradation of 125I-labelled asialo-fetuin by isolated rat hepatocytes. Biochim Biophys Acta. 1977 Aug 25;499(1):73–84. doi: 10.1016/0304-4165(77)90230-6. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T. The effect of leupeptin on intracellular digestion of asialofetuin in rat hepatocytes. Exp Cell Res. 1981 Jul;134(1):207–217. doi: 10.1016/0014-4827(81)90478-x. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H. Binding and internalization of asialo-glycoproteins by isolated rat hepatocytes. Int J Biochem. 1981;13(1):45–51. doi: 10.1016/0020-711x(81)90135-x. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Subunit structure of external invertase from Saccharomyces cerevisiae. J Biol Chem. 1977 Jun 25;252(12):4409–4412. [PubMed] [Google Scholar]
- Ullrich K., Basner R., Gieselmann V., Von Figura K. Recognition of human urine alpha-N-acetylglucosaminidase by rat hepatocytes. Involvement of receptors specific for galactose, mannose 6-phosphate and mannose. Biochem J. 1979 May 15;180(2):413–419. doi: 10.1042/bj1800413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr G. A. A macrophage receptor for (mannose/glucosamine)-glycoproteins of potential importance in phagocytic activity. Biochem Biophys Res Commun. 1980 Apr 14;93(3):737–745. doi: 10.1016/0006-291x(80)91139-0. [DOI] [PubMed] [Google Scholar]
- Winkler J. R., Segal H. L. Inhibition by swainsonine of the degradation of endocytosed glycoproteins in isolated rat liver parenchymal cells. J Biol Chem. 1984 Feb 10;259(3):1958–1962. [PubMed] [Google Scholar]


