Abstract
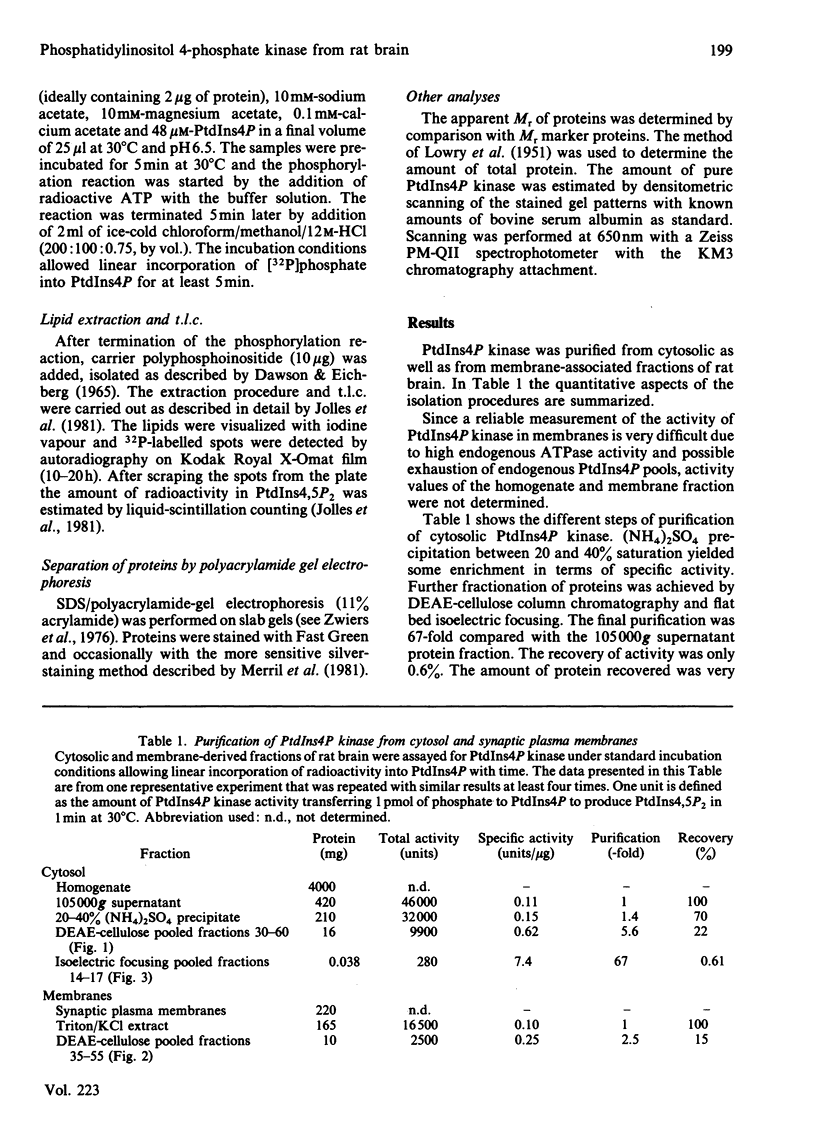
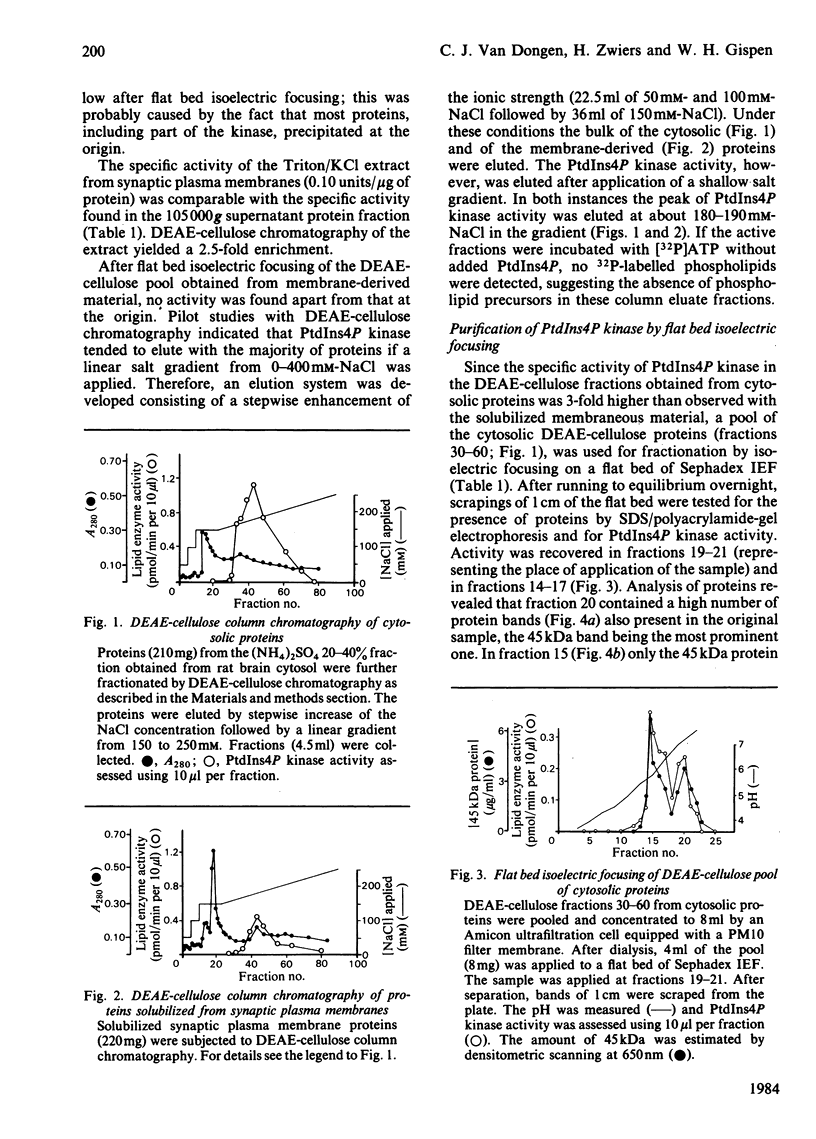
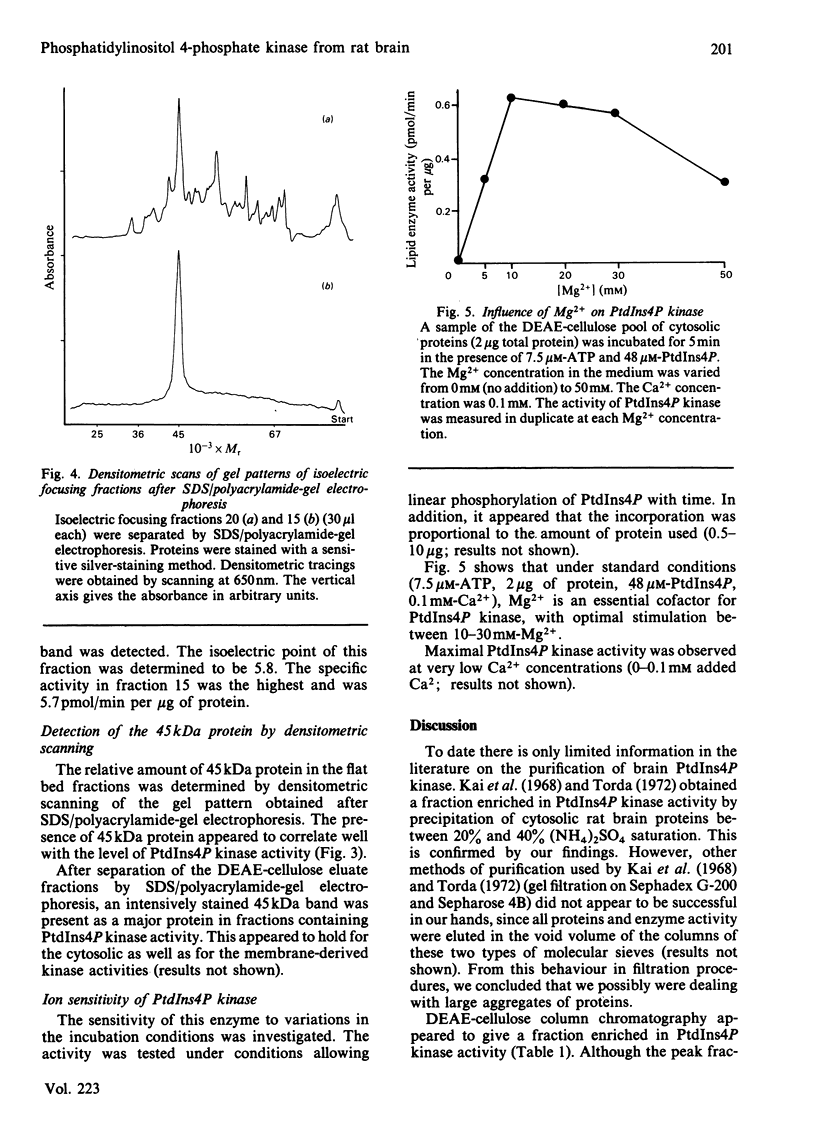
Phosphatidylinositol 4-phosphate (PtdIns4P) kinase was purified from cytosolic and particulate material of rat brain. The purification procedure of the enzyme from cytosol consisted of (NH4)2SO4 precipitation. DEAE-cellulose column chromatography and preparative isoelectric focusing. Other methods after DEAE-cellulose column chromatography failed to achieve further purification of the PtdIns4P kinase, probably caused by the tendency of the enzyme to aggregate with contaminating proteins. The final purification was 67-fold, and the recovery was 0.6%. After isoelectric focusing the fraction containing the highest PtdIns4P kinase activity showed only one protein as visualized by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis and silver staining. The apparent Mr of this protein was 45 kDa and the isoelectric point about 5.8. The activity of PtdIns4P kinase was dependent on the concentration of divalent cations in the incubation medium. PtdIns4P kinase activity was found to be optimal at 10-30 mM-Mg2+. In an attempt to compare the cytosolic with the membrane-derived kinase activity, a Triton/KCl extract from synaptic membranes was subjected to the same purification procedure as the cytosolic enzyme. A difference in isoelectric focusing was observed, possibly due to a higher tendency to form aggregates. However, we tend to conclude that also in the membranes the PtdIns4P kinase activity is present as a 45 kDa protein, identical with that found in the cytosol.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Green K., Smith J. P. Sympathetic denervation and the triphosphoinositide effect in the iris smooth muscle: a biochemical method for the determination of alpha-adrenergic receptor denervation supersensitivity. J Neurochem. 1979 Jan;32(1):225–228. doi: 10.1111/j.1471-4159.1979.tb04532.x. [DOI] [PubMed] [Google Scholar]
- Akhtar R. A., Taft W. C., Abdel-Latif A. A. Effects of corticotropin-(1-24)-tetracosapeptide on polyphosphoinositide metabolism and protein phosphorylation in rabbit iris subcellular fractions. J Neurochem. 1983 Nov;41(5):1460–1468. doi: 10.1111/j.1471-4159.1983.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Bostwick J. R., Eichberg J. Detergent solubilization and hydrophobic chromatography of rat brain phosphatidylinositol kinase. Neurochem Res. 1981 Oct;6(10):1053–1065. doi: 10.1007/BF00964412. [DOI] [PubMed] [Google Scholar]
- Cooper P. H., Hawthorne J. N. Phosphatidylinositol kinase and diphosphoinositide kinase of rat kidney cortex: properties and subcellular localization. Biochem J. 1976 Oct 15;160(1):97–105. doi: 10.1042/bj1600097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Eichberg J. Diphosphoinositide and triphosphoinositide in animal tissues. Extraction, estimation and changes post mortem. Biochem J. 1965 Sep;96(3):634–643. doi: 10.1042/bj0960634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh D. S., Kuizon S., Brockerhoff H. Mutual stimulation by phosphatidylinositol-4-phosphate and myelin basic protein of their phosphorylation by the kinases solubilized from rat brain myelin. Life Sci. 1984 Jan 16;34(3):259–264. doi: 10.1016/0024-3205(84)90597-6. [DOI] [PubMed] [Google Scholar]
- Downes P., Michell R. H. Phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: lipids in search of a function. Cell Calcium. 1982 Oct;3(4-5):467–502. doi: 10.1016/0143-4160(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Griffin H. D., Hawthorne J. N. Calcium-activated hydrolysis of phosphatidyl-myo-inositol 4-phosphate and phosphatidyl-myo-inositol 4,5-bisphosphate in guinea-pig synaptosomes. Biochem J. 1978 Nov 15;176(2):541–552. doi: 10.1042/bj1760541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., Dekker A., Wirtz K. W., Gispen W. H. Corticotropin-(1--24)-tetracosapeptide affects protein phosphorylation and polyphosphoinositide metabolism in rat brain. Biochem J. 1981 Jan 15;194(1):283–291. doi: 10.1042/bj1940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., van Dongen C. J., Schotman P., Wirtz K. W., Gispen W. H. Modulation of brain polyphosphoinositide metabolism by ACTH-sensitive protein phosphorylation. Nature. 1980 Aug 7;286(5773):623–625. doi: 10.1038/286623a0. [DOI] [PubMed] [Google Scholar]
- Jork R., de Graan P. N., van Dongen C. J., Zwiers H., Matthies H., Gispen W. H. Dopamine-induced changes in protein phosphorylation and polyphosphoinositide metabolism in rat hippocampus. Brain Res. 1984 Jan 16;291(1):73–81. doi: 10.1016/0006-8993(84)90652-8. [DOI] [PubMed] [Google Scholar]
- Kai M., Salway J. G., Hawthorne J. N. The diphosphoinositide kinase of rat brain. Biochem J. 1968 Feb;106(4):791–801. doi: 10.1042/bj1060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Oestreicher A. B., Van Dongen C. J., Zwiers H., Gispen W. H. Affinity-purified anti-B-50 protein antibody: interference with the function of the phosphoprotein B-50 in synaptic plasma membranes. J Neurochem. 1983 Aug;41(2):331–340. doi: 10.1111/j.1471-4159.1983.tb04747.x. [DOI] [PubMed] [Google Scholar]
- Radola B. J. Isoelectric focusing in layers of granulated gels. I. Thin-layer isoelectric focusing of proteins. Biochim Biophys Acta. 1973 Feb 21;295(2):412–428. doi: 10.1016/0005-2795(73)90037-8. [DOI] [PubMed] [Google Scholar]
- Rodnight R. Aspects of protein phosphorylation in the nervous system with particular reference to synaptic transmission. Prog Brain Res. 1982;56:1–25. doi: 10.1016/S0079-6123(08)63766-8. [DOI] [PubMed] [Google Scholar]
- Shaikh N. A., Palmer F. B. Phosphoinositide kinases in chick brain and sciatic nerve, a developmental study. J Neurochem. 1977 Feb;28(2):395–402. doi: 10.1111/j.1471-4159.1977.tb07760.x. [DOI] [PubMed] [Google Scholar]
- Torda C. Cyclic AMP-dependent diphosphoinositide kinase. Biochim Biophys Acta. 1972 Dec 29;286(2):389–395. doi: 10.1016/0304-4165(72)90275-9. [DOI] [PubMed] [Google Scholar]
- Tou J. S., Hurst M. W., Huggins C. G., Foor W. E. Biosynthesis of triphosphoinositide in rat kidney cortex. Arch Biochem Biophys. 1970 Oct;140(2):492–502. doi: 10.1016/0003-9861(70)90093-7. [DOI] [PubMed] [Google Scholar]
- Zwiers H., Schotman P., Gispen W. H. Purification and some characteristics of an ACTH-sensitive protein kinase and its substrate protein in rat brain membranes. J Neurochem. 1980 Jun;34(6):1689–1699. doi: 10.1111/j.1471-4159.1980.tb11262.x. [DOI] [PubMed] [Google Scholar]


