Abstract
Multilocular cystic nephroma (MLCN) is an unusual, benign slow-growing renal cystic neoplasm which mimics other cystic renal lesions and has such clinical, radiological, and morphological features that causes diagnostic dilemma. MLCN lies in the spectrum of mixed epithelial and stromal tumor (MEST) family of kidney. According to World Health Organization (WHO 2016 classification), MEST encompasses spectrum of tumors ranging from predominantly cystic tumors, adult cystic nephroma (ACN) to tumors that are variably solid (MEST), thus creating diagnostic dilemma. Moreover, it has several benign and malignant differentials due to its several overlapping histomorphological features which when not cautiously dealt with may result in misdiagnosing it as malignant lesion. We hereby present a case of a woman in late twenties who presented with left flank swelling and pain since 6 months which was misdiagnosed as renal cell carcinoma on radiology which turned out to be ACN on histology and further verified on immunohistochemistry.
Keywords: Multilocular cystic nephroma, Mixed epithelial and stromal tumor histomorphological, Immunohistochemistry, Adult
Introduction
Multilocular cystic nephroma (MLCN)/cystic nephroma (CN) has historically been problematic in the field of renal neoplastic diseases. It is a rare, non-hereditary, benign cystic neoplasm of the kidney, first described by Edmunds in the year 1893 and termed a cystic adenoma. About 200 cases have been reported so far [1]. CN has two distinct lesions due to a bimodal peak of distribution, most commonly seen in the first 2 years of life with male preponderance, termed pediatric cystic nephroma, considered to be a part of the spectrum of cystic lesions and second above 30 years of age with female predominance, called adult cystic nephroma (ACN) (suggesting an association with circulatory hormones) and is considered to be a highly cystic end of the spectrum of mixed epithelial and stromal tumors (MEST). ACN is considered a separate entity, classified under soft tissue tumors of the kidney [2]. World Health Organization (WHO) 2016 classification encompasses this spectrum of tumors ranging from predominantly cystic tumors, ACN to variably solid and cystic MEST [3]. We hereby, present a case report, of a female in her late 20 s with ACN verified on immunohistochemistry (IHC).
Case report
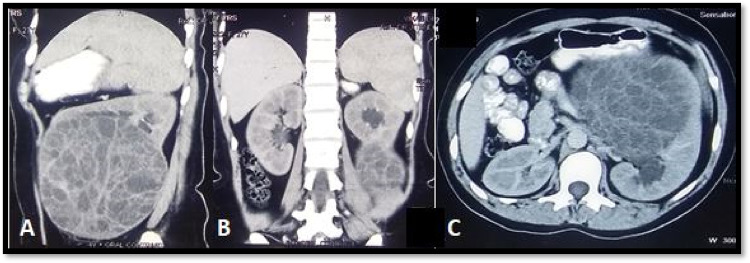
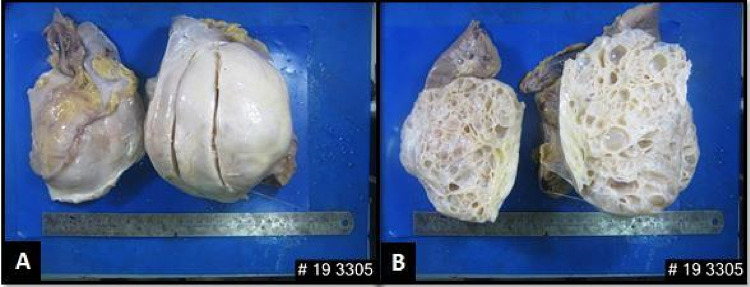
A female in her late 20 s presented in the urology department with left flank mass and pain for 6 months. She was afebrile, with stable vitals. On examination, per abdomen was soft, and tender, and a palpable, firm, non-tender, ballotable lump was identified moving with respiration. Her creatinine levels were within normal limits (0.7 mg/dl) and her estimated glomerular filtration rate was 120 ml/min/1.73/m2. On contrast-enhanced computed tomography (CECT) abdomen, a large, left renal diffusely cystic mass measuring 13 × 13 × 4.5 cm with multiple thickened, faint contrast-enhancing internal septations, and loculations and areas of linear calcification was identified, radiologically consistent with Bosniak IV type cyst probability of cystic renal cell carcinoma was considered (Fig. 1A–C). No retroperitoneal lymph node or ascites were reported. The patient was admitted for open radical nephrectomy. Intra-operatively omentum and mesocolon was stretched over the surface of the cystic tumor mass arising from the lower and mid pole of left kidney, displacing kidney superolaterally. Mass was limited to the gerota’s space. Single renal artery and single renal vein with free adrenals were noted. Single ureter was seen with two firm lymph nodes, largest 2 × 1 cm in size along left para-aortic border inferior to the renal hilum. Kidney was opened in the coronal plane and entire mid and lower pole along with the pelvicalyceal system was replaced by a huge cystic mass lesion with preserved upper pole parenchyma. Left open radical nephrectomy was performed and specimen was sent to the department of pathology for histopathological examination (HPE). Grossly, the entire mid pole and lower pole with the pelvicalyceal system were replaced by a 20 × 20 cm multicystic mass, with a honeycomb appearance of the cut surface. The cysts had thick shaggy septations and were filled with an admixture of clear fluid with gelatinous material. No definite solid mass was identified on gross inspection (Fig. 2A, B). Margins were free from tumor infiltration grossly. Two enlarged hilar lymph nodes were identified.
Fig. 1.
A On contrast-enhanced computed tomography (CECT) abdomen, (sagittal section) a large, left renal mass ms. 13 × 13 × 4.5 cm, predominantly cystic, displaying diffusely distributed, multiple thickened, internal septations, and loculations with calcification anteriorly consistent with Bosniak IV type cyst and radiological impression of cystic renal cell carcinoma was made. B CECT abdomen (coronal section) showing left cystic tumor at the lower pole of kidney with a residual normal kidney at upper pole. C CECT abdomen (cross section) showing left kidney multilocular cystic tumor
Fig. 2.
A Left nephrectomy specimen measuring 20 × 15 × 13 with smooth, shiny and bosselated outer surface. B Cut surface shows multiple variable-sized cysts filled with serous fluid ranging from 0.5 to 4 cm. Residual kidney at upper pole shows maintained corticomedullary junction. No solid component was identified. A part of residual kidney was seen at the upper pole
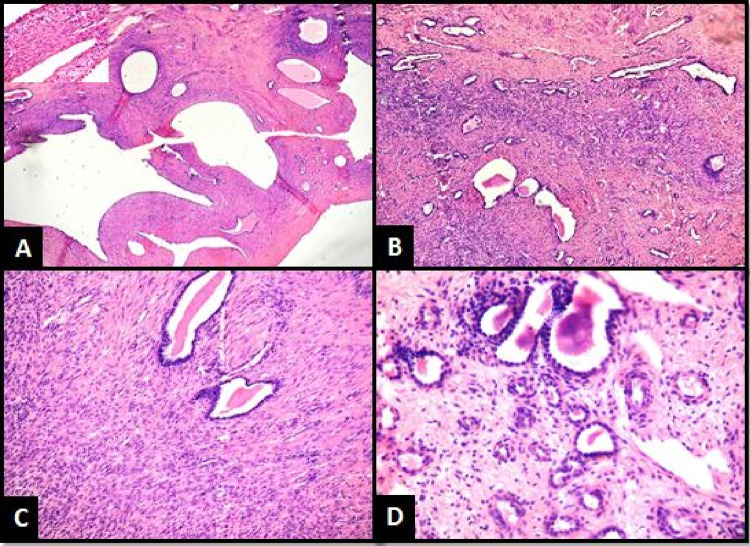
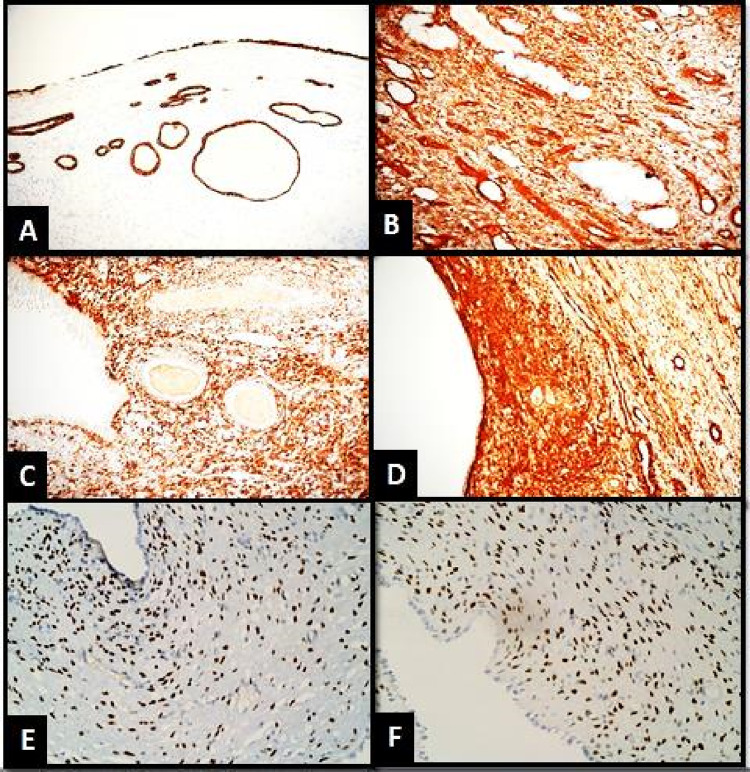
HPE showed a well-defined cystic tumor displaying biphasic components with epithelial elements in the form of tubules and multiple variable-sized cystic spaces lined by flattened to cuboidal epithelium displaying clearing and hobnail appearance. Mesenchymal elements were identified in the form of fibroblastic ovarian stroma (Fig. 3A–D). Various histological differentials of cystic renal tumors in an adult are considered. Lymph nodes showed reactive lymphoid hyperplasia. On IHC, tumor cells displayed cytokeratin (CK) positivity in the epithelial lining and estrogen receptor (ER), progesterone (PR), smooth muscle actin (SMA), desmin, vimentin positivity, and calretinin negativity in the stromal cells (Fig. 4A–F).
Fig. 3.
A Multicystic neoplasm, showing thick intervening septations with inset showing hobnail lining epithelium (×100, H&E). B Entrapped tubules within stroma (×100, H&E). C Ovarian like stroma with cystic tubules (×200, H&E). D Cystic tubules and glands (×400, H&E)
Fig. 4.
A CK positivity in the lining epithelium of the cyst glands and tubules (×100, IHC). B Vimentin positivity in the stroma (×200, IHC). C Desmin positivity in the stroma (×200, IHC). D SMA positivity in the stroma (×200, IHC). E ER positivity in the stromal cells (×400, IHC). F PR positivity in the stromal cells (×400, IHC)
Discussion
Michal and Syrucek first described MEST family tumors in 1998 and included them in the WHO 2002 classification subsequently [4]. ACN and MEST were considered distinct entities in the WHO classification (2004) of renal neoplasms [5]. Recently various studies have proposed several overlapping clinic–pathological features including age and sex distribution, molecular, IHC profile, and histological features which symbolize similar pathogenesis with varied morphology [6]. In 2016, WHO proposed a spectrum of tumors ranging from predominantly cystic tumors (ACN) to tumors that are variably solid (MESTs).
ACN is a rare, benign, cystic slow-growing, multilocular renal tumor, described by various terminologies including MLCN, cystadenoma, solitary multilocular cyst, benign multilocular cyst, benign cystic nephroma (CN), cystic hamartoma, multilocular renal cyst, multilocular CN, and multi-CN [7]. Since its etiopathogenesis remains unknown, multiple theories have been put forward that wherever developmental defects become predominant, tumors develop from ureteric buds [7]. Since 1892, more than 200 cases have been reported in literature around the globe [8]. Due to its overlapping features, it portends a diagnostic dilemma in the diagnosis.
Mostly MLCN cases are asymptomatic and are detected incidentally during radiological examinations for some other ailments. Symptomatic adult patients present with flank pain, gross hematuria, abdominal mass, and urinary tract infection. They tend to be unilateral, though very rarely bilateral cases have also been reported. Most patients have silent lesions and remain asymptomatic unless incidentally diagnosed on imaging for another cause.
Imaging studies, ultrasonography (USG), and CT may aid in the differential diagnosis of MLCN. CN appears as a well-circumscribed multilocular cystic mass with multiple variably thickened septae, and solid components showing contrast enhancement, with serous fluid inside the cyst. Sometimes, obstructive symptoms like hydronephrosis and hemorrhage may be seen due to the extension of the neoplasm into the renal pelvis. Usually, Bosniak classification for CT is utilized to determine the risk of malignancy in which definitive discrimination of type 2 and 3 cysts is very difficult. Usually Category III and above are assigned to MLCN, and the malignant potential is greater than 54% [9]. A preoperative diagnosis based on imaging studies is difficult, and several differentials are considered including multicystic renal cell carcinoma (RCC); hence, total or partial nephrectomy is the only feasible and definitive method depending upon the tumor size, for reaching a particular diagnosis.
Histology of cystic nephroma was described by Edmunds in 1892 followed by Powel et al. who described two cystic nephroma cases and collated with the previous thirteen cases reported in literature [10]. Thereafter, Joshi and Beckwith established the histological criteria of MLCN in 1989 [11] and hence, redefined the diagnostic criteria which included the presence of a well-circumscribed firmly encapsulated mass with thick fibrous pseudo capsule, markedly distinct from renal parenchyma. It comprises a predominantly cystic component without any solid component; multilocular, solitary, unilateral cysts, with intervening thick and thin septae; a definite cystic lining epithelium exhibiting flattened, cuboidal or ‘hobnail’ appearing cells; septae formed by well-differentiated renal tubular or fibrous tissue. No nephron in the interlobular septa, and normal residual renal tissue. No communication between the cystic lesion and the renal pelvis is identified. A list of differentials of cystic renal neoplasms in the adult age group should be kept in mind while diagnosing such kidney lesions (Table 1) [12]. Our case meets the proposed criteria. Stromal content present alters between dense paucicellular collagen to distinct cellular spindle cell bundles mimicking ovarian stroma [13].
Table 1.
Benign and Malignant differentials of Cystic Nephroma in adult
| S. no. | Differential diagnosis | Age | Gross Findings | Microscopy | IHC |
|---|---|---|---|---|---|
| 1 | Adult cystic nephroma (ACN) |
> 30 years Females predominate (8:1) |
Completely cystic with no solid component Cyst contains hemorrhage or clear fluid Cysts range from microscopic to > 5 cm Septa are thin (< 5 mm), translucent and uniform |
Multilocular with absence of communication between the cyst and the renal tissue, filled with clear fluid and no communication between the locules Cysts are lined by flattened, cuboidal or hobnail epithelium with lining cells displaying pale, clear cytoplasm Stromal content ranges from dense pauci-cellular collagen to distinct cellular bundles of spindle cells very closely resembling ovarian stroma |
Epithelial lining cysts are positive for CK Septal stromal cells stain mostly with CD10, calretinin, inhibin, estrogen, and progesteron receptors, Positive reaction for SMA is common |
| 2 | Mixed epithelial and stromal tumor (MEST) |
Broad age range including pre-pubertal children Female predominance (4–5:1) |
Variable solid and cystic areas, with typically predominant solid areas and some cysts Varies from well circumscribed to infiltrative Grows into lumen of renal pelvis and even into ureter as a polypoidal mass Development of domed nodules protruding into lumen of large cysts or renal pelvis |
Variable admixture of epithelial and stromal elements Epithelial component is heterogenous ranging from simple ducts to complex branched glandular formations to florid complex papillary structures Epithelial cells range from flattened to cuboidal to columnar cells with clear to eosinophilic cytoplasm. Ciliated and mucin secreting cells are also seen Mesenchymal component ranges from hypocellular, fibrotic to more cellular fibroblastic and myofibroblastic foci to more cellular spindle cell stroma |
Stromal cells express ER, PR Epithelial cells show PAX2 and PAX8 positivity |
| 3 | Adult polycystic kidney disease | 30–40 years of age |
Markedly enlarged kidneys with bosselated surface (up to 8 kg) composed of sub-capsular cysts up to 4 cm Cysts contain clear to brown fluid Most commonly bilateral |
Saccular expansions or diverticula of all portions of renal tubule and glomerular capsule that later become disconnected and filled with fluid Cysts are lined by cuboidal or flattened epithelium, may have papillary projections or polyps Functional nephrons exist between cysts with areas of global sclerosis, tubular atrophy, interstitial fibrosis and chronic inflammation Infants may show primarily cystic dilatation of Bowman's space |
NAD |
| 4 | Medullary sponge kidney | 30–40 years |
Normal sized kidneys with multiple, small cysts in medullary pyramids and papillae, giving medulla a sponge-like appearance Most often bilateral |
Medullary cysts lined by cuboidal epithelium or urothelium May have concretions adherent to cyst wall Often severe inflammation and scarring in interstitium, often with tubular atrophy near papillary tips |
NAD |
| Malignant lesions | |||||
| 1 | Tubulo-cystic Renal carcinoma |
30–94 years Male predominance (7:1) |
Well circumscribed invested by pseudocapsule Size range from 0.5 to 17 cm. Cut surface is gray white to spongy |
Cystic spaces and tubules lie within bland connective tissue stroma which varies in amount. Cysts are lined by flat cuboidal and sometimes hobnail-type cells with eosinophilic cytoplasm. Marked nuclear pleomorphism is evident with nucleolar prominence in the range of Fuhrman Grade 2 or 3. Septal structures do not harbor clusters of clear cells | Epithelial lining shows positivity for CK 8, 18, 19, CD10, AMACR. CK 7 is usually focal |
| 2 | Multicystic renal cell carcinoma (MCRC) |
40–60 years of age Male > Females |
Usually unilateral and < 5 cm Well circumscribed with fibrous pseudo-capsule Variably sized cysts with thin septa Cysts filled with clear, serous, gelatinous or hemorrhagic fluid No mural solid nodules or areas of necrosis seen |
CN, there are focally distributed clear cells in the surface of the septa, hobnail epithelium, ovarian-like stroma, and mature tubules in the septa, whereas evident solid areas in cystic mass or extensile nodules of clear cells favor MCRCC | cysts lined epithelial cells and the clear tumor cell clusters were positive for epithelium markers like CKpan (19/19), EMA (16/19) and CK7 (15/19), CA-IX (17/19) and PAX8 (15/19), and a low percentage staining for CD10 (7/19) |
| 3 | Multilocular cystic renal neoplasm of low malignant potential | Adult age group |
Usually unilateral and < 5 cm (mean size: 4 cm) Well circumscribed with fibrous pseudocapsule Variably sized cysts with thin septa Cysts filled with clear, serous, gelatinous or hemorrhagic fluid No mural solid nodules or areas of necrosis |
Exclusively cystic, multiloculated renal tumor Devoid of any expansile solid growth Clear cells lining with low grade nuclei Thin, fibrous septa lined by clear cells. Lining of cysts may show focal multilayering, cells with granular cytoplasm and small intracystic papillations Septa may contain calcification or ossification Presence of necrosis, frequent or atypical mitoses |
CAIX, EMA, CAM5.2PAX8, PAX2, CD10, RCC CK7 and vimentin variable AMACR in 80% |
We came across a literature review by Granja MF et al. 2015 where they utilized electronic databases and reviewed ninety three articles if they contained approximately 50% or more of the demographic information [14]. Overall 179 unique cases were analyzed where majority of the cases were from US followed by India, UK, Spain, and others. Out of 179, 105 cases were above 11 years of age and rest 74 was 10 years of age or below. MLCN was more common in females (84/105, 80%) in patients 11 years old or older. The most common presentation was abdominal mass, followed by incidental imaging finding, abdominal pain, and hematuria. Most common presentation in males above 11 years of age was abdominal pain whereas it was incidental imaging finding in females. Of 151 reports, the median MLCN size reported was 90 mm with right sided presentation in 83 cases and left sided in 80 while rest were bilateral. The most common surgical intervention carried out was total nephrectomy followed by partial sparing nephrectomy. We further came across nine adult cystic nephroma cases and a case series of six, accounting for a total of fifteen cases with a brief overview enlisted in Table 2 [9, 15–23]. Age ranged from 20 to 66 years of age with a mean age of 45.8 years.
Table 2.
List of adult cystic nephroma cases reported in literature
| S. no. | Author | Age (y/gender) | History | Size | CT finding/USG | Surgical procedure | IHC performed |
|---|---|---|---|---|---|---|---|
| 1 | Cavıldak et al. [15] | 48 y/F | Lumbar disc hernia | 4.6 × 3.8 × 4.5 cm | Well defined, lobulated, encapsulated cystic lesion with multiple thin septa in lower pole of left kidney | Open partial nephrectomy | NA |
| 2 | Sharma et al. [16] | 25 y/F | intermittent dull aching left-sided flank pain of 7 days' duration | 6 cm × 5 cm | Large well defined lesion of the size 9.9 cm × 8.2 cm × 8.1 cm arising from left lower pole of kidney | Open left partial nephrectomy | NA |
| 3 | Ajit et al. [17] | 28 y/F | Progressively increasing right abdomen lump with flank pain and haematuria for two years | 31 × 19 × 19.6 cm | Multicystic enhancing tumour of 31 × 19 × 19.6 cm size arising from right kidney, was crossing the midline and was pushing the duodenum, head of pancreas and IVC to the left | Radical nephrectomy | Positive for ER, PR, SMA, WT1 and CK; negative for HMB-45 and S-100 |
| 4 | Dell et al. [18] | 66 y/M |
Dysuria, frequency and recent episode of UTI H/O of HT, DM II and underwent appendectomy 50 years ago |
4.2 × 2.6 cm |
Unilocular, well-circumscribed cyst arising upper pole the left kidney Numerous calcifications on the walls and variably thin and thick septa. No solid components seen CT scan showed a poorly enhancing cystic lesion protruding into sinus |
Laparoscopic Nephrectomy |
Lining epithelial cells keratin and EMA +ve F VIII, CD34, and vimentin −ve |
| 5 | Dong et al. [19] | 30 y/M | Intermittent right-flank pain and gross hematuria since 2 years | 5 × 4.5 × 3.5-cm nephrectomy specimen with a 4.5 × 3 × 2-cm tumoral mass |
Well-circumscribed, polycystic and heterogeneous mass with areas of calcifications arising from the upper pole of the right kidney No solid components evident |
Laparoscopic Nephron sparing surgery | NA |
| 6 | Ozturk et al. [20] | 59 y/F | Left flank pain and abdominal pain | 22 × 10.9 × 8.2 cm | 24.5 × 11.9 × 9.8 cm multilocular cystic renal mass in the left kidney with hypointense appearance in T1-weighted images and hyperintense in T2-weighted images, and a multicystic appearance in ureter projection | Transperitoneal radical nephroureterectomy | NA |
| 7 | Qiu et al. [21] | 20 y/F | Left renal cyst for 1 year with pain in her left flank one month ago | – | – | Laparoscopic Partial Nephrectomy | NA |
| 8 | Abrol et al. [22] | 27 y/F | Left loin pain and fever | Size not mentioned |
Well-defined fluid attenuating round lesion with multiple daughter cysts in segments five and eight of the liver A similar-looking multi-septated cystic mass was seen in the upper pole of the left kidney There was no solid component or calcification |
Partial Nephrectomy with surgery of liver cyst Diagnosis of hydatid cyst of the liver and left kidney was made |
NA |
| 9 | Mohanty et al. [23] |
45 y/F 25 y/F |
Intermittent, non-colicky pain in right hypochondrium and lumbar region of 12-months duration Persistent dull aching pain in the right lumbar region for six months with no urinary complaints |
6.6 × 6.5 × 4.4 cm 6.5 × 6.0 × 4.2 cm |
Cystic mass with fine internal septations and delayed enhancement of the wall, involving the mid-pole of the right kidney compressing the pelvis and upper ureter Non-enhancing multi-locular cystic mass involving upper pole of the right kidney extending into the middle part, with no solid component. No vascular invasion, ascites or lymphadenopathy was seen |
Right nephrectomy and cholecystectomy was performed Radical nephrectomy |
NA NA |
| 10 |
Wilkinsen et al. [9] Case series of 6 |
35/M 39/F 60/F 62/F 53/F 65/F |
Loin pain with hematuria and Recurrent urinary tract infection | Avg size 8.1 cm | Laparoscopic radical nephrectomy with case 2 underwent Open nephrectomy | ||
| 11 | Index case | 29/F | Left Flank mass and pain | 13 × 13 × 4.5 cm | Predominantly cystic, with diffusely distributed, multiple thickened, faint contrast-enhancing internal septations, and loculations with areas of linear calcification anteriorly was identified consistent with Bosnaik IV type cyst and radiological impression of cystic renal cell carcinoma | Open radical nephrectomy | CK +ve in the cyst lining epithelium ER, PR, SMA, desmin, vimentin +ve in the stromal cells and calretinin negative |
NA not available, UTI urinary tract infection, H/O history of, HT hypertension, DM diabetes mellitus
IHC has not been well studied in establishing its diagnosis; however, it may provide some useful information. Studies reveal that stromal contents express mostly CD10, calretinin, inhibin, estrogen, and progesterone receptors, while the epithelial component has an increased affinity toward CK [16]. Doros et al., 2013 conducted genetic studies and suggested that DICER1 mutations play an important role in the development of CN [24].
Nephrectomy remains the standard of care or conventional treatment modality, depending upon the tumor size whether total or partial; however, if a mass is smaller than 4 cm, unilateral, solitary, and localized, or when MLCN is considered preoperatively, nephron-sparing surgery should be preferred in combination with an intraoperative frozen section for verification [25]. Renal function is also preserved by partial nephrectomy, either conventional or laparoscopic surgery. In the index case, the tumor involved the mid and lower pole with a residual kidney at the upper pole. Mass was very large and located centrally near the renal hilum, and not suitable for nephron-sparing surgery. As a result, a radical nephrectomy was performed.
No consensus is available on the postoperative follow-up of the patient with MLCN. MLCN has been reported to develop cystic renal cell carcinoma recommending postoperative follow-up of such cases. Patients who underwent partial nephrectomy developed local recurrence or metastasis which is related to incomplete resection as stated by the published literature [6]. On the contrary, Castillo et al., 1991 in a series of 29 cases did not encounter any postoperative local recurrence or metastasis [26]. Also in our case, we did not observe any local recurrence or metastasis.
Conclusion
CN is a rarely observed benign gradually progressing entity with a good prognosis. It is suspected in a patient presenting with complex cystic renal mass. Although these tumors are predominantly benign and prognosis is excellent, long-term follow-up for local recurrence is still mandatory since incomplete tumor resection can lead to recurrence and, in rare cases, malignant transformation can occur with a grim prognosis. Hence, tumor excision with clear margins is a standard of care and a careful long-term follow-up is warranted.
Author contributions
RV carried out concepts and design, literature search. JV carried out data acquisition, data analysis, manuscript preparation concepts and designing, literature search and contributed to manuscript preparation and clinical study and will stand as guarantor also. NG helped in data acquisition, data analysis, and clinical study. All the authors have read and approved the final manuscript.
Funding
Nil.
Availability of data and materials
All the data regarding the findings are available within the manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflicting interests.
Compliance with ethical standards
This case report was conducted by the fundamental principles of the Declaration of Helsinki.
Consent for publication
Consent for the publication and use of images and any related information was taken from the patient involved in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Edmunds W. Cystic adenoma of kidney. Trans Pathol Soc Lond. 1892;43:89–90. [Google Scholar]
- 2.Jevremovic D, Lager DJ, Lewin M. Cystic nephroma (multilocular cyst) and mixed epithelial stromal tumor of the kidney: a spectrum or the same entity? Ann Diagn Pathol. 2006;10:77–82. [DOI] [PubMed] [Google Scholar]
- 3.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs—part a: renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. [DOI] [PubMed] [Google Scholar]
- 4.Michal M, Syrucek M. Benign mixed epithelial and stromal tumor of the kidney. Pathol Res Pract. 1998;194:445–8. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Lin Y, Xiang H, Fang D, Jiang P, Shen B. Mixed epithelial and stromal tumour of the kidney: report of eight cases and literature review. World J Surg Oncol. 2013;11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dell’Atti L. An unusual presentation of cystic nephroma in an adult man. Rare Tumors. 2015;7:5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, et al. The International Society of Urological Pathology (ISUP) Vancouver classification of renal neoplasia. Am J Surg Pathol. 2013;37:1469–89. [DOI] [PubMed] [Google Scholar]
- 8.Sacher P, Willi UV, Niggli F, Stallmach T. Cystic nephroma: a rare benign renal tumor. Pediatr Surg Int. 1998;13:197–9. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson C, Palit V, Bardapure M, Thomas J, Browning AJ, Gill K, Biyani CS. Adult multilocular cystic nephroma: report of six cases with clinical, radio-pathologic correlation and review of literature. Urol Ann. 2013;5(1):13–7. 10.4103/0974-7796.106958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell T, Shackman R, Johnson HD. Multilocular cysts of the kidney. Br J Urol. 1951;23(2):142–52. 10.1111/j.1464-410x.1951.tb02576.x. [DOI] [PubMed] [Google Scholar]
- 11.Joshi VV, Beckwith JB. Multilocular cyst of the kidney (cystic nephroma) and cystic, partially differentiated nephroblastoma. Terminology and criteria for diagnosis. Cancer. 1989;64:466–79. [DOI] [PubMed] [Google Scholar]
- 12.Foteini Karasavvidou and others. Mixed epithelial and stromal tumor—adult cystic nephroma of the kidney: a case report with immunohistochemical analysis. J Surg Case Reports. 2022;2022(9):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sodhi KS, Suri S, Samujh R, Rao KL, Vaiphei K, Saxena AK. Case report: bilateral multilocular cystic nephromas: a rare occurrence. Br J Radiol. 2005;78:450–2. [DOI] [PubMed] [Google Scholar]
- 14.Granja MF, O’Brien AT, Trujillo S, Mancera J, Aguirre DA. Multilocular cystic nephroma: a systematic literature review of the radiologic and clinical findings. AJR Am J Roentgenol. 2015;205(6):1188–93. 10.2214/AJR.15.14548. [DOI] [PubMed] [Google Scholar]
- 15.Cavıldak İK, Çakıcı MÇ, Karakoyunlu N, Ersoy H. Cystic nephroma: a case report in adult patients. Turk J Urol. 2018;44(4):373–6. 10.5152/tud.2017.56957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma A, Andankar M, Pathak H. A rare presentation of cystic nephroma in a young adult. Asian J Urol. 2017;4(2):128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawant AS, Savalia AJ, Pawar P, Narwade S, Chaudhari R. A case report of largest documented multilocular cystic nephroma removed by thoracoabdominal approach. J Clin Diagn Res. 2017;11(7):10–2. 10.7860/JCDR/2017/25612.10244. (Epub 2017 Jul 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dell’ Atti L. An unusual presentation of cystic nephroma in an adult man. Rare Tumors. 2015;18:7(2):5860. 10.4081/rt.2015.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong B, Wang Y, Zhang J, Fu Y, Wang G. Multilocular cystic nephroma treated with laparoscopic nephron-sparing surgery: a case report. Can Urol Assoc J. 2014;8(7–8):E545–7. 10.5489/cuaj.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozturk H, Karaaslan S. Uretheral invagination of multilocular cystic nephroma; a case report of a new pathologic variant. Int J Clin Exp Pathol. 2014;15:7(8):5271–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu M, Lu J, Ma LL, Yan Y, Zhang SD. Retroperitoneal laparoscopic partial nephrectomy for treatment of cystic nephroma: one case report. Beijing Da Xue Xue Bao Yi Xue Ban. 2014;46(4):650–2 (Chinese). [PubMed] [Google Scholar]
- 22.Abrol N, Gupta N, Arava S, Ray R. Cystic nephroma masquerading as hydatid cyst of the kidney. Indian J Pathol Microbiol. 2010;53:877–9. [DOI] [PubMed] [Google Scholar]
- 23.Mohanty D, Jain BK, Agrawal V, Gupta A. Cystic nephroma: a diagnostic dilemma. Saudi J Kidney Dis Transpl. 2010;21(3):518–20. [PubMed] [Google Scholar]
- 24.Doros LA, Rossi CT, Yang J, Field A, Williams GM, Messinger Y, Cajaiba MM, Perlman EJ, Schultz KA, Cathro HP, et al. DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Mod Pathol. 2014;27:1267–80. 10.1038/modpathol.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antic T, Perry KT, Harrison K, Zaytsev P, Pins M, Campbell SC, et al. Mixed epithelial and stromal tumors of the kidney and cystic nephroma share overlapping features: reappraisal of 15 lesions. Arch Pathol Lab Med. 2006;130:80–5. [DOI] [PubMed] [Google Scholar]
- 26.Castillo OA, Boyle ET Jr, Kramer SA. Multilocular cysts of kidney. A study of 29 patients and review of literature. Urology. 1991;37:156–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data regarding the findings are available within the manuscript.