Abstract
Background
Curcumin, a compound in turmeric, shows potential in cancer treatment but is hindered by low bioavailability and solubility. Nanocurcumin, enhanced through nanotechnology, addresses these limitations, offering potential in oncological applications. This review systematically examines the efficacy, bioavailability, and safety of nanocurcumin in cancer treatment, collating data from in vitro, in vivo, and clinical studies.
Methods
A comprehensive systematic search was conducted across four major databases: PubMed (Medline), Scopus, Web of Science, and Embase (up to February 2024). The selection criteria were based on the PICOT structure, and studies were assessed for risk of bias using the Cochrane bias risk tool for clinical studies and related checklists for in vitro and in vivo studies. Statistical analyses were performed in STATA software version 17.
Results
In total, 8403 articles were identified and assessed, and then only 61 articles were found eligible to be included. Nanocurcumin formulations, especially with Poly (lactic-co-glycolic acid) (PLGA), displayed superior solubility and therapeutic efficacy. In vitro studies highlighted its enhanced cellular uptake and anti-proliferative effects, particularly against cervical cancer cells. In vivo studies confirmed its chemopreventive efficacy and potential synergy with other cancer therapies. Though in early stages, clinical trials showed promise in reducing side effects and improving efficacy in cancer treatments.
Conclusion
Nanocurcumin shows promise as an innovative approach in cancer therapy, potentially offering improved efficacy and reduced side effects compared to traditional treatments. Early clinical trials indicate its potential to enhance the quality of life for cancer patients by mitigating treatment-related toxicities and improving therapeutic outcomes. However, larger randomized controlled trials are necessary to definitively establish its clinical efficacy, optimal dosing regimens, and long-term safety profile across various cancer types. As research progresses, nanocurcumin could become a valuable addition to the oncologist's toolkit, particularly in combination therapies or for patients intolerant to conventional treatments. Future clinical studies should focus on optimizing treatment protocols, identifying responsive patient populations, and assessing long-term outcomes to facilitate the translation of these promising findings into standard clinical practice.
Keywords: Nanocurcumin, Cancer Treatment, Bioavailability, PLGA
Introduction
Cancer is characterized by uncontrolled cell growth, manifesting either as solid tumors or liquid cancers (such as those affecting blood or bone marrow). The main cancer treatment methods are surgery, chemotherapy, and radiotherapy. Although there have been notable improvements in cancer diagnosis and therapy, there is still an urgent want for the development of treatment methods that are both safe and effective. Traditional cancer treatments, such as chemotherapy and radiotherapy, frequently result in harmful side effects and the development of drug resistance. These constraints have stimulated the exploration of innovative therapeutic strategies specifically targeting cancer cells while limiting harm to normal tissues [1, 2]. Compared with previous standards of care (including chemotherapy, radiotherapy, and surgery), cancer immunotherapy has brought significant improvements for patients in terms of survival and quality of life. Immunotherapy has now firmly established itself as a novel pillar of cancer care, from the metastatic stage to the adjuvant and neoadjuvant settings in numerous cancer types [3].
Curcumin, a biologically active chemical extracted from the rhizome of Curcuma longa (turmeric), has attracted considerable interest due to its possible anti-cancer capabilities [4]. Multiple preclinical studies have shown that curcumin can influence various cellular pathways in cancer advancement, such as cell proliferation, apoptosis, angiogenesis, and metastasis [5, 6]. Nevertheless, the practical application of curcumin in medical settings has been impeded by its limited capacity to dissolve in water, its low rate of absorption into the body, and its quick breakdown by metabolic processes [7–9].
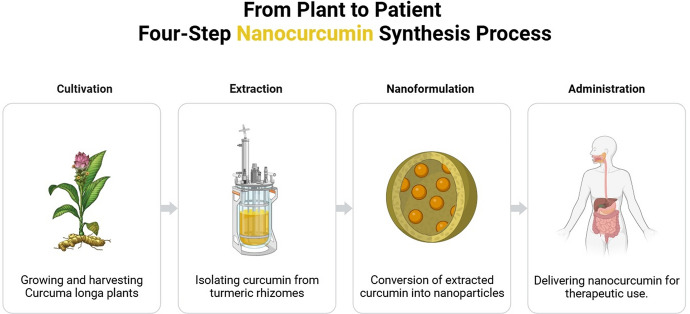
Nanotechnology has arisen as a viable approach to address the constraints of traditional medication delivery techniques [8]. Peptides and nanoparticles work synergistically in cancer treatment. Nanoparticles functionalized with peptides can target tumor cells better. Combining peptides and medicines can create self-assembled nanoparticles for targeted cancer treatment[9]. Nanocurcumin, nanoformulations of curcumin, have been created to improve its solubility, stability, and bioavailability (Fig. 1) [10]. The nanoformulations consist of different platforms, including polymeric nanoparticles, liposomes, micelles, and nanoemulsions. Each platform has distinct physicochemical features and biological consequences [11].
Fig. 1.
Nanocurcumin from cultivation to administration
The combination of nanotechnology and curcumin has created new possibilities for cancer treatment. Formulations of nanocurcumin have shown more bioavailability, enhanced absorption by cells, gradual release, and specific transport to tumor locations [12]. Furthermore, nanocurcumin has demonstrated superior effectiveness in inhibiting cancer growth than free curcumin in laboratory and animal studies [13, 14]. The positive results have generated enthusiasm for using nanocurcumin in clinical settings to treat cancer.
This systematic review offers a thorough assessment of the existing knowledge regarding using nanocurcumin in cancer treatment and compiling and examining data obtained from in vitro, in vivo, and clinical trials. We collected data from laboratory experiments, animal research, and experiments related to human patients, and we aim to investigate the effectiveness, ability to be absorbed by the body, and safety of nanocurcumin in different forms of cancer. In addition, we will review the obstacles and prospects of nanocurcumin as a promising therapeutic agent in oncology.
Method
Search strategy
A thorough and organized search followed the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Various electronic databases, namely PubMed (Medline), Scopus, Web of Science, and Embase, were examined to identify relevant studies published until February 2024. The search strategy utilized a combination of specific terms and Medical Subject Headings (MeSH) such as “nanocurcumin,” “curcumin nano-formulation,” “cancer,” and “tumor.” A manual search was performed to verify that no relevant studies were overlooked. This entailed reviewing the references and sources cited in the selected papers to locate additional studies that may not have been discovered during the electronic search.
The inclusion criteria for this review were as follows: [1] original research articles investigating the efficacy, bioavailability, or safety of nanocurcumin in cancer; [2] studies conducted in vitro, in vivo, or as clinical trials; [3] studies comparing nanocurcumin with free curcumin or other cancer therapies; and [4] articles published in English. Exclusion criteria included: [1] review articles, editorials, conference abstracts, and book chapters; [2] studies not related to cancer; and [3] studies using curcumin without nano-formulation. Table 1 shows the PICOT Framework and Eligibility Criteria for Study Selection.
Table 1.
PICOT framework and eligibility criteria for study selection
| Population | Cells, animals, and humans with cancer diagnosis |
|---|---|
| Intervention | Nanocurcumin |
| Comparison | Other treatments |
| Outcomes | Apoptosis of cancer cells and prevention of disease progression |
| Search terms | Nanocurcumin, Cancer |
| Inclusion criteria | (1) original research articles investigating the efficacy, bioavailability, or safety of nanocurcumin in cancer; (2) studies conducted in vitro, in vivo, or as clinical trials; (3) studies comparing nanocurcumin with free curcumin or other cancer therapies; and (4) articles published in English |
| Exclusion criteria | (1) review articles, editorials, conference abstracts, and book chapters; (2) studies not related to cancer; and (3) studies using curcumin without nano-formulation |
| Research question | Is nanocurcumin effective for cancer treatment or not? |
Study selection and data extraction
Two independent reviewers (MB and AM) screened the titles and abstracts of the retrieved articles for eligibility. Full-text articles were then assessed for inclusion based on the predefined criteria. Any reviewer discrepancies were resolved through discussion or consultation with a third reviewer (RRD).
Data extraction was performed using a standardized form, which included the following information: first author, year of publication, study type (in vitro, in vivo, or clinical), cancer type, nanocurcumin formulation, comparison groups, outcome measures, and critical findings. For in vitro studies, additional data on cell lines, assays, and molecular mechanisms were extracted. In vivo studies were further characterized by animal models, tumor characteristics, and treatment regimens. Patient demographics, dosing schedules, and adverse events were recorded for clinical trials.
Quality assessment
The studies included in the analysis were evaluated for quality using suitable assessment tools corresponding to their respective study designs. Randomized controlled trials were assessed using the Cochrane Risk of Bias tool, while non-randomized studies were evaluated using the Newcastle–Ottawa Scale. In vitro studies underwent assessment using a modified version of the NIH National Cancer Institute's Framework for Assessing the Quality of In-Vitro Studies. The quality assessment was conducted independently by two reviewers (PH and NM), and any disagreements were resolved through consensus or by consulting a third reviewer (YM).
Data synthesis and analysis
The extracted data was qualitatively synthesized, explicitly focusing on assessing the efficacy, bioavailability, and safety of nanocurcumin in cancer. The studies were categorized based on their design (in vitro, in vivo, or clinical) and the type of cancer investigated. Subgroup analyses were conducted by considering different nanocurcumin formulations and comparison groups. Due to variations in study designs, outcome measures, and data reporting, performing a quantitative synthesis (meta-analysis) was not feasible. However, whenever possible, effect sizes and 95% confidence intervals were calculated for individual studies to provide a standardized measure of the observed effects' magnitude.
Result
Study selection
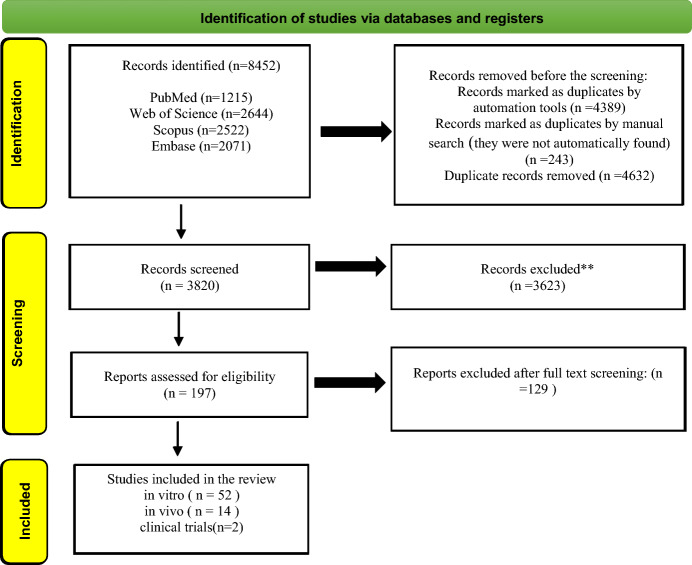
The initial search yielded 8403 records from the electronic databases. After removing duplicates, 5627 unique records were screened for eligibility based on their titles and abstracts. Of these, 5482 records were excluded as they did not meet the inclusion criteria. The remaining 190 articles were retrieved for full-text assessment, of which 129 were further excluded due to lack of relevant outcomes, use of curcumin without nano-formulation, or being review articles. Finally, 61 studies were included in the qualitative synthesis, comprising 47 in vitro, 12 in vivo, and two clinical trials. The PRISMA flow diagram depicting the study selection process is presented in Fig. 2.
Fig. 2.
Identification of studies via databases and registers
Characteristics of included studies
In vitro studies
The 47 in vitro studies (Table 2) utilized various cancer cell lines, including breast, colon, lung, prostate, and liver cancer. The most common nanocurcumin formulations were polymeric nanoparticles, such as PLGA, PEG, and chitosan. These studies employed techniques like MTT assay, flow cytometry, and Western blot to assess the anti-cancer effects of nanocurcumin.
Table 2.
In vitro studies
| Authors name | Year | Source of cells | Analyze | Method synthesis | |
|---|---|---|---|---|---|
| [15] | Bisht, S | 2007 | human pancreatic cancer cell | dynamic laser light scattering and TEM | |
| [16] | Lim, K.J., et al | 2011 | embryonal tumor-derived lines DAOY and D283Med, glioblastoma neurosphere lines HSR-GBM1 and JHH -GBM14 | ||
| [17] | Prasanth, R | 2011 | nasopharyngeal cancer cells | AFM | |
| [13] | Nair, K.L., et | 2012 | human epithelial cervical cancer cells (HeLa) | MTT, Annexin V/propidium iodide staining, cleavage of poly (ADP-ribose) polymerase (PARP), and reduction of clonogenic capacity | |
| [14] | Milano, F., et | 2013 | esophageal adenocarcinoma (EAC) | ||
| [12] | Basniwal, R.K | 2014 | |||
| [18] | Chaudhari, P.D | 2015 | breast cancer cell line culture, MCF-7 | DSC, XRD, and TEM analyses were conducted. A study conducted in living organisms showed higher bioavailability in Wistar rats than in unprocessed curcumin, as determined by HPLC. Additionally, an MT assay was performed | |
| [19] | Dhivya, R. et al., | 2015 | breast cancer cell line culture, MCF-7 | DSC. XRD. TEM. In vivo, the study revealed increased bio-availability in Wistar rats compared to raw Curcumin by HPLC. MT assay | |
| [20] | Hossain, D.M., et al | 2015 | breast cancer | ||
| [21] | Hu, B., et | 2015 | human hepatocellular carcinoma (HCC) | ||
| [22] | Pillai, J.J., et | 2015 | The MTT assay depicted a high amount of cytotoxicity of PPF nanocurcumin in HeLa cells | Characterized by FT-IR and 1H-NMR techniques. TEM and DLS. DCS. The MTT AO/EB staining, DAPI staining, and clonogenic | |
| [23] | Xie, M. et | 2015 | Colorectal cancer cells (HCT116) | ||
| [24] | Chamani, F., et | 2016 | Hepatocellular carcinomaHCC cell lines, HepG2 and Huh7 | .MTT, PCR | |
| [25] | Keshavarz, R., et al | 2016 | Glioblastoma | PCR Annexin-V-FLUOS staining followed by flow cytometry and real-time | |
| [26] | Khan, M.A., et al | 2016 | Cervical cancer line SiHa, HeLa, CasKi, and C33A | (TEM), (DLS), HPLC, MALDI-TOF, FT-IR, XRD and UV–vis.Cell metabolic assay by MTT.Detection of apoptosis by DAP | tripolyphosphate (TPP) cross-linking method. drug entrapment efficiency was ≈85% |
| [27] | Khosropanah, M.H., et al | 2016 | Human breast adenocarcinoma cell line (MDA-MB231) | MTT) and dye exclusion assay. TEM(particle diameter was between 150 to 200 nm) | selfassembly |
| [28] | Paunovic, V., et al | 2016 | U251 glioma, B16 melanoma, and H460 lung cancer cells | Photoexcited nanocurcumin can trigger apoptosis independent of oxidative stress but relies on JNK and caspase pathways | tetrahydrofuran/water solvent exchange, |
| [29] | Aldahoun, M.A., et al | 2017 | Prostate cancer cell line (PC3) | Nanocurcumin combined with the magnetic field (NANOCUR-MF) and control against PC3 was 35.93%, which is three times higher compared to curcumin combined with the magnetic field (CUR-MF) | Magnetic field |
| [30] | Dash, T.K. and VSB | 2017 | COLO205 cells | hydroxypropyl-β-cyclodextrin (HP-β-CD) | |
| [31] | Mahjoub, M.A., et al | 2017 | MDA-MB-231 metastatic breast cancer cells | qRT-PCR. MTT assay, Annexin V-FITC staining. lowcytomety and wound healing assay | |
| [32] | Mishra, D. et al., | 2017 | Breast cancer cell line (GILM2) | SEM.DLS | |
| [33] | Athira, G.K., | 2018 | Anti-cancer potential to HeLa cells | FTIR), (SEM), (TEM), (DLS), (AFM), (XRD) | wet grinding method |
| [34] | Bagheri, R., Z. Sanaat, and N. Zarghami | 2018 | SW480 Colorectal Cancer Cell Line | (SEM) and FTIR Spectroscopy. MTT. qRT-PCR method | |
| [35] | Baghi, N., et al | 2018 | MDA-MB-231 breast cancer cells | MTT. real-time PCR. on DNC-related cytotoxicity. Annexin-V/PI staining followed by flow cytometry and wound healing assay | |
| [36] | Hashemzehi, M., et al | 2018 | Human breast cancer cell line MDA-MB-231 | qRT-PCR and Western blotting. (MDA), (SOD), (CAT), (T-SH) | |
| [37] | Hosseini, S., et al | 2018 | Esophageal Squamous Cell Carcinoma (KYSE-30) | ||
| [38] | Nguyen, N.T., et al | 2018 | MCF-7 breast cancer cell | (FT-IR). (TEM), DLS. Via HPLC along with AES-ICP to evaluate the high drug loading | |
| [39] | Shariati, M., et al | 2018 | Hepatocellular carcinoma cell line (Huh7) | MTT. RT-PCR | |
| [40] | Srivastava, S., et al | 2018 | OCC(oral cancer cells) | Employing homogenization with high-energy sonication | |
| [41] | Dang, L.H., et al., | 2019 | Transmission electron microscopy, variable UV–visible spectrophotometry, as well as fluorescence spectroscopy, DLS | Ultrasonication to control the self-assembly of phosphocasein | |
| [42] | Harini, L., et al., | 2019 | Breast cancer (MCF-7) cells | WST | Maximum of 23 µM was released from CUR-MSNAP at 96 h. CUR-MSNAP released 13 µM of drug, and then a sustained pattern of release was observed till 96 h |
| [43] | Hosseini, S., et al | 2019 | Breast cancer cells (MCF7) | MTT. PCR | |
| [44] | Seyed Hosseini, E., et al | 2019 |
Ovarian cancer OVCAR3 SKOV3 |
MTT assay and flow cytometry. real-time PCR | |
| [45] | Yang, R. et al | 2019 | MCF-7 cancer cells | Western blot.MTT. fluorescent image | The release of cumulative amounts of CUR after 72 h at pH 5.0 (58.2%) was also significantly higher than that at pH 7.0 (16.0%) |
| [46] | Cheng, T. et al | 2020 | Pancreatic adenocarcinoma cells. PDAC. Cell line 399. T3M4, MIA PaCa-2 and PANC-1 | SEM, fluorescence microscopy, Fourier transform infrared spectroscopy, X-ray diffraction, and HPLC.MTT | |
| [47] | Hanna, D.H. and G.R. Saad, | 2020 | Human Hep-2 cancer cells | Fourier transform infrared spectroscopy, TEM, X-ray diffraction, and zeta potential analysis. Neutral red uptake.Flow cytometry. Real-time PCR. Annexin V/PI staining assay. LDH). AO/EB staining assay. Comet assay.Cell cycle arrest | sol-oil method |
| [48] | Kuo, I.M., et al | 2020 | CT26 colon cancer | Annexin V–fluorescein isothiocyanate (FITC) apoptosis detection (BD Biosciences).flow cytometer.Western blot. direct immunofluorescence | |
| [49] | Pandit, A.H., et al | 2020 | SUM149 human breast cancer |
Zeta potential DLC, TEM, XTT assay Fluorescent microscopy Xenograft tumor growth assay |
|
| [50] | Ghaderi, S., et al | 2021 | OVCAR-3 cells | Real-time PCR and western blotting | |
| [51] | Sadoughi, A., et al | 2021 | MCF-7, MDA-MB-231 breast cancer cells &human fibroblast cells | Spectrophotometry, SEM, MTT, and Annexin V.real-time PCR | |
| [52] | Wozniak, M. et al., | 2021 | Melanoma (MugMel2), squamous cell carcinoma (SCC-25), and normal human keratinocytes (HaCaT) cell lines | MTT, healing assay, flow cytometry, and immunocytochemistry | |
| [53] | Alam, J., et al., | 2022 | Gastric cancer AGS. PI/Cyto9 staining | MTT.HPLC.flow cytometry | |
| [54] | Atia, MM, et al | 2022 | Hepatic cancer HepG2 and Huh-seven cancer cell | TEM.DLS.Acridine orange/ethidium bromide (EB/AO) assays.Animal experiments and treatments.Western blot analysis.RT-PCR | |
| [55] | Essawy, M.M., et al., | 2022 | Oral cancer squamous cell carcinoma cell line |
Wound closure autofluorescence |
|
| [56] | Mohammadi, H., et al | 2022 | HT29 and Hct116 Colon Cancer Cell Lines | ||
| [57] | Mukherjee, D., et al | 2022 | Oral Squamous Cancer Cells. KB 3–1 cell) | Oral Squamous Cancer Cells. KB 3–1 cell) | |
| [58] | Sadeghi, R.V., et al | 2022 | Human cervical cancer cell line (HeLa cell line RRID: CVCL_003 | MTT assay and flow cytometry real-time RT-PCR and western blot | |
| [59] | Krishnaveni P, et al | 2023 | DAL, A72 and HT29 | Cell block technique, AO/PI staining, TUNEL assay, immunocytochemistry, immunofluorescence, and Real-time PCR. AO/PI staining and TUNEL assay | |
| [60] | Moawad, Mahmoud, et al | 2023 | Hep-G2 | (qRT-PCR), flow cytometry | Ball milling |
| [61] | Subandi, Subandi, and Sigit Purbadi Purbadi S | 2023 | BeWo choriocarcinoma cell line (ATCC CCL-98) | Real-Time PCR, flow cytometry | |
| [62] | Seyed Hosseini, Elahe, et al | 2023 | SKOV3 and OVCAR3 | Real-time PCR and Western blot | |
| [63] | Viraaj, V., et al | 2023 | KB-3–1 Cell line, oral cancer | AO assay, MTT Assay, Trypan blue assay (TB assay), Haemolytic assay | solvent-antisolvent method |
| Authors name | Year | Carrier | Gen | Cellular result | |||
|---|---|---|---|---|---|---|---|
| [15] | Bisht, S | 2007 | N-isopropyl acrylamide (NIPAAM) co-polymers, along with N-vinyl-2-pyrrolidone (VP) and poly(ethylene glycol)monoacrylate (PEG-A), are used in various applications | Triggering cell death, preventing NFκB activation, and reducing baseline levels of various inflammatory cytokines such as IL-6, IL-8, and TNFα | |||
| [16] | Lim, K.J., et al | 2011 |
Combination of G 2/M arrest and apoptotic induction Downregulation of the insulin-like growth factor pathway in DAOY medulloblastoma cells. Levels of STAT3 were also attenuated |
||||
| [17] | Prasanth, R | 2011 | |||||
| [13] | Nair, K.L., et | 2012 | Two PLGA combinations, 50:50 and 75:25, with varying ratios of lactide to glycolide, were utilized | ||||
| [14] | Milano, F., et | 2013 | Colloidal nanoparticles, named Theracurmin | Up-regulated the expression of the co-stimulatory molecule CD86 in DCs | Basic levels of T cell-induced cytotoxicity of 6.4 and 4.1% increased to 15 and 13%, respectively | ||
| [12] | Basniwal, R.K | 2014 | Nanoparticles r2–40 nm and aqueous solubility of up to a maximum of 3 mg/mL | ||||
| [18] | Chaudhari, P.D | 2015 | Curcumin was formed into a stable microdispersion through melt granulation with Gelucire® 50/13, a hydrophilic carrier, and subsequent adsorption onto Aeroperl® 300 Pharma | ||||
| [19] | Dhivya, R. et al., | 2015 | Nanocomposite of Curcumin with ZnO nanoparticle. The average particle size of ZnO nanoparticle and nanocomposite from XRD was 21.44 nm and 24.66 nm, respectively. Nanocomposite was found to have a narrow particle size of 53 nm | ||||
| [20] | Hossain, D.M., et al | 2015 | FoxP3 | ||||
| [21] | Hu, B., et | 2015 | Polymeric nanoparticle formulation of Curcumin (NFC) | NFC and sorafenib synergistically down-regulated the expression of MMP9 via NF-κB/p65 signaling pathway | Decreased the population of CD133-positive HCC cells | ||
| [22] | Pillai, J.J., et | 2015 | Nanoparticles of PLGA-PEG co-polymer, which were conjugated with folic acid (PPF co-polymer) | ||||
| [23] | Xie, M. et | 2015 | Cell cycle arrest in G2/M phase. CM NPs exhibited reduced cytotoxicity on normal cells (NCM460) compared to CM-DMSO and 5-Fu | ||||
| [24] | Chamani, F., et | 2016 | Dendrosomal nanocurcumin (DNC) | mir-34 family and DNA methyltransferases (DNMT1, DNMT3A and 3B) | |||
| [25] | Keshavarz, R., et al | 2016 | Dendrosomal curcumin (DNC) | Enhanced expression of GADD45 and a reduced expression of NF-κB and c-Myc | Enhance the number of apoptotic cells (90%) compared with their application alone (15% and38% for p53 overexpression and DNC, respectively) | ||
| [26] | Khan, M.A., et al | 2016 | Chitosan nanoparticles (CsNPs) | ||||
| [27] | Khosropanah, M.H., et al | 2016 | Myristic acid-chitosan (MA-chitosan) nanogels | ||||
| [28] | Paunovic, V., et al | 2016 | Curcumin nanoparticles. size of curcumin nanocrystals was approximately 250 nm | Mitochondrial depolarization, caspase-3 activation, and cleavage of poly (ADP-ribose) polymerase, indicating apoptotic cell death | |||
| [29] | Aldahoun, M.A., et al | 2017 | The IC50 of nanocurcumin in combination with the magnetic field (NANOCUR-MF) and the control group against PC3 was 35.93%, which was three times higher than curcumin combined with the magnetic field (CUR-MF), at 10.77%. However, their E% against HEK was insignificant, with 1.4% for NANOCUR-MF and 1.95% for CUR-MF | ||||
| [30] | Dash, T.K. and VSB | 2017 | Optimizing organic solvent selection through freeze-drying enhanced encapsulation efficiency (60%) and a particle size of approximately 40 nm when acetone was used in PVA-stabilized dispersion | Curcumin reversed DOX resistance in COLO205 cells at low concentrations, and the presence of PVA enhanced curcumin encapsulation in HP-β-CD. Further, it was observed that prepared HP-β-CD-encapsulated Curcumin is equi-efficacious to nano-dispersed curcumin | |||
| [31] | Mahjoub, M.A., et al | 2017 | Dendrosomal nanocurcumin | CXCL12/CXCR4 axis and Hedgehog pathway genes | Lowcytomety and wound healing assay | ||
| [32] | Mishra, D. et al., | 2017 | Silk fibroin, also known as SF, has an average diameter of 127.7 ± 6.8 nm and exhibits a bimodal distribution, with most particles ranging between 70 and 110 nm | Initial burst release within the first 24 h and continued release up to 7 days | |||
| [33] | Athira, G.K., | 2018 | octenyl succinylated cassava starch-curcumin < 50 nm | C max of nanocurcumin (110.89 ± 0.921 ng/ml) was significantly higher when compared to that of native curcumin (82.94 ± 1.128 ng/ml) | |||
| [34] | Bagheri, R., Z. Sanaat, and N. Zarghami | 2018 | PLGA-PEG nanoparticles | telomerase gene.hTERT | |||
| [35] | Baghi, N., et al | 2018 | Dendrosomal nanocurcumin | p53.EMT- ZEB1 and BMI1 | When p53 overexpression and DNC are combined in treatment, the percentage of apoptotic cells significantly increases to 92%. Cells treated with a p53-expressing vector enhance 38%, while those treated with DNC enhance to 86% | ||
| [36] | Hashemzehi, M., et al | 2018 | Phytosomal-encapsulated | CyclinD1,GSK3a/b, P-AMPK, MMP9, and E-cadherin | Phytosomal-curcumin inhibits cell growth and movement triggered by thrombin via AMP-Kinase in breast cancer | ||
| [37] | Hosseini, S., et al | 2018 | nano-micelle | Cyclin D1 | Cell proliferation in the KYSE-30 cell line decreased by 71.09%, a more significant reduction than Paclitaxel (61.30%) and Carboplatin (62.32%). The IC50 of nano-curcumin in KYSE-30 was notably lower at 1.87 mg/mL compared to free drugs (Paclitaxel 7.5 mg/mL and Carboplatin 40 mg/mL) in KYSE-30 and nano-curcumin (10 mg/mL) in normal cells | ||
| [38] | Nguyen, N.T., et al | 2018 | (nanogel)thermosensitive co-polymer heparin-Pluronic F127 (Hep-F127) co-delivering cisplatin (CDDP) and curcumins (Cur) (Hep-F127/CDDP/Cur).size:129.3 ± 3.8 nm | The IC50 concentrations of each formulation showed that 100 ppm of Hep-F127/Cur resulted in a 63.10 ± 1.91% reduction in cell growth. Treatment with Hep-F127/CDDP led to cell inhibition of 88.57 ± 1.38%, while Hep-F127/Cur/CDDP treatment resulted in a notable 95.32 ± 2.57% inhibition | |||
| [39] | Shariati, M., et al | 2018 | Smad3 and E2F1. Smad7 | The best results were obtained from 72 h of experiments with 12.5 µM IC50 | |||
| [40] | Srivastava, S., et al | 2018 | up to 200 nm | Blc2, and Bax | IC (50) value for growth inhibition was calculated as 47.89 and 26.19 μg/ml, respectively, for nano-CU and nano-FU | ||
| [41] | Dang, L.H., et al., | 2019 | Micelles based on cationic amphiphilic block co-polymer | ||||
| [42] | Harini, L., et al., | 2019 | Non-spherical mesoporous silica nanoparticles (MSNAs) | Activation of caspase 9, 6, 12, PARP, CHOP, and PTEN. protein Akt1 | MSNAP causes more effective cell death at 30 µM curcumin than free curcumin. PEI-coated MSNA increased drug loading to 80%. The LD50 of MCM-41P was ten µg/mL, whereas the LD50 of MSNAP was 80 µg/mL | ||
| [43] | Hosseini, S., et al | 2019 | Nano-micelle | cyclinD1 | Nano-curcumin decreased cell proliferation by 83.6%; at a curcumin concentration of 162.87 mmol/L, cell viability reduced to 16% | ||
| [44] | Seyed Hosseini, E., et al | 2019 | Dendrosomal nanocurcumin | LSINCT5. ABO73614 | CCAT2. ANRIL. BC200. FAL1. MALAT1. XIST. OVAAL. GAPDH | The DNC treatment showed an inhibitory effect at a 0.088-fold rate. The IC50 of DNC for SKOV3 cells was 25 μM at 24 h, reduced to 22 μM at 48 h, and decreased to 17.5 μM at 72 h. The concentrations were 20 μM at 24 h, 15 μM at 48 h, and ten μM at 72 h | |
| [45] | Yang, R. et al | 2019 | A coral shaped nano-transporter DNA-FeS (2)-DA | PKM2 and FASN |
Without NIR:CUR IC50 = 250.6 μg·mL−1), CUR@DNA-FeS2-DA (IC50 = 222.0 μg·mL−1) with NIR:CUR@DNA-FeS2-DA (IC50 = 114.4 μg·mL−1). CUR (IC50 = 229.3 μg·mL−1) |
||
| [46] | Cheng, T. et al | 2020 | Curcumin/gelatin-blended nanofibrous |
p-STAT3 Bip/PERK/elf2a |
The colony number dropped by around 60% in the group that received a conditioned Cc/Glt NM (CM-Cc/Glt NM) medium. In comparison, DMEM vs. CM-Glt NM vs. CM-Cc/Glt NM showed 75.2 ± 7.7, 77.2 ± 13.3, and 29.6 ± 6.5, respectively | ||
| [47] | Hanna, D.H. and G.R. Saad, | 2020 | 28 nm | P53, Bax, and Caspase-3, Bcl-XL | An IC50 value of 17 ± 0.31 μg ml − 1 was achieved after 48 h. This resulted in cell cycle arrest in the G2/M phase and a rise in apoptotic cells in the sub-G1 phase | ||
| [48] | Kuo, I.M., et al | 2020 | Cyclin D1 and Cyclin A.Hsp70 | The IC50 values for curcumin, resveratrol, and their combined treatment on CT26 cells were determined to be 26.76 ± 1.06 and 88.76 ± 1.07 μM | |||
| [49] | Pandit, A.H., et al | 2020 | The size of nanocurcumin was < 100 nm | ||||
| [50] | Ghaderi, S., et al | 2021 | BAX/Bcl-2 | ||||
| [51] | Sadoughi, A., et al | 2021 | Tri-polyphosphate chitosan nanoparticles 48 nm | TP53. VEGF | IC50 of nano Cur-chitosan-TPP vs free curcumin 15 μg/mL at 72 h vs 20 μg/mL at 48 h. led to an induction of apoptosis (79.93%) and cell cycle arrest (at S and G2M) | ||
| [52] | Wozniak, M. et al., | 2021 | All subsequent biological studies chose curcumin in the concentration of 10 μM | Flow cytometry: The combination of liposomal Curcumin and PDT increased apoptosis to 40% and 30% in SCC-25 and MUG-Mel2 | |||
| [53] | Alam, J., et al., | 2022 | Emulsifier TPGS1000 in the PLGA based formulationCurcumin loaded diameter of ~ 175 nm, | Nano-curcumin significantly increased the inhibition rate from 7 to 69% after 24 h, from 11 to 87% after 48 h, and from 16 to 97% after 72 h. The IC50 values for Curcumin and Nano-curcumin were 24.20 µM and 18.78 µM, respectively, after 72 h. The sub-G0 population rose from 4.1% in the control group to 24.5% and 57.8% when treated with curcumin and nano-curcumin, respectively | |||
| [54] | Atia, MM, et al | 2022 |
YP2E1, P53, cleaved caspase-3, and COL1A1 |
Curcumin was reduced by 44.95% and 62.36%, respectively, while Niacin Curcumin was reduced by 66.21% and 47.85% compared to the AC group. Cleaved caspase-3 Niacin Curcumin showed a 57.5% reduction compared to the AC group. Retreatment with curcumin led to a 49.67% reduction in expression, and Niacin Curcumin was reduced by 65.29% compared to AC-treated mice | |||
| [55] | Essawy, M.M., et al., | 2022 | The 60.8 µg/mL concentration was more effective in inhibiting the migration of cancer cells by 25% compared to the native curcumin particle concentration of 212.4 µg/mL | ||||
| [56] | Mohammadi, H., et al | 2022 | Nano-micelle | IC50 values of Nanocurcumin in HT29, HCT116, and HGF were 70.63, 123.9, and 168.53 μg/ml, respectively | |||
| [57] | Mukherjee, D., et al | 2022 | NC ~ 200 nm size | The nano-curcumin IC50 for the HeLa cell line after 48 h was approximately 15 μM. Flow cytometry data indicated a 46.5% result | |||
| [58] | Sadeghi, R.V., et al | 2022 | Oleic acid-derived dendrosome nano-carrier( OA400 Nanoparticle) | E6, E7, P53, and Rb mRNA | The IC50 value of nano-curcumin for the HeLa cell line within 48 h was about 15 μM. Flow cytometry results showed that 46.5% | ||
| [59] | Krishnaveni P, et al | 2023 | Curcumin solid lipid nanoparticles | Bax and Caspase 8,Bcl2, Cyclin D1 and PCNA, miR181a, pre-miR-182, miR155 | solid lipid nanoparticles conveyed curcumin to cancer cells successfully and expanded the restorative impact by applying its capacities through miRNAs, acceptance of apoptosis as well as hindrance of metastasis | ||
| [60] | Moawad, Mahmoud, et al | 2023 | Nanocapsules | p53, Bcl-2, Bax, and Bax |
Curcumin nanocapsules significantly increased the apoptotic cell population in a dose- and time-dependent manner mRNA expression analysis showed that proapoptotic Bax, Caspase-3, and tumor suppressor gene p53 were up-regulated during the process initiated by curcumin nanocapsules and reduced the rate of Bcl-2/bax |
||
| [61] | Subandi, Subandi, and Sigit Purbadi Purbadi S | 2023 | NF-κB | Nanocurcumin and MTX reduced telomerase expression, NF-κB expression, and BrdU proliferation index in BeWo carcinoma cell line cultures more rapidly than MTX alone | |||
| [62] | Seyed Hosseini, Elahe, et al | 2023 | Dendrosomal nano carrier | AKT, PI3K, PKC, JNK, P38 and MMPs mRNAs | The matrigel invasion, as well as cell viability of ovarian cancer cell lines SKOV3 and OVCAR3 by dendrosomal nano curcumin alone or in combination with oxaliplatin, was inhibited significantly | ||
| [63] | Viraaj, V., et al | 2023 |
The low proliferation index of the bulk is due to its large size and low permeability Furthermore, due to a threefold reduction in bulk, the chemically synthesized nanocurcumin exhibited a better proliferation index than the green synthetic nanocurcumin. This demonstrated improved uptake of nanocurcumin by the cell lines |
||||
Nanocurcumin demonstrated superior cellular uptake and anti-proliferative activity compared to free curcumin. For instance, in human cervical cancer cells (HeLa), nanocurcumin exhibited enhanced cytotoxicity, as evidenced by increased apoptosis and downregulation of clonogenic potential. Nanocurcumin also modulated vital signaling pathways, such as PI3K/Akt, JAK/STAT3, and NF-κB, and regulated the expression of apoptosis-related genes, including p53, Bcl-2, and caspases.
In vivo studies
The 12 in vivo studies (Table 3) investigated the efficacy of nanocurcumin in various animal cancer models. These studies predominantly used xenograft models and chemically-induced carcinogenesis models. The nanocurcumin formulations were administered through different routes, such as oral, intraperitoneal, and intratumoral.
Table 3.
Invivo studies
| Authors | Title | Year | Objectives of study | Type of cancer | Duration of study | Sample size | Carcinogenic | Cell type of cancer | |
|---|---|---|---|---|---|---|---|---|---|
| [64] | Alizadeh, A.M., et al. | Chemoprevention of azoxymethane-initiated colon cancer in rats by using a novel polymeric nanocarrier–curcumin | 2012 | Preventive effects of polymeric nanocarrier–curcumin on colon carcinogenesis in an AOM-induced rat tumor. | Normal colonic mucosa, mild to severe,dysplasia and colonic adenocarcinoma. | 18 weeks | 40Male Wistar rats (100–120 g) | azoxymethane(AOM).15 mg/kg, s.c- weekly for two consecutive weeks | |
| [65] | Chang, C.Z., et al., | Curcumin, encapsulated in nano-sized PLGA, down-regulates nuclear factor κb (p65) and subarachnoid hemorrhage-induced early brain injury in a rat model. | 2015 | Examine the efficacy of nanocurcumin, a diarylheptanoid, on a SAH-induced EBI model. | |||||
| [66] | Chaudhari, P.D. and P.N. Kendre | An emerging trend in addressing the challenges to oral nanocurcumin delivery is to improve the quality of life of cancer patients. | 2015 |
Enhancement of solubility, bioavailability, and anti-cancer activity of curcumin |
Breast cancer | FT-IR, DSC, and XRD-MT assay | |||
| [67] | El-Azab, N.E.E., M.Y. Salem, and S. Abd El-Salam | A histological and immunohistochemical study of different therapeutic modalities for experimentally induced ulcerative colitis in rats. | 2016 | UC(Ulcerative colitis ) | 2 weeks | 65adult male rats | |||
| [68] | Mukerjee, A., A.P. Ranjan, and J.K. Vishwanatha | Targeted Nanocurcumin Therapy Using Annexin A2 Antibody Improves Tumor Accumulation and Therapeutic Efficacy Against Highly Metastatic Breast Cancer. | 2016 | Assess the in vivo targeting efficacy and examine the combined therapeutic effects of new Annexin A2 (AnxA2) antibody-linked curcumin-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles (AnxA2-CPNP) for metastatic breast cancer through xenograft trials in mice. | Metastatic breast cancer |
CF10A, MCF10AT and MCF10CA1 |
|||
| [69] | Arozal, W., et al. | Pharmacokinetic Profile of Curcumin and Nanocurcumin in Plasma, Ovary, and Other Tissues. | 2019 | Ovary | 15 days | 10 Female Sprague Dawley rats 180–260 g | |||
| [70] | Vijayakurup, V., et al. | Chitosan Encapsulation Enhances the Bioavailability and Tissue Retention of Curcumin and Improves its Efficacy in Preventing B a P-induced Lung Carcinogenesis | 2019 | Evaluate whether encapsulation of curcumin in chitosan nanoparticles can improve the cellular uptake and prolong the tissue retention of curcumin, yielding better chemoprevention | Lung Carcinogenesis | H1299 | |||
| [71] | Abuelezz, N.Z., et al., | Nanocurcumin alleviates insulin resistance and pancreatic deficits in polycystic ovary syndrome rats: Insights on PI3K/Akt/mTOR and TNF-α modulations. | 2020 | This study investigated TNF-α and pancreatic PI3K/AKT/mTOR levels in a PCOS animal model and evaluated their effects on developed pancreatic deficits. Secondly, we explored the impact of nanocurcumin as a potent anti-inflammatory supplement against these developed pancreatic pathologies | ovary | 15 days | 60 Virgin, adult female Wistar rats (7 weeks old and 160–200 g body weight)in 6 group | ||
| [72] | Wang, G.N. and S. Sukumar | Characteristics and anti-tumor activity of polysorbate 80 curcumin micelles preparation by cloud point cooling. | 2020 | Xenografts in mice | breast cancer | Six-week-old female Nod/SCID/gamma strain of mice | Micell(polysorbate 80) 20nm | Utilizing the process of micelle formation that occurs during slow cooling below its cloud point | |
| [73] | immunological responses and anti-tumor effects of HPV16/18 L1-L2-E7 multiepitope fusion construct along with curcumin and nanocurcumin in C57BL/6 mouse model. | 2021 | Two multiepitope DNA and peptide-based vaccine constructs (L1-L2-E7 and HSP70-L1-L2-E7) were used along with curcumin and nanocurcumin to evaluate immune responses and protective/therapeutic effects in tumor mouse mode | 96C57BL/6 mice aged five to seven weeks(12group) | |||||
| [74] | Lakshmanan, A. et al., | Nanocurcumin-Loaded UCNPs for Cancer Theranostics: Physicochemical Properties, In Vitro Toxicity, and In Vivo Imaging Studies | 2021 | UCNPs accumulate in the liver, lungs, intestines, and spleen four hours after administration. | Lewis Lung Cancer Mouse Model/female BDF1 mice | Upconversion nanoparticles (UCNPs) using Poly (lactic-co-glycolic acid) (PLGA) 350nm | nanocarrier: reverse microemulsion/loading method:solvent-antisolvent method | ||
| [75] | Curcumin Nanoparticle Enhances the Anticancer Effect of Cisplatin by Inhibiting PI3K/AKT and JAK/STAT3 Pathway in Rat Ovarian Carcinoma Induced by DMBA | 2021 | Investigate the mechanism of curcumin nanoparticles given in combination with cisplatin in rat ovarian carcinoma | Ovarian carcinoma | 25 female Wistar rats | dimethylbenz(a)anthracene (DMBA) | |||
| [76] | Nanocurcumin preserves kidney function and hematology parameters in DMBA-induced ovarian cancer treated with cisplatin via its antioxidative and anti-inflammatory effect in rats | 2023 | Nanocurcumin, which has a good effect on kidney function in rats with ovarian cancer | Ovarian | 4 weeks | 20Wistar female rats aged eight weeks and weighing 150–200 g | Dimethylbenz(a)anthracene (DMBA) | Urea and creatinine colorimetric analysis at 534 nm,(TNF)-α,(NGAL) (FSH) ELISA Kit | |
| [77] | Abdelgawad LM,etal | Document details–Influence of Nanocurcumin and Photodynamic Therapy Using Nanocurcumin in Treatment of Rat Tongue Oral Squamous Cell Carcinoma Through Histological Examination and Gene Expression of BCL2 and Caspase-3 | 2023 | comparing the effects of using Nanocurcumin and photodynamic therapy (PDT), alone or together, in treating OSCC in rats. | Tongue Oral Squamous Cell Carcinoma | Four months | Forty Wister male rats8 weeks with a weight range of 120- 160 g | dimethylbenz anthracene0.5 percent three times per week for four weeks | DAL, A72 and HT29 cell lines |
| Authors | Analyse of efficacy | Administration | Dosing | Duration of treatment | Control | Curcumin free | Compare nano with curcumin free | |||
|---|---|---|---|---|---|---|---|---|---|---|
| [64] | Alizadeh, A.M., et al. |
Histological assay.Hematoxylin and eosin examinations- vidin–biotin immunoperoxidase |
Two weeks before till 14 weeks after the last injection of AOM | 0.2% and PNCC | Reductions in tumor incidence 70 to 85 compared to control | Reductions in tumor incidence by 33% compared to control | ||||
| [65] | Chang, C.Z., et al., | Western blot(, caspases)rt-PCR | 75/150/300 μg/kg/day via osmotic mini-pump post-SAH | |||||||
| [66] | Chaudhari, P.D. and P.N. Kendre | 300 μg/kg | SAH-induced EBI model | Nanocurcumin treatment demonstrated neuroprotective effects by increasing NF-κB (p65) expression and decreasing caspase-9a expression related to mitochondria. These effects were observed in nanocurcumin nanoparticles, which may help mitigate EBI induced by SAH. | ||||||
| [67] | El-Azab, N.E.E., M.Y. Salem, and S. Abd El-Salam | histological and immunohistochemical techniques | a daily oral dose of nanocurcumin starting three days after induction of colitis for two weeks;2 | 2 Weeks | Nanocurcumin is more effective than pentoxifylline in the treatment of UCin rats. | |||||
| [68] | Mukerjee, A., A.P. Ranjan, and J.K. Vishwanatha | AnxA2-CPNPs effectively block cell proliferation, invasion, and migration, critical factors in cancer progression and spreading. | ||||||||
| [69] | Arozal, W., et al. | Cell viability, plasmin generation, and wound healing-Live animal imaging -angiogenesis assay | a single oral dose of 500 mg/kg | Curcumin concentrations in ovaries in the nanocurcumin group were 3.6 times higher than those in the curcumin group. They conclude that reducing curcumin's particle size did not alter its pharmacokinetic profile. However, it increased the distribution of curcumin in some tissues, although not optimal for use in ovarian cancer. | ||||||
| [70] | Vijayakurup, V., et al. | chronic toxicity model in Swiss albino mice.MTT.Clonogenic assay/ immunoassay.Western blotting.HOMA assessments and immunohistochemistry. | Oraly 25 mg/kg Aqueous suspension of chitosan nanocurcumin or free curcumin dissolved in corn oil | Free curcumin reduced tumor incidence by 22% and tumor multiplicity by 46.8%. In comparison, a quarter of the dose of chitosan nanocurcumin led to a 52% decrease in tumor incidence and a 71.4% decrease in tumor multiplicity compared to the B[a]P group. | ||||||
| [71] | Abuelezz, N.Z., et al., | TEM.FTIR | HOMA.Western blotting. Histopathology. immunohistochemistry | 100 mg and 200 mg/kg | ||||||
| [72] | Wang, G.N. and S. Sukumar | U.V.TEM | SUM149 human breast cancer | MTT.Fluorescent microscopy and image analysis.Xenograft tumor growth assay | MC (100 mg/kg body weight), used at half the dose of FC (200 mg/kg body weight), | Achieved a statistically significant reduction in tumor size compared to vehicle-treated mice | ||||
| [73] | immunological responses and anti-tumor effects of HPV16/18 L1-L2-E7 multiepitope fusion construct along with curcumin and nanocurcumin in C57BL/6 mouse model. | HEK-293 T cell line(normal).c3(tumor) | Granzyme B assay.Immunological assay.Monitoring tumor growth.Cytokine secretion.Antibody assay | three times with a two-week interval | 20 μM | Curcumin and nanocurcumin inhibited the growth of C3 tumors in mice, resulting in 60% and 80% survival rates, respectively. Combining homologous multiepitope peptides and heterologous multiepitope DNA prime/multiepitope peptide boost regimens and curcumin and nanocurcumin provided complete protection against C3 tumors, leading to 100% tumor-free mice. | ||||
| [74] | Lakshmanan, A. et al., | XRD.FTIR. | Rat glioma C6 cells. | MTT Assay.MTS Assay.Anti-Stokes Photoluminescence Microscopy and Confocal Fluorescence Microscopy | PLGA-UCNPs showed 60–80% cell viability at 0.12–0.02 mg/mL in the rat C6 glioma cell medium. | |||||
| [75] | Curcumin Nanoparticle Enhances the Anticancer Effect of Cisplatin by Inhibiting PI3K/AKT and JAK/STAT3 Pathway in Rat Ovarian Carcinoma Induced by DMBA | ELISA, Western Blot Analysis, Quantitative RT-PCR Analysis | Administered for four weeks | Nanocurcumin (100 mg/kg BW every day), cisplatin (4 mg/kg BW every week) | Nanocurcumin improves the anti-cancer activity of cisplatin better than conventional curcumin. Cisplatin-curcumin and cisplatin-nanocurcumin showed a | A remarkable increase in apoptotic markers, the ratio of Bax/BCl2 mRNA expressions of caspase-3 and caspase-9 | ||||
| [76] | Nanocurcumin preserves kidney function and hematology parameters in DMBA-induced ovarian cancer treated with cisplatin via its antioxidative and anti-inflammatory effect in rats | 4weeks | Drug vehicle only, cisplatin only (4 mg/kg BW weekly), cisplatin (4 mg/kg BW weekly) with curcumin (100 mg/kg BW daily), and cisplatin (4 mg/kg BW weekly) with nanocurcumin (100 mg/kg BW daily) | Cisplatin and nanocurcumin in a rat model of ovarian cancer provide added benefits in preserving renal functio | ||||||
| [77] | Abdelgawad LM, et. al | Bcl2 and Caspase-3 expression levels measurements by ELISA, Body weight, Tumor volume, Histopathology observation | Nanocurcumin200nm and 94% purity was given orally in distilled water with a concentration of 0.1-2 ml/mg/kg+A continuous 650 nm wavelength (6 mm diameter) was generated using gallium aluminum arsenide diode laser equipment | PDT using nanocurcumin photosensitizer was influential in the treatment of OSCC regarding clinical | ||||||
Nanocurcumin demonstrated superior tumor growth inhibition and bioavailability compared to free curcumin. In a xenograft model of breast cancer, nanocurcumin significantly reduced tumor volume and increased survival. Additionally, nanocurcumin exhibited chemopreventive effects in a rat colon carcinogenesis model, reducing tumor incidence and multiplicity. Notably, nanocurcumin enhanced the efficacy of conventional chemotherapeutic agents, such as cisplatin and doxorubicin, in ovarian and breast cancer models.
Clinical trials
Two clinical trials (Table 4) investigated the safety and efficacy of nanocurcumin in cancer patients. Two phase II trials evaluated nanocurcumin as an adjunct therapy in prostate cancer patients undergoing radiotherapy and bladder cancer patients receiving chemotherapy [10, 11]. These trials reported improved clinical outcomes, such as reduced radiation-induced toxicities and enhanced tumor response rates, in the nanocurcumin-treated groups. However, the sample sizes in these trials were relatively small and more significant, well-designed studies are needed to validate the findings.
Table 4.
clinical trials
| Authors | Publication year | Title | Type of study | Cancer | Assay | Pat | Dose | Administrate | Efficacy | |
|---|---|---|---|---|---|---|---|---|---|---|
| [78] | Saadipoor, A., et al | 2019 | Randomized, double-blind, placebo-controlled phase II trial of nanocurcumin in prostate cancer patients undergoing radiotherapy | Randomized, Double-blind | prostate cancer | CTCAE v.4.03 grading criteria | 64 | 120 mg/day for | Three days before and during the RT course | Proctitis was observed in 18 out of 31 (58.1%) patients who received the placebo, compared to 15 out of 33 (45.5%) patients treated with nanocurcumin |
| [79] | Sandoughdaran, S., et al., | 2020 | Randomized, Double-blind Pilot Study of Nanocurcumin in Bladder Cancer Patients Receiving Induction Chemotherapy | Randomized, Double-blind | muscle-invasive bladder cancer (MIBC) | 26 | 180 mg/day | Clinical response rates were 30.8 and 50% in the placebo and nanocurcumin. No significant difference was found between the two groups concerning grade 3/4 renal and hematologic toxicities and hematologic nadirs | secondary: nephrotoxicity, hematologic nadirs, and toxicities | The entire clinical response assessment will be conducted up to four weeks after treatment completion |
Synthesis of results
The collective evidence from in vitro, in vivo, and clinical studies suggests that nanocurcumin is a promising therapeutic agent for cancer treatment. Nanocurcumin consistently demonstrated enhanced bioavailability, cellular uptake, and anti-cancer efficacy compared to free curcumin. The improved physicochemical properties of nanocurcumin, such as increased solubility and stability, contribute to its superior performance.
Nanocurcumin exerts its anti-cancer effects through multiple mechanisms, including the induction of apoptosis, cell cycle arrest, and modulation of cancer-related signaling pathways. The ability of nanocurcumin to sensitize cancer cells to conventional therapies highlights its potential as an adjuvant treatment option.
However, it is essential to acknowledge the limitations of the current evidence. Most of the studies were preclinical, and the clinical trials had small sample sizes. The variability in nanocurcumin formulations, cancer types, and dosing regimens across studies makes direct comparisons challenging. Therefore, further research, particularly in large-scale, well-designed clinical trials, is necessary to establish the clinical efficacy and long-term safety of nanocurcumin in cancer treatment.
Discussion
The exploration of nanocurcumin in cancer therapy, as evidenced by the results of this systematic review, underscores its burgeoning role in oncological treatments. The distinctive physicochemical properties of nanocurcumin, notably in formulations using PLGA combinations (50:50 and 75:25), have been pivotal in enhancing its therapeutic efficacy. The 50:50 PLGA nanocurcumin, with its smaller particle size and higher encapsulation efficiency, demonstrates a favorable pharmacokinetic profile characterized by a more rapid release rate and sustained release over a week, thereby augmenting its anti-cancer capabilities [13].
In vitro studies have highlighted the superior cellular uptake and anti-proliferative activity of nanocurcumin compared to free curcumin, especially in human epithelial cervical cancer cells (HeLa). These research projects, using techniques such as MTT assay and Annexin V/propidium iodide staining, demonstrate the improved ability of nanocurcumin to fight tumors. This is supported by the findings of PARP cleavage and decreased clonogenic potential in HeLa cells [13]. Furthermore, the electrophoretic mobility shift assay and immunocytochemical analysis confirm the higher effectiveness of nanocurcumin, highlighting its potential in fighting different types of cancer. [14]. Mona M Atia and colleagues found that both curcumin and nanocurcumin decreased the viability of HepG2 and Huh-7 cancer cells and increased the occurrence of apoptosis, regardless of the presence or absence of acrylamide. Moreover, nanocurcumin exhibited superior anti-tumor effectiveness compared to curcumin. The administration of acrylamide in mice resulted in a significant rise in the expression of CYP2E1, p53, cleaved caspase-3, and COL1A1 in the liver, as well as elevated levels of blood alanine aminotransferase and aspartate aminotransferase activity. Nanocurcumin and curcumin successfully counteracted these effects, with nanocurcumin exhibiting a reduction in the histopathology and fibrosis resulting from acrylamide and effectively correcting the acrylamide-induced glycogen depletion. Using nanoparticle formulation can enhance the effectiveness of curcumin in fighting cancer and protecting the liver [54]. Another study has demonstrated that the combination of dendrosomal nanocurcumin and exogenous p53 work synergistically to provide anti-cancer effects on MDA-MB-231 breast cancer cells [6]. The nanocurcumin effectively inhibited the proliferation of Hep-2 cancer cells by causing cell cycle arrest in the G2/M phase and inducing apoptosis. This apoptotic process relied on the activation of caspase-3 and p53 [53].
In vivo studies complement these findings, illustrating nano curcumin’s synergistic effect with other therapeutic agents, such as in the multiepitope HSP70-L1-L2-E7 vaccine construct against HPV-related C3 tumor cells. These studies also demonstrate the enhanced chemopreventive efficacy of curcumin-loaded chitosan nanoparticles compared to free curcumin in lung carcinogenesis models, suggesting broader applicability in different cancer types [18, 21]. Melva Louisa and colleagues showed that nanocurcumin mitigates the elevation of renal function indicators and alterations in hematological indices in rats subjected to cisplatin treatment. When comparing rats treated with cisplatin, plasma urea, creatinine, and neutrophil gelatinase-associated lipocalin (NGAL) levels were significantly reduced in the nanocurcumin-treated group. In addition, nanocurcumin resulted in an increase in glutathione activities, a decrease in lipid peroxidation, and a reduction in plasma TNF-α levels. Combining nanocurcumin with cisplatin in a rat model of ovarian cancer may offer supplementary advantages as a prophylactic drug against renal dysfunction and the hematological damage caused by cisplatin [80]. Gemini nano-curcumin (Gemini-Cur) effectively reaches the cells and hinders cell division in a manner that is influenced by both time and dosage. Annexin V/FITC verified the apoptotic impact on 4T1 cells. Furthermore, in vivo experiments demonstrated that the growth of tumors in mice treated with Gemini-Cur was inhibited compared to the control group. Expression analyses revealed the regulation of genes associated with programmed cell death and the spread of cancer, such as Bax, Bcl-2, MMP-9, VEGF, and COX-2, in mice that received treatment. The studies presented here provide evidence of the potent anti-cancer effects of Gemini-Cur in mouse models. Nevertheless, additional molecular and cellular investigations are necessary to determine this therapeutic benefit [81] definitively. A study confirmed the targeting ability of a new Annexin A2 antibody-linked curcumin-filled poly (lactic-co-glycolic acid) (PLGA) nanoparticles (AnxA2-CPNP) against metastatic breast cancer in living organisms. The findings demonstrated that AnxA2-CPNPs successfully hindered cell growth, invasion, migration, tumor growth, and metastasis. Live imaging of animals illustrated that AnxA2-PNPs and AnxA2-CPNPs accurately aimed and gathered in the tumor. Experiments involving xenografts in mice displayed notable regression of breast tumors due to the precise targeting, gathering, and continual release of curcumin. [68].
Clinical trials, though limited, offer a glimpse into nanocurcumin’s potential in actual patient settings. Studies involving patients undergoing radiotherapy for prostate cancer and chemotherapy for muscle-invasive bladder cancer indicate nanocurcumin's tolerability and efficacy. While not conclusively definitive, these trials emphasize nanocurcumin's role in improving clinical response rates and mitigating radiation-induced complications in cancer patients [15–17]. Saleh Sandoughdaran designed a study to assess the practicality and possible effectiveness of adding nanocurcumin supplements to the treatment of patients with localized muscle-invasive bladder cancer (MIBC) who are undergoing induction chemotherapy. Their data suggest that adding nanocurcumin as a supplemental therapy may be helpful for MIBC patients and provide justification for doing more extensive research in the future.
Furthermore, this study offers a significant translational understanding to bridge the gap between experimental research and clinical use in the field [79]. Researchers carried out an additional investigation to analyze the effects of nanocurcumin on prostate cancer patients undergoing radiotherapy. The study found no notable distinction between the two groups concerning radiation-induced cystitis, duration of radiation side effects, blood count lows, and tumor reaction. Nonetheless, these results provide valuable insights to bridge the divide between laboratory studies and practical clinical use [77]. Despite these positive indications, more significant, well-designed clinical trials are necessary to conclusively determine nanocurcumin's ability to effectively target and kill cancer cells in humans across various cancer types and stages. Future research should focus on optimizing dosing regimens, identifying responsive cancer types, and assessing long-term outcomes to establish nanocurcumin's clinical efficacy fully.
The convergence of these findings from various studies forms a compelling narrative for nanocurcumin’s role in cancer therapy. It highlights nanocurcumin’s ability to enhance curcumin's bioavailability and therapeutic efficacy, chiefly through improved solubility, stability, and targeted delivery. While preclinical studies paint a promising picture, the need for extensive clinical trials to ascertain nanocurcumin’s safety and efficacy in humans is paramount.
The safety profile of nanocurcumin is a critical consideration in its development as a cancer therapeutic. While bulk curcumin has a well-established safety record, nanoformulations may exhibit different biological interactions and potential toxicities. Recent studies have shown promising results regarding nanocurcumin’s safety. For instance, Saadipoor et al. (2019) reported no significant adverse effects in prostate cancer patients receiving nanocurcumin during radiotherapy [77]. Similarly, Sandoughdaran et al. (2020) observed no increased toxicity when combining nanocurcumin with chemotherapy in bladder cancer patients [78]. However, these studies had relatively small sample sizes and short durations. Preclinical research by Abuelezz et al. (2020) demonstrated the safety of nanocurcumin in animal models, with no observed toxicity at therapeutic doses [70]. Nevertheless, comprehensive long-term safety studies and more extensive clinical trials are essential to fully elucidate nanocurcumin's safety profile across various cancer types and treatment regimens before widespread clinical adoption.
The path to regulatory approval for nanocurcumin as a mainstream cancer treatment presents significant challenges. Nanoformulations, including nanocurcumin, face unique regulatory hurdles due to their complex nature and potential for novel biological interactions [81]. The US Food and Drug Administration (FDA) and European Medicines Agency (EMA) have established specific guidelines for nanomedical products, requiring extensive characterization of physicochemical properties, biodistribution, and potential toxicity [82]. For nanocurcumin, demonstrating consistent manufacturing processes, stability, and batch-to-batch reproducibility is crucial [83]. Additionally, regulatory bodies demand robust clinical efficacy data from well-designed trials. While early-phase studies have shown promise [77, 78], more significant, randomized controlled trials are necessary to meet regulatory standards. The regulatory landscape for nanomedicines is evolving, and nanocurcumin developers must navigate these changing requirements to achieve approval, a process that may take several years and substantial resources [84].
Conclusion
This systematic review demonstrates the immense potential of nanocurcumin as a promising therapeutic agent in cancer treatment. The findings from numerous in vitro, in vivo, and clinical studies consistently highlight nanocurcumin's superior bioavailability, enhanced anti-cancer efficacy, and favorable safety profile compared to native curcumin. Nanocurcumin formulations, particularly those involving PLGA polymers, exhibit distinct physicochemical properties that facilitate improved solubility, stability, and targeted delivery, thereby potentiating their therapeutic effects. The clinical implications of these findings are significant, suggesting that nanocurcumin could potentially improve treatment efficacy while reducing side effects commonly associated with traditional chemotherapies, thus enhancing the quality of life for cancer patients. The observed synergistic effects with conventional therapies indicate a potential for reducing drug dosages and minimizing toxicity. However, while certain limitations exist, such as variability in study designs and cancer types investigated and the need for larger-scale clinical trials to fully establish safety and efficacy, the collective evidence strongly advocates for the continued exploration of nanocurcumin in oncological applications. As research in nanotechnology and cancer biology advances, nanocurcumin holds immense promise to revolutionize cancer treatment strategies, offering improved outcomes and enhanced quality of life for patients. Extensive interdisciplinary collaborations and well-designed translational studies are crucial to fully harness nanocurcumin's therapeutic potential and facilitate its clinical implementation in cancer care, potentially making it a valuable addition to the oncologist's toolkit shortly.
Limitations and future directions
Despite the promising results observed in nanocurcumin research, future studies need to address several limitations and challenges. One significant limitation is the high cost of developing and producing nanocurcumin formulations. The complex manufacturing processes and specialized equipment required for nanoparticle synthesis contribute to increased production costs [85]. This economic barrier could potentially limit the accessibility of nanocurcumin-based treatments for patients. Future research should optimize production methods and explore cost-effective formulation strategies to make nanocurcumin more affordable and widely available.
Another critical area for future investigation is the potential development of resistance to nanocurcumin in cancer cells. While curcumin has shown promise in overcoming drug resistance in various cancer types [86], the long-term efficacy of nanocurcumin and its ability to prevent or delay resistance development needs further study. Research by Ghalandarlaki et al. (2014) suggests that nanocurcumin may have advantages in overcoming multidrug resistance due to its enhanced cellular uptake and retention [87]. However, more comprehensive studies are required to elucidate potential resistance mechanisms and develop strategies to counteract them.
Future directions should include:
Conducting large-scale, long-term clinical trials to establish the efficacy and safety profile of nanocurcumin across various cancer types.
Investigating combination therapies with nanocurcumin to enhance its anti-cancer effects and potentially overcome resistance.
Developing and evaluating cost-effective production methods for nanocurcumin to improve its economic viability.
Exploring the molecular mechanisms of nanocurcumin's action in different cancer types to optimize its use and predict potential resistance pathways.
Assessing the long-term effects of nanocurcumin treatment on cancer cell populations to understand and mitigate resistance development.
Investigating personalized nanocurcumin formulations based on individual patient characteristics and tumor profiles to maximize efficacy and minimize resistance.
Addressing these limitations and pursuing these research directions will be crucial in realizing the full potential of nanocurcumin as a mainstream cancer treatment option.
Abbreviations
- PLGA
Poly (lactic-co-glycolic acid)
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyse
- PEG
Poly (ethylene glycol)
- MeSH
Medical Subject Headings
- TEM
Transmission electron microscopy
- PARP
Poly (ADP-ribose) polymerases
- NGAL
Neutrophil gelatinase-associated lipocalin
- MIBC
Muscle-invasive bladder cancer
- NIPAAM
N-isopropyl acrylamide
- AFM
Atomic force microscopy
- EAC
Esophageal adenocarcinoma
- HPLC
High-performance liquid chromatography
- DSC
Differential scanning calorimetry
- HCC
Hepatocellular carcinoma
- NFC
Nanoparticle formulation of curcumin
- DLS
Dynamic Light Scattering
- DNC
Dendrosomal nanocurcumin
- PCR
Polymerase chain reaction
- TPP
Tripolyphosphate
- XRD
X-ray diffraction
- CsNPs
Chitosan nanoparticles
- PVA
Polyvinyl alcohol
- SEM
Scanning electron microscopy
- FTIR
Fourier-transform infrared spectroscopy
Author contributions
R.R.D. A.K.M and M.B. contributed to the design and conception. N.M.A., P.H., and S.A.S contributed to the search. R.R.D. and S.A.S. and A.K.M contributed to the screening. R.R.D, Y.M., and M.B. contributed data, statistical analysis, and interpretation of data. R.R.D. was responsible for overall supervision. A.K.M, M.B., and A.P qualitatively evaluated the studies and their reports based on the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement. M.B, P.H and N.M.A drafted the manuscript, which R.R.D., A.P and Y.M. revised. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This article reviews the existing literature and contains no studies with human participants or animals performed by authors. Therefore, no ethical approval was required for this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arruebo M, Vilaboa N, Sáez-Gutierrez B, Lambea J, Tres A, Valladares M, et al. Assessment of the evolution of cancer treatment therapies. Cancers. 2011;3(3):3279–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nussbaumer S, Bonnabry P, Veuthey J-L, Fleury-Souverain S. Analysis of anticancer drugs: a review. Talanta. 2011;85(5):2265–89. [DOI] [PubMed] [Google Scholar]
- 3.Esfahani K, et al. A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol. 2020;27(s2):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mei L, Zhang Z, Zhao L, Huang L, Yang X-L, Tang J, et al. Pharmaceutical nanotechnology for oral delivery of anti-cancer drugs. Adv Drug Deliv Rev. 2013;65(6):880–90. [DOI] [PubMed] [Google Scholar]
- 5.Wolf CPJG, Rachow T, Ernst T, Hochhaus A, Zomorodbakhsch B, Foller S, et al. Interactions in cancer treatment considering cancer therapy, concomitant medications, food, herbal medicine and other supplements. J Cancer Res Clin Oncol. 2021;148:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khodavirdipour A, Zarean R, Safaralizadeh R. Evaluation of the anti-cancer effect of Syzygium cumini ethanolic extract on HT-29 colorectal cell line. J Gastrointest Cancer. 2021;52:575–81. [DOI] [PubMed] [Google Scholar]
- 7.Bachmeier BE, Killian PH, Melchart D. The role of curcumin in prevention and management of metastatic disease. Int J Mol Sci. 2018;19(6):1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansouri K, Rasoulpoor S, Daneshkhah A, Abolfathi S, Salari N, Mohammadi M, et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. 2020;20:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai J, Ashrafizadeh M, Aref AR, Sethi G, Ertas YN. Peptide-functionalized, -assembled and -loaded nanoparticles in cancer therapy. Drug Discov Today. 2024;29(7):103981. 10.1016/j.drudis.2024.103981. [DOI] [PubMed] [Google Scholar]
- 10.Islam MR, Islam F, Nafady MH, Akter M, Mitra S, Das R, et al. Natural small molecules in breast cancer treatment: understandings from a therapeutic viewpoint. Molecules. 2022;27(7):2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scazzocchio B, Minghetti L, D’Archivio M. Interaction between gut microbiota and curcumin: a new key of understanding for the health effects of curcumin. Nutrients. 2020;12(9):2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabet S, Rashidinejad A, Melton LD, McGillivray DJ. Recent advances to improve curcumin oral bioavailability. Trends Food Sci Technol. 2021;110:253–66. [Google Scholar]
- 13.Nair KL, Thulasidasan AK, Deepa G, Anto RJ, Kumar GS. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anti-cancer activity with dependence on the combination of the carrier. Int J Pharm. 2012;425(1–2):44–52. [DOI] [PubMed] [Google Scholar]
- 14.Basniwal RK, Khosla R, Jain N. Improving the anti-cancer activity of curcumin using nanocurcumin dispersion in water. Nutr Cancer. 2014;66(6):1015–22. [DOI] [PubMed] [Google Scholar]
- 15.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, et al. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. J Nanobiotechnol. 2007;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim KJ, Bisht S, Bar EE, Maitra A, Eberhart CG. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol Ther. 2011;11(5):464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasanth R, Nair G, Girish CM. Enhanced endocytosis of nano-curcumin in nasopharyngeal cancer cells: AN atomic force microscopy study. Appl Physics Lett. 2011;99:16. [Google Scholar]
- 18.Chaudhari PD, Kendre PN. Emerging trend in adressing the challenges to oral nanocurcumin delivery to improve quality of life of patients suffering from cancer. Indian Drugs. 2015;52(9):21–31. [Google Scholar]
- 19.Dhivya R, Ranjani J, Rajendhran J, Rajasekaran M, Annaraj J. pH responsive curcumin/ZnO nanocomposite for drug delivery. Adv Mater Lett. 2015;6(6):505–12. [Google Scholar]
- 20.Hossain DM, Panda AK, Chakrabarty S, Bhattacharjee P, Kajal K, Mohanty S, et al. MEK inhibition prevents tumour-shed transforming growth factor-β-induced T-regulatory cell augmentation in tumour milieu. Immunology. 2015;144(4):561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu B, Sun D, Sun C, Sun YF, Sun HX, Zhu QF, et al. A polymeric nanoparticle formulation of curcumin in combination with sorafenib synergistically inhibits tumor growth and metastasis in an orthotopic model of human hepatocellular carcinoma. Biochem Biophys Res Commun. 2015;468(4):525–32. [DOI] [PubMed] [Google Scholar]
- 22.Pillai JJ, Thulasidasan AKT, Anto RJ, Devika NC, Ashwanikumar N, Kumar GSV. Curcumin entrapped folic acid conjugated PLGA-PEG nanoparticles exhibit enhanced anti-cancer activity by site specific delivery. RSC Adv. 2015;5(32):25518–24. [Google Scholar]
- 23.Xie M, Fan D, Zhao Z, Li Z, Li G, Chen Y, et al. Nano-curcumin prepared via supercritical: Improved anti-bacterial, anti-oxidant and anti-cancer efficacy. Int J Pharm. 2015;496(2):732–40. [DOI] [PubMed] [Google Scholar]
- 24.Chamani F, Sadeghizadeh M, Masoumi M, Babashah S. Evaluation of miR-34 family and DNA methyltransferases 1, 3A, 3B gene expression levels in hepatocellular carcinoma following treatment with dendrosomal nanocurcumin. Asian Pac J Cancer Prev. 2016;17:219–24. [DOI] [PubMed] [Google Scholar]
- 25.Keshavarz R, Bakhshinejad B, Babashah S, Baghi N, Sadeghizadeh M. Dendrosomal nanocurcumin and p53 overexpression synergistically trigger apoptosis in glioblastoma cells. Iran J Basic Med Sci. 2016;19(12):1353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan MA, Zafaryab M, Mehdi SH, Ahmad I, Rizvi MM. Characterization and anti-proliferative activity of curcumin loaded chitosan nanoparticles in cervical cancer. Int J Biol Macromol. 2016;93(Pt A):242–53. [DOI] [PubMed] [Google Scholar]
- 27.Khosropanah MH, Dinarvand A, Nezhadhosseini A, Haghighi A, Hashemi S, Nirouzad F, et al. Analysis of the anti-proliferative effects of curcumin and nanocurcumin in MDA-MB231 as a breast cancer cell line. Iranian J Pharmaceut Res. 2016;15(1):231–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Paunovic V, Ristic B, Markovic Z, Todorovic-Markovic B, Kosic M, Prekodravac J, et al. c-Jun N-terminal kinase-dependent apoptotic photocytotoxicity of solvent exchange-prepared curcumin nanoparticles. Biomed Microdevices. 2016;18:2. [DOI] [PubMed] [Google Scholar]
- 29.Aldahoun MA, Jaafar MS, Al-Akhras MAH, Bououdina M. Enhanced nanocurcumin toxicity against (PC3) tumor and microbial by using magnetic field in vitro. Artificial Cells Nanomed Biotechnol. 2017;45(4):843–53. [DOI] [PubMed] [Google Scholar]
- 30.Dash TK, Konkimalla VSB. Selection and optimization of nano-formulation of P-glycoprotein inhibitor for reversal of doxorubicin resistance in COLO205 cells. J Pharm Pharmacol. 2017;69(7):834–43. [DOI] [PubMed] [Google Scholar]
- 31.Mahjoub MA, Bakhshinejad B, Sadeghizadeh M, Babashah S. Combination treatment with dendrosomal nanocurcumin and doxorubicin improves anti-cancer effects on breast cancer cells through modulating CXCR4/NF-kappa B/Smo regulatory network. Mol Biol Rep. 2017;44(4):341–51. [DOI] [PubMed] [Google Scholar]
- 32.Mishra D, Iyyanki TS, Hubenak JR, Zhang Q, Mathur AB. Silk fibroin nanoparticles and cancer therapy. nanotechnology in cancer. Elsevier: Amsterdam; 2017. [Google Scholar]
- 33.Athira GK, Jyothi AN, Vishnu VR. Water soluble octenyl succinylated cassava starch-curcumin nano-formulation with enhanced bioavailability and anti-cancer potential. Starch-Starke. 2018;70:7–8. [Google Scholar]
- 34.Bagheri R, Sanaat Z, Zarghami N. Synergistic effect of free and nano-encapsulated chrysin-curcumin on inhibition of hTERT gene expression in sw480 colorectal cancer cell line. Drug Res (Stuttg). 2018;68(6):335–43. [DOI] [PubMed] [Google Scholar]
- 35.Baghi N, Bakhshinejad B, Keshavarz R, Babashah S, Sadeghizadeh M. Dendrosomal nanocurcumin and exogenous p53 can act synergistically to elicit anti-cancer effects on breast cancer cells. Gene. 2018;670:55–62. [DOI] [PubMed] [Google Scholar]
- 36.Hashemzehi M, Behnam-Rassouli R, Hassanian SM, Moradi-Binabaj M, Moradi-Marjaneh R, Rahmani F, et al. Phytosomal-curcumin antagonizes cell growth and migration, induced by thrombin through AMP-Kinase in breast cancer. J Cell Biochem. 2018;119(7):5996–6007. [DOI] [PubMed] [Google Scholar]
- 37.Hosseini S, Chamani J, Rahimi H, Azmoodeh N, Ghasemi F, Abadi PH. An in vitro study on curcumin delivery by nano-micelles for esophageal squamous cell carcinoma (KYSE-30). Rep Biochem Mol Biol. 2018;6(2):137–43. [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen NT, Nguyen NNT, Tran NTN, Le PN, Nguyen TBT, Nguyen NH, et al. Synergic activity against MCF-7 breast cancer cell growth of nanocurcumin-encapsulated and cisplatin-complexed nanogels. Molecules. 2018;23:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shariati M, Hajigholami S, Veisi Malekshahi Z, Entezari M, Bodaghabadi N, Sadeghizadeh M. Nanocurcumin-mediated down-regulation of telomerase via stimulating TGFβ1 signaling pathway in hepatocellular carcinoma cells. Iran Biomed J. 2018;22(3):171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srivastava S, Mohammad S, Gupta S, Mahdi AA, Dixit RK, Singh V, et al. Chemoprotective effect of nanocurcumin on 5-fluorouracil-induced-toxicity toward oral cancer treatment. Natl J Maxillofac Surg. 2018;9(2):160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dang LH, Vu MT, Chen J, Nguyen CK, Bach LG, Tran NQ, et al. Effect of ultrasonication on self-assembled nanostructures formed by amphiphilic positive-charged co-polymers and negative-charged drug. ACS Omega. 2019;4(3):4540–52. [Google Scholar]
- 42.Harini L, Srivastava S, Gnanakumar GP, Karthikeyan B, Ross C, Krishnakumar V, et al. An ingenious non-spherical mesoporous silica nanoparticle cargo with curcumin induces mitochondria-mediated apoptosis in breast cancer (MCF-7) cells. Oncotarget. 2019;10(11):1193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosseini S, Chamani J, Hadipanah MR, Ebadpour N, Hojjati AS, Mohammadzadeh MH, et al. Nano-curcumin’s suppression of breast cancer cells (MCF7) through the inhibition of cyclinD1 expression. Breast Cancer-Targets Ther. 2019;11:137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seyed Hosseini E, Alizadeh Zarei M, Babashah S, Nakhaei Sistani R, Sadeghizadeh M, Haddad Kashani H, et al. Studies on combination of oxaliplatin and dendrosomal nanocurcumin on proliferation, apoptosis induction, and long non-coding RNA expression in ovarian cancer cells. Cell Biol Toxicol. 2019;35(3):247–66. [DOI] [PubMed] [Google Scholar]
- 45.Yang R, Fang XL, Zhen Q, Chen QY, Feng C. Mitochondrial targeting nano-curcumin for attenuation on PKM2 and FASN. Colloids Surf B Biointerfaces. 2019;182: 110405. [DOI] [PubMed] [Google Scholar]
- 46.Cheng T, Zhang Z, Shen H, Jian Z, Li J, Chen Y, et al. Topically applicated curcumin/gelatin-blended nanofibrous mat inhibits pancreatic adenocarcinoma by increasing ROS production and endoplasmic reticulum stress mediated apoptosis. J Nanobiotechnology. 2020;18(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanna DH, Saad GR. Nanocurcumin: preparation, characterization and cytotoxic effects towards human laryngeal cancer cells. RSC Adv. 2020;10(35):20724–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuo IM, Lee JJ, Wang YS, Chiang HC, Huang CC, Hsieh PJ, et al. Potential enhancement of host immunity and anti-tumor efficacy of nanoscale curcumin and resveratrol in colorectal cancers by modulated electro- hyperthermia. BMC Cancer. 2020;20(1):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandit AH, Mazumdar N, Imtiyaz K, Rizvi MMA, Ahmad S. Self-healing and injectable hydrogels for anti-cancer drug delivery: a study with multialdehyde gum arabic and succinic anhydride chitosan. ACS Appl Bio Mater. 2020;3(12):8460–70. [DOI] [PubMed] [Google Scholar]
- 50.Ghaderi S, Babaei E, Hussen BM, Mahdavi M, Azeez HJ. Gemini curcumin suppresses proliferation of ovarian cancer OVCAR-3 Cells via induction of apoptosis. Anti-cancer Agents Med Chem. 2021;21(6):775–81. [DOI] [PubMed] [Google Scholar]
- 51.Sadoughi A, Irani S, Bagheri-Khoulenjani S, Atyabi SM, Olov N. Cold atmospheric plasma modification of curcumin loaded in tri-phosphate chitosan nanoparticles enhanced breast cancer cells apoptosis. Polym Adv Technol. 2021;32(1):31–40. [Google Scholar]
- 52.Wozniak M, Nowak M, Lazebna A, Wiecek K, Jablonska I, Szpadel K, et al. The Comparison of in vitro photosensitizing efficacy of curcumin-loaded liposomes following photodynamic therapy on melanoma MUG-Mel2, squamous cell carcinoma scc-25, and normal keratinocyte HaCaT cells. Pharmaceuticals. 2021;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alam J, Dilnawaz F, Sahoo SK, Singh DV, Mukhopadhyay AK, Hussain T, et al. Curcumin encapsulated into biocompatible co-polymer PLGA nanoparticle enhanced anti-gastric cancer and anti-helicobacter pylori effect. Asian Pac J Cancer Prev. 2022;23(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atia MM, Abdel-Tawab HS, Mostafa AM, Mobarak SA. Nanocurcumin and curcumin prevent N, N’-methylenebisacrylamide-induced liver damage and promotion of hepatic cancer cell growth. Sci Rep. 2022;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Essawy MM, Mohamed MM, Raslan HS, Rafik ST, Awaad AK, Ramadan OR. The theranostic potentialities of bioavailable nanocurcumin in oral cancer management. BMC Complement Med Therapies. 2022;22:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohammadi H, Shokrzadeh M, Akbari-Dafsari O, Amani N, Modanlou M, Mohammadpour A, et al. Evaluation of curcumin nano-micelle on proliferation and apoptosis of HT29 and Hct116 colon cancer cell lines. Middle East J Cancer. 2022;13(1):99–109. [Google Scholar]
- 57.Mukherjee D, Dash P, Ramadass B, Mangaraj M. Nanocurcumin in oral squamous cancer cells and its efficacy as a chemo-adjuvant. Cureus J Med Sci. 2022;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sadeghi RV, Parsania M, Sadeghizadeh M, Haghighat S. Investigation of Curcumin-Loaded OA400 Nanoparticle’s Effect on the Expression of E6 and E7 Human Papilloma-Virus Oncogenes and P53 and Rb Factors in HeLa Cell Line. Ir J Pharmaceut Res. 2022;21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krishnaveni P, Thangapandiyan M, Raja P, Rao GVS. Pathological and molecular studies on anti-tumor effect of curcumin and curcumin solid lipid nanoparticles. Pak Vet J. 2023;43(2):315–20. [Google Scholar]
- 60.Moawad M, Nasr GM, Osman AS, Shaker ES. Curcumin nanocapsules effect in apoptotic processes, gene expression, and cell cycle on Hep-G2 cell lines. Int J Immunopathol Pharmacol. 2023;37:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Purbadi S. Effect of nanocurcumin in combination with methotrexate on telomerase activity, NF-kb expression, and proliferation index of bewo choriocarcinoma cells. Indones J Obstet Gynecol. 2023;11(2):105–11. [Google Scholar]
- 62.Seyed Hosseini E, Alizadeh Zarei M, Tarrahimofrad H, Zamani J, Haddad Kashani H, Ahmad E, et al. Synergistic effects of dendrosomal nanocurcumin and oxaliplatin on oncogenic properties of ovarian cancer cell lines by down-expression of MMPs. Biol Res. 2023;56:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viraaj V, Khodabux RJ, Indhuja J, Sudharsan S, Thamizhchelvan H, Deepa PV. Comparing the efficacy of nanocurcumin and its bulk counterpart to understand the permeability-an in vitro approach. J Integrat Sci Technol. 2023;11:3. [Google Scholar]
- 64.Alizadeh AM, Khaniki M, Azizian S, Mohaghgheghi MA, Sadeghizadeh M, Najafi F. Chemoprevention of azoxymethane-initiated colon cancer in rat by using a novel polymeric nanocarrier-curcumin. Eur J Pharmacol. 2012;689(1–3):226–32. [DOI] [PubMed] [Google Scholar]
- 65.Chang CZ, Wu SC, Lin CL, Kwan AL. Curcumin, encapsulated in nano-sized PLGA, down-regulates nuclear factor κb (p65) and subarachnoid hemorrhage induced early brain injury in a rat model. Brain Res. 2015;1608:215–24. [DOI] [PubMed] [Google Scholar]
- 66.El-Azab NEE, Salem MY, Abd E-S. A histological and immunohistochemical study of different therapeutic modalities for experimentally induced ulcerative colitis in rats. Egyptian J Histol. 2016;39(1):12–24. [Google Scholar]
- 67.Mukerjee A, Ranjan AP, Vishwanatha JK. Targeted nanocurcumin therapy using annexin A2 anitbody improves tumor accumulation and therapeutic efficacy against highly metastatic breast cancer. J Biomed Nanotechnol. 2016;12(7):1374–92. [DOI] [PubMed] [Google Scholar]
- 68.Arozal W, Ramadanty WT, Louisa M, Satyana RPU, Hartono G, Fatrin S, et al. Pharmacokinetic profile of curcumin and nanocurcumin in plasma, ovary, and other tissues. Drug Res. 2019;69(10):559–64. [DOI] [PubMed] [Google Scholar]
- 69.Vijayakurup V, Thulasidasan AT, Shankar GM, Retnakumari AP, Nandan CD, Somaraj J, et al. Chitosan encapsulation enhances the bioavailability and tissue retention of curcumin and improves its efficacy in preventing B a P-induced lung carcinogenesis. Cancer Prev Res. 2019;12(4):225–36. [DOI] [PubMed] [Google Scholar]
- 70.Abuelezz NZ, Shabana ME, Abdel-Mageed HM, Rashed L, Morcos GNB. Nanocurcumin alleviates insulin resistance and pancreatic deficits in polycystic ovary syndrome rats: Insights on PI3K/AkT/mTOR and TNF-α modulations. Life Sci. 2020;256: 118003. [DOI] [PubMed] [Google Scholar]
- 71.Wang GN, Sukumar S. Characteristics and anti-tumor activity of polysorbate 80 curcumin micelles preparation by cloud point cooling. J Drug Delivery Sci Technol. 2020. 10.1016/j.jddst.2020.101871. [Google Scholar]
- 72.Kayyal M, Bolhassani A, Noormohammadi Z, Sadeghizadeh M. Immunological responses and anti-tumor effects of HPV16/18 L1–L2-E7 multiepitope fusion construct along with curcumin and nanocurcumin in C57BL/6 mouse model. Life Sci. 2021. 10.1016/j.lfs.2021.119945. [DOI] [PubMed] [Google Scholar]
- 73.Lakshmanan A, Akasov RA, Sholina NV, Demina PA, Generalova AN, Gangadharan A, et al. Nanocurcumin-loaded ucnps for cancer theranostics: physicochemical properties, in vitro toxicity, and in vivo imaging studies. Nanomaterials. 2021;11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sandhiutami NMD, Arozal W, Louisa M, Rahmat D, Wuyung PE. Curcumin nanoparticle enhances the anticancer effect of cisplatin by inhibiting PI3K/AKT and JAK/STAT3 pathway in rat ovarian carcinoma induced by DMBA. Front Pharmacol. 2021. 10.3389/fphar.2020.603235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Louisa M, Wanafri E, Arozal W, Sandhiutami NMD, Basalamah AM. Nanocurcumin preserves kidney function and haematology parameters in DMBA-induced ovarian cancer treated with cisplatin via its antioxidative and anti-inflammatory effect in rats. Pharm Biol. 2023;61(1):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdelgawad LM, Abdelaziz AA, El-Begawey MB, Saafan AM. Influence of nanocurcumin and photodynamic therapy using nanocurcumin in treatment of rat tongue oral squamous cell carcinoma through histological examination and gene expression of BCL2 and Caspase-3. Reports Biochem Mol Biol. 2023;11(4):730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saadipoor A, Razzaghdoust A, Simforoosh N, Mahdavi A, Bakhshandeh M, Moghadam M, et al. Randomized, double-blind, placebo-controlled phase II trial of nanocurcumin in prostate cancer patients undergoing radiotherapy. Phytother Res. 2019;33(2):370–8. [DOI] [PubMed] [Google Scholar]
- 78.Sandoughdaran S, Razzaghdoust A, Tabibi A, Basiri A, Simforoosh N, Mofid B. Randomized, double-blind pilot study of nanocurcumin in bladder cancer patients receiving induction chemotherapy. Urol J. 2020;18(3):295–300. [DOI] [PubMed] [Google Scholar]
- 79.Louisa M, Putera AM, Sidqi AA, Mahaputra DK, Firmansyah ER, Ammar MF, et al. Attenuation of cisplatin-induced hepatotoxicity by nanocurcumin through modulation of antioxidative and anti-inflammatory pathways. J Appl Pharmaceut Sci. 2023;13(3):060–70. [Google Scholar]
- 80.Kandjani BZ, Hesari FS, Babaei E. Gemini curcumin inhibits 4T1 cancer cell proliferation and modulates the expression of apoptotic and metastatic genes in Balb/c mice model. Pathology-Res Practice. 2023;243: 154344. [DOI] [PubMed] [Google Scholar]
- 81.Tinkle S, McNeil SE, Mühlebach S, Bawa R, Borchard G, Barenholz YC, et al. Nanomedicines: addressing the scientific and regulatory gap. Ann N Y Acad Sci. 2014;1313:35–56. [DOI] [PubMed] [Google Scholar]
- 82.Reflect Scientific. FDA Guidance on Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation. 2018.
- 83.Sainz V, Conniot J, Matos AI, Peres C, Zupančič E, Moura L, et al. Regulatory aspects on nanomedicines. Biochem Biophys Res Commun. 2015;468(3):504–10. [DOI] [PubMed] [Google Scholar]
- 84.Hafner A, Lovrić J, Lakoš GP, Pepić I. Nanotherapeutics in the EU: an overview on current state and future directions. Int J Nanomedicine. 2014;9:1005–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yadav D, Suri S, Choudhary AA, Sikender M, Hemant BMN, et al. Novel approach: Herbal remedies and natural products in pharmaceutical science as nano drug delivery systems. Int J Pharm Technol. 2011;3:3092–116. [Google Scholar]
- 86.Nouri Z, Fakhri S, Nouri K, Wallace CE, Farzaei MH, Bishayee A. Targeting multiple signaling pathways in cancer: The rutin therapeutic approach. Cancers (Basel). 2020;12(8):2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghalandarlaki N, Alizadeh AM, Ashkani-Esfahani S. Nanotechnology-applied curcumin for different diseases therapy. Biomed Res Int. 2014;2014: 394264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.