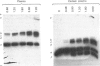
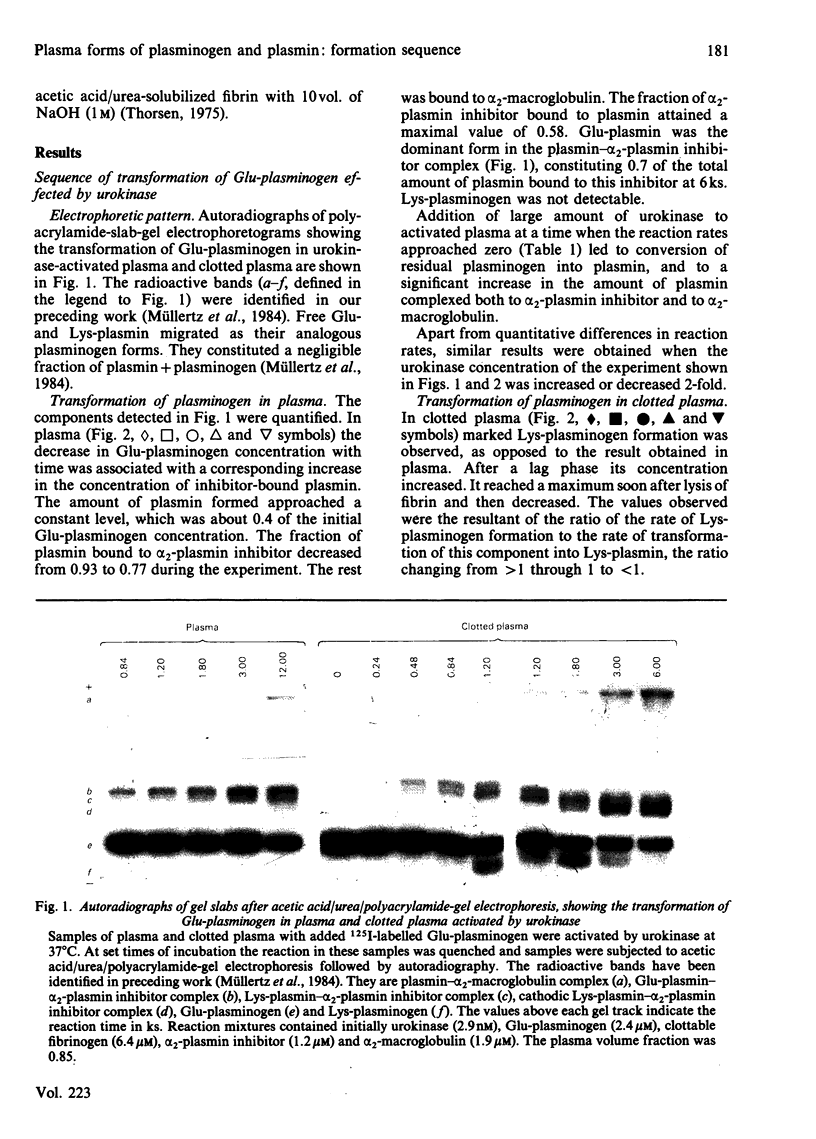
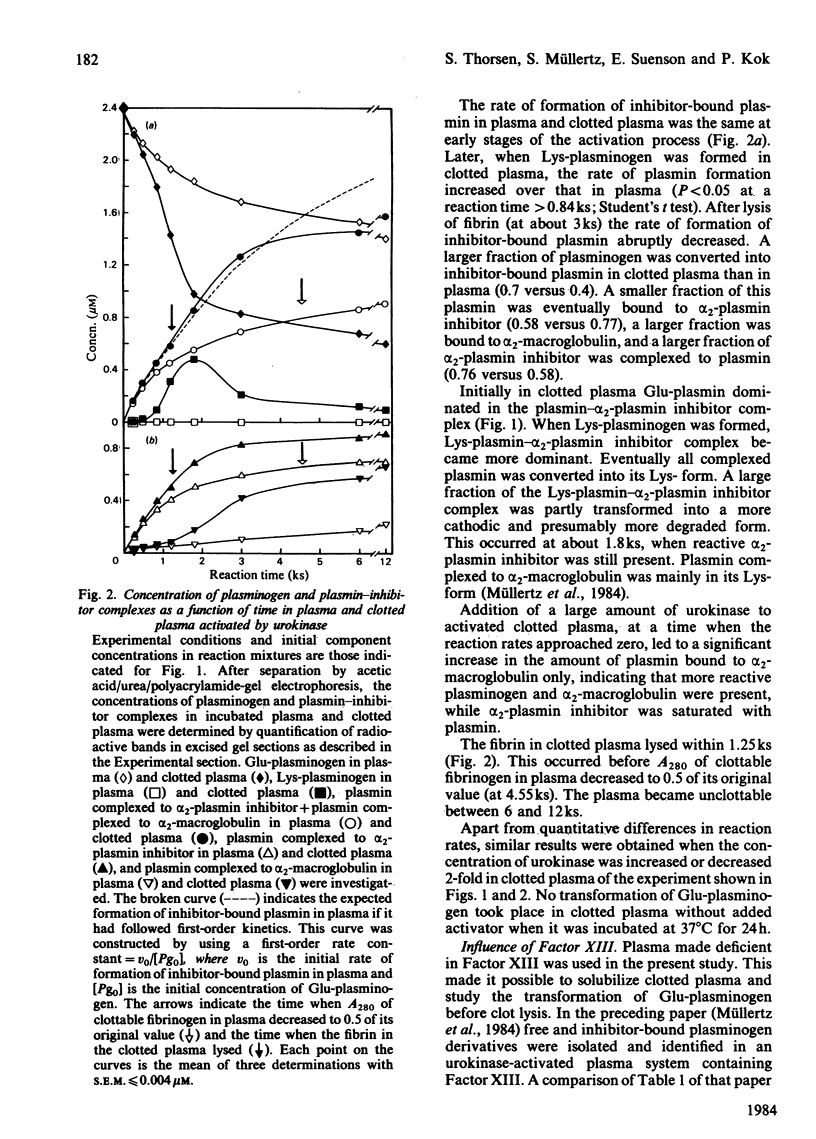
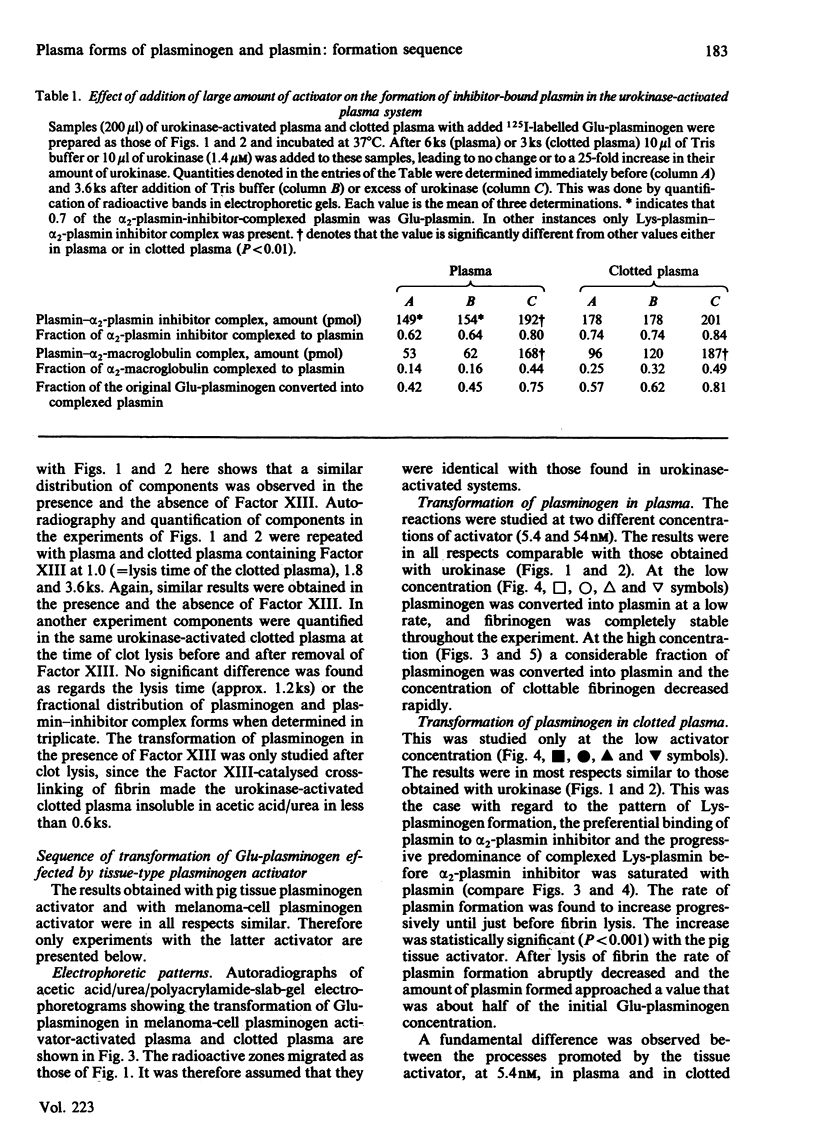
Abstract
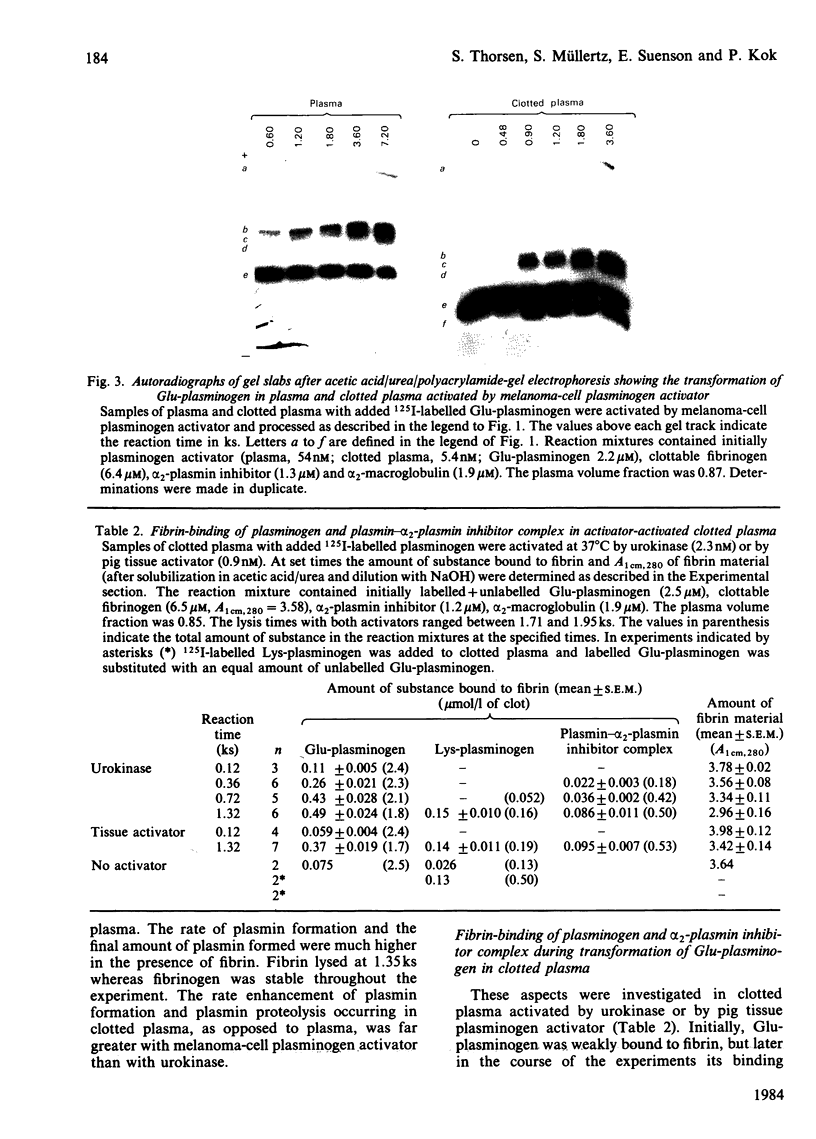
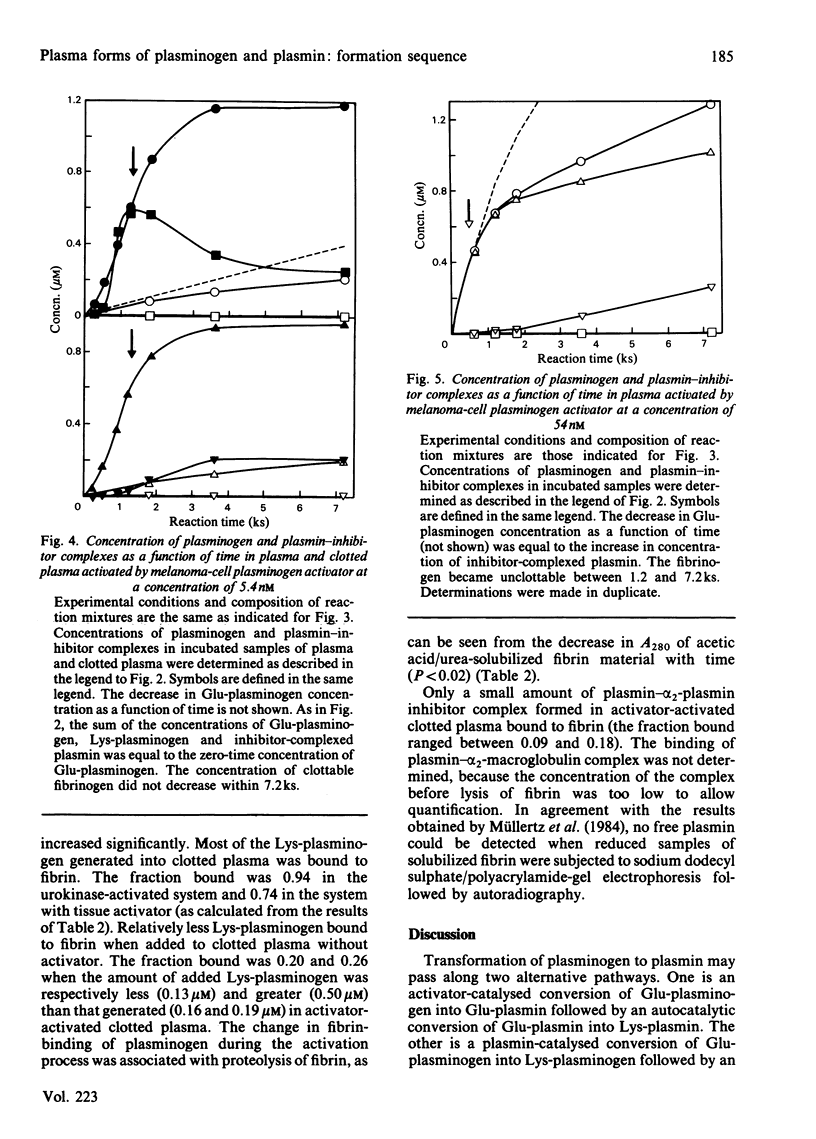
The pathway of plasminogen transformation was studied in plasma, particularly in relation to fibrin formation and the subsequent stimulation of plasminogen activation. Plasminogen was activated by urokinase (low fibrin-affinity) or tissue-type plasminogen activator (high fibrin-affinity). Formation of 125I-labelled free and inhibitor-bound plasminogen derivatives was quantified after their separation by acetic acid/urea/polyacrylamide-gel electrophoresis. In plasma activator converted Glu-plasminogen (residues 1-790) into Glu-plasmin, which was complexed to alpha 2-plasmin inhibitor. When this inhibitor was saturated, Glu-plasmin was autocatalytically converted into Lys-plasmin (residues 77-790). No plasmin-catalysed Lys-plasminogen formation was observed. Upon fibrin formation, activation initially followed the same Glu-plasminogen-into-Glu-plasmin conversion pathway, and stimulation of plasminogen activation was only observed with tissue-type plasminogen activator. In agreement with the emergence of novel effector function, on early plasmin cleavage of fibrin [Suenson, Lützen & Thorsen (1984) Eur. J. Biochem. 140, 513-522] the fibrin-binding of Glu-plasminogen increased when solid-phase fibrin showed evident signs of degradation. This was associated with the formation of considerable amounts of the more easily activatable Lys-plasminogen, most of which was fibrin-bound. At the same time the rate of plasmin formation with urokinase increased over that in unclotted plasma and the rate of plasmin formation with tissue-type plasminogen activator accelerated. Altogether these processes favoured enhanced fibrin degradation. The rates of Lys-plasminogen and plasmin formation abruptly decreased after lysis of fibrin, probably owing to a compromised effector function on further fibrin degradation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camiolo S. M., Thorsen S., Astrup T. Fibrinogenolysis and fibrinolysis with tissue plasminogen activator, urokinase, streptokinase-activated human globulin, and plasmin. Proc Soc Exp Biol Med. 1971 Oct;138(1):277–280. doi: 10.3181/00379727-138-35878. [DOI] [PubMed] [Google Scholar]
- Christensen U., Clemmensen I. Purification and reaction mechanisms of the primary inhibitor of plasmin from human plasma. Biochem J. 1978 Nov 1;175(2):635–641. doi: 10.1042/bj1750635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen U., Holmberg L., Bladh B., Astedt B. Kinetics of the reaction between urokinase and an inhibitor of fibrinolysis from placental tissue. Thromb Haemost. 1982 Aug 24;48(1):24–26. [PubMed] [Google Scholar]
- Clemmensen I., Christensen F. Inhibition of urokinase by complex formation with human alpha1-antitrypsin. Biochim Biophys Acta. 1976 Apr 8;429(2):591–599. doi: 10.1016/0005-2744(76)90307-7. [DOI] [PubMed] [Google Scholar]
- Collen D. Identification and some properties of a new fast-reacting plasmin inhibitor in human plasma. Eur J Biochem. 1976 Oct 1;69(1):209–216. doi: 10.1111/j.1432-1033.1976.tb10875.x. [DOI] [PubMed] [Google Scholar]
- Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980 Jun 18;43(2):77–89. [PubMed] [Google Scholar]
- Günzler W. A., Steffens G. J., Otting F., Kim S. M., Frankus E., Flohé L. The primary structure of high molecular mass urokinase from human urine. The complete amino acid sequence of the A chain. Hoppe Seylers Z Physiol Chem. 1982 Oct;363(10):1155–1165. doi: 10.1515/bchm2.1982.363.2.1155. [DOI] [PubMed] [Google Scholar]
- Harpel P. C. Alpha2-plasmin inhibitor and alpha2-macroglobulin-plasmin complexes in plasma. Quantitation by an enzyme-linked differential antibody immunosorbent assay. J Clin Invest. 1981 Jul;68(1):46–55. doi: 10.1172/JCI110253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschen A. On the structure of functional sites in fibrinogen. Thromb Res Suppl. 1983;5:27–39. [PubMed] [Google Scholar]
- Holmberg L., Kristoffersson A. C., Lecander I., Wallén P., Astedt B. Immunoradiometric quantification of tissue plasminogen activator secreted by fetal organs. Comparison with urokinase. Scand J Clin Lab Invest. 1982 Jun;42(4):347–354. [PubMed] [Google Scholar]
- Holmberg L., Lecander I., Astedt B. Binding of urokinase to plasma proteinase inhibitors. Scand J Clin Lab Invest. 1980;40(8):743–747. doi: 10.3109/00365518009095590. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Kok P., Astrup T. Isolation and purification of a tissue plasminogen activator and its comparison with urokinase. Biochemistry. 1969 Jan;8(1):79–86. doi: 10.1021/bi00829a013. [DOI] [PubMed] [Google Scholar]
- Kok P. Separation of plasminogen activators from human plasma and a comparison with activators from human uterine tissue and urine. Thromb Haemost. 1979 Jun 30;41(4):734–744. [PubMed] [Google Scholar]
- Korninger C., Collen D. Neutralization of human extrinsic (tissue-type) plasminogen activator in human plasma: no evidence for a specific inhibitor. Thromb Haemost. 1981 Oct;46(3):662–665. [PubMed] [Google Scholar]
- Lucas M. A., Straight D. L., Fretto L. J., McKee P. A. The effects of fibrinogen and its cleavage products on the kinetics of plasminogen activation by urokinase and subsequent plasmin activity. J Biol Chem. 1983 Oct 25;258(20):12171–12177. [PubMed] [Google Scholar]
- Matsuo O., Rijken D. C., Collen D. Comparison of the relative fibrinogenolytic, fibrinolytic and thrombolytic properties of tissue plasminogen activator and urokinase in vitro. Thromb Haemost. 1981 Jun 30;45(3):225–229. [PubMed] [Google Scholar]
- Mattsson C., Nyberg-Arrhenius V., Wallén P. Dissolution of thrombi by tissue plasminogen activator, urokinase and streptokinase in an artificial circulating system. Thromb Res. 1981 Mar 15;21(6):535–545. doi: 10.1016/0049-3848(81)90254-1. [DOI] [PubMed] [Google Scholar]
- Moroi M., Aoki N. Inhibition of plasminogen binding to fibrin by alpha2-plasmin inhibitor. Thromb Res. 1977 Jun;10(6):851–856. doi: 10.1016/0049-3848(77)90142-6. [DOI] [PubMed] [Google Scholar]
- Moroi M., Aoki N. Isolation and characterization of alpha2-plasmin inhibitor from human plasma. A novel proteinase inhibitor which inhibits activator-induced clot lysis. J Biol Chem. 1976 Oct 10;251(19):5956–5965. [PubMed] [Google Scholar]
- Müllertz S., Clemmensen I. The primary inhibitor of plasmin in human plasma. Biochem J. 1976 Dec 1;159(3):545–553. doi: 10.1042/bj1590545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllertz S. Different molecular forms of plasminogen and plasmin produced by urokinase in human plasma and their relation to protease inhibitors and lysis of fibrinogen and fibrin. Biochem J. 1974 Nov;143(2):273–283. doi: 10.1042/bj1430273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllertz S., Thorsen S., Sottrup-Jensen L. Identification of molecular forms of plasminogen and plasmin-inhibitor complexes in urokinase-activated human plasma. Biochem J. 1984 Oct 1;223(1):169–177. doi: 10.1042/bj2230169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Petersen L. C., Clemmensen I. Kinetics of plasmin inhibition in the presence of a synthetic tripeptide substrate. The reaction with pancreatic trypsin inhibitor and two forms of alpha 2-plasmin inhibitor. Biochem J. 1981 Oct 1;199(1):121–127. doi: 10.1042/bj1990121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijken D. C., Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981 Jul 10;256(13):7035–7041. [PubMed] [Google Scholar]
- Rijken D. C., Hoylaerts M., Collen D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J Biol Chem. 1982 Mar 25;257(6):2920–2925. [PubMed] [Google Scholar]
- Rijken D. C., Juhan-Vague I., Collen D. Complexes between tissue-type plasminogen activator and proteinase inhibitors in human plasma, identified with an immunoradiometric assay. J Lab Clin Med. 1983 Feb;101(2):285–294. [PubMed] [Google Scholar]
- Rijken D. C., Wijngaards G., Welbergen J. Immunological characterization of plasminogen activator activities in human tissues and body fluids. J Lab Clin Med. 1981 Apr;97(4):477–486. [PubMed] [Google Scholar]
- Rånby M. Studies on the kinetics of plasminogen activation by tissue plasminogen activator. Biochim Biophys Acta. 1982 Jun 24;704(3):461–469. doi: 10.1016/0167-4838(82)90068-1. [DOI] [PubMed] [Google Scholar]
- Suenson E., Lützen O., Thorsen S. Initial plasmin-degradation of fibrin as the basis of a positive feed-back mechanism in fibrinolysis. Eur J Biochem. 1984 May 2;140(3):513–522. doi: 10.1111/j.1432-1033.1984.tb08132.x. [DOI] [PubMed] [Google Scholar]
- Suenson E., Thorsen S. Secondary-site binding of Glu-plasmin, Lys-plasmin and miniplasmin to fibrin. Biochem J. 1981 Sep 1;197(3):619–628. doi: 10.1042/bj1970619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen S., Clemmensen I., Sottrup-Jensen L., Magnusson S. Adsorption to fibrin of native fragments of known primary structure from human plasminogen. Biochim Biophys Acta. 1981 May 29;668(3):377–387. doi: 10.1016/0005-2795(81)90171-9. [DOI] [PubMed] [Google Scholar]
- Thorsen S. Differences in the binding to fibrin of native plasminogen and plasminogen modified by proteolytic degradation. Influence of omega-aminocarboxylic acids. Biochim Biophys Acta. 1975 May 30;393(1):55–65. doi: 10.1016/0005-2795(75)90216-0. [DOI] [PubMed] [Google Scholar]
- Thorsen S., Glas-Greenwalt P., Astrup T. Differences in the binding to fibrin of urokinase and tissue plasminogen activator. Thromb Diath Haemorrh. 1972 Aug 31;28(1):65–74. [PubMed] [Google Scholar]
- Thorsen S., Kok P., Astrup T. Reversible and irreversible alterations of human plasminogen indicated by changes in susceptibility to plasminogen activators and in response to epsilon-aminocaproic acid. Thromb Diath Haemorrh. 1974 Dec 31;32(2-3):325–340. [PubMed] [Google Scholar]
- Wiman B., Collen D. On the kinetics of the reaction between human antiplasmin and plasmin. Eur J Biochem. 1978 Mar 15;84(2):573–578. doi: 10.1111/j.1432-1033.1978.tb12200.x. [DOI] [PubMed] [Google Scholar]
- Wun T. C., Schleuning W. D., Reich E. Isolation and characterization of urokinase from human plasma. J Biol Chem. 1982 Mar 25;257(6):3276–3283. [PubMed] [Google Scholar]