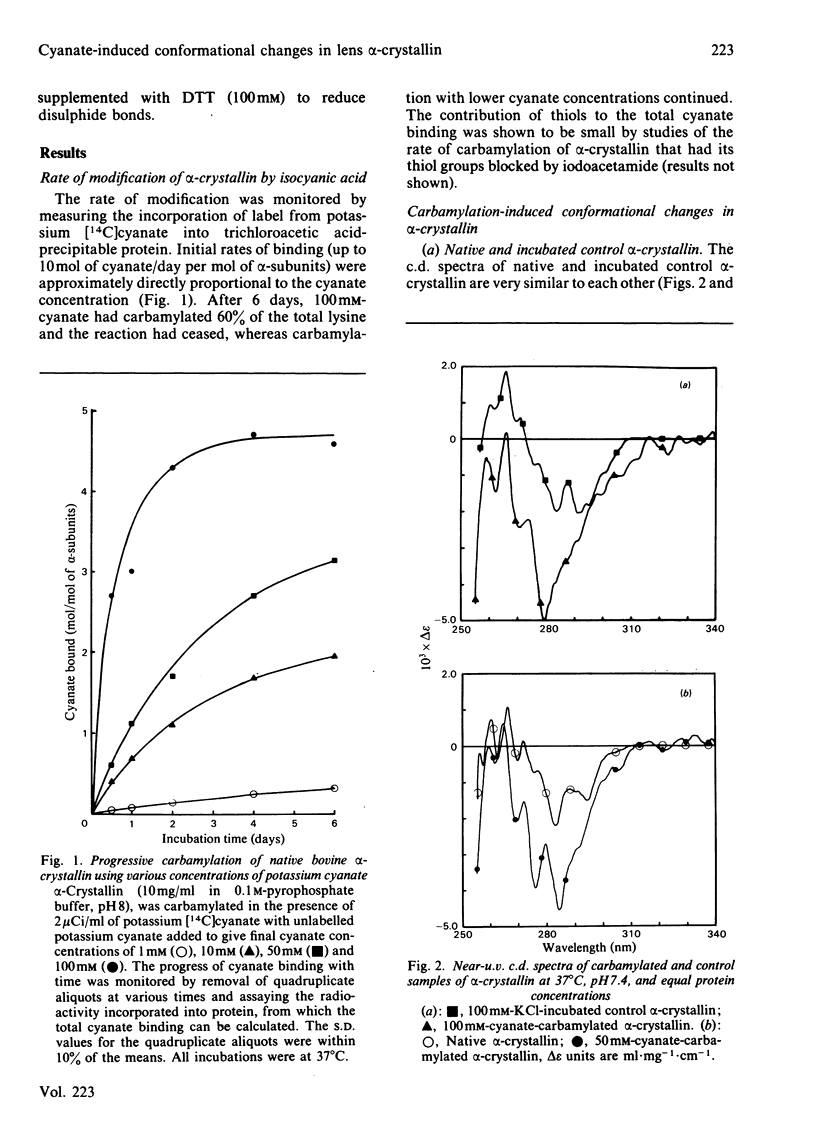
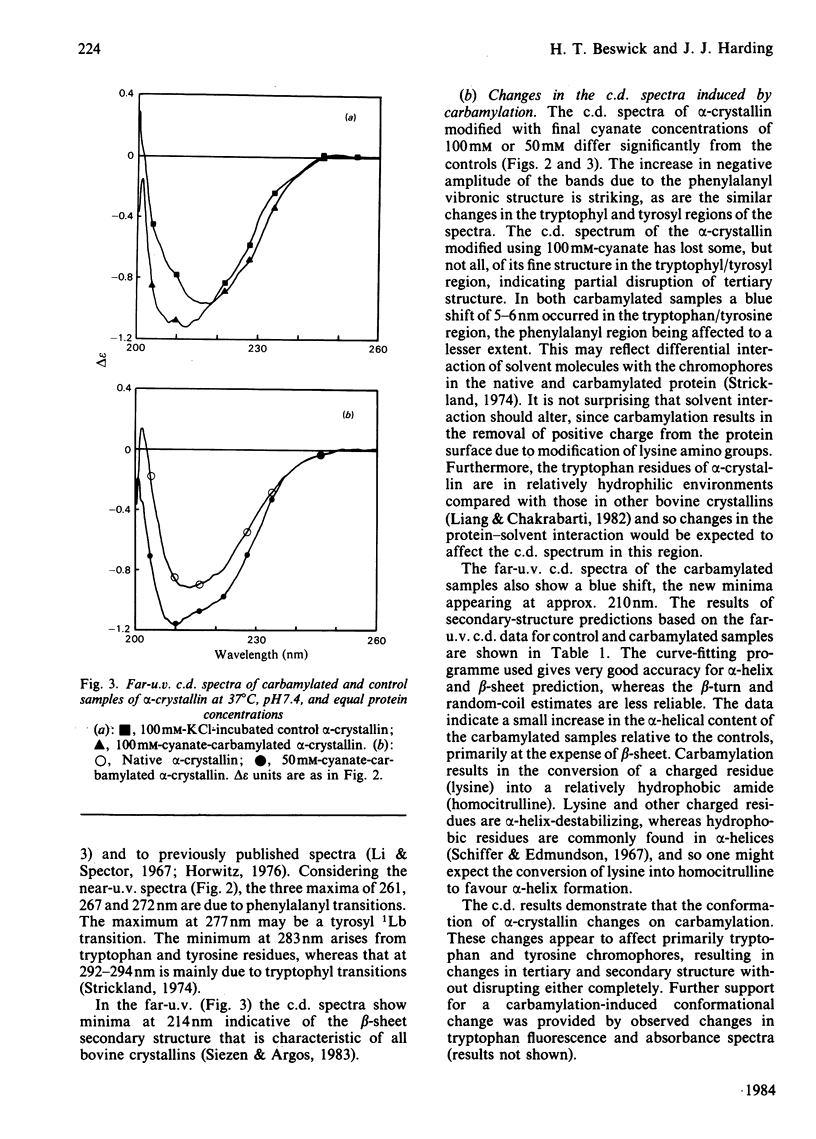
Abstract
Carbamylation of lens proteins may contribute to cataractogenesis in certain medical conditions where blood urea is elevated for prolonged periods. This paper reports on the effects of carbamylation on the physicochemical properties of one of the major lens structural proteins, alpha-crystallin. In particular it is shown that carbamylation alters the tertiary and secondary structure of the protein, leading to an increased reactivity of protein thiols, resulting in interchain disulphide bonding.
Full text
PDF

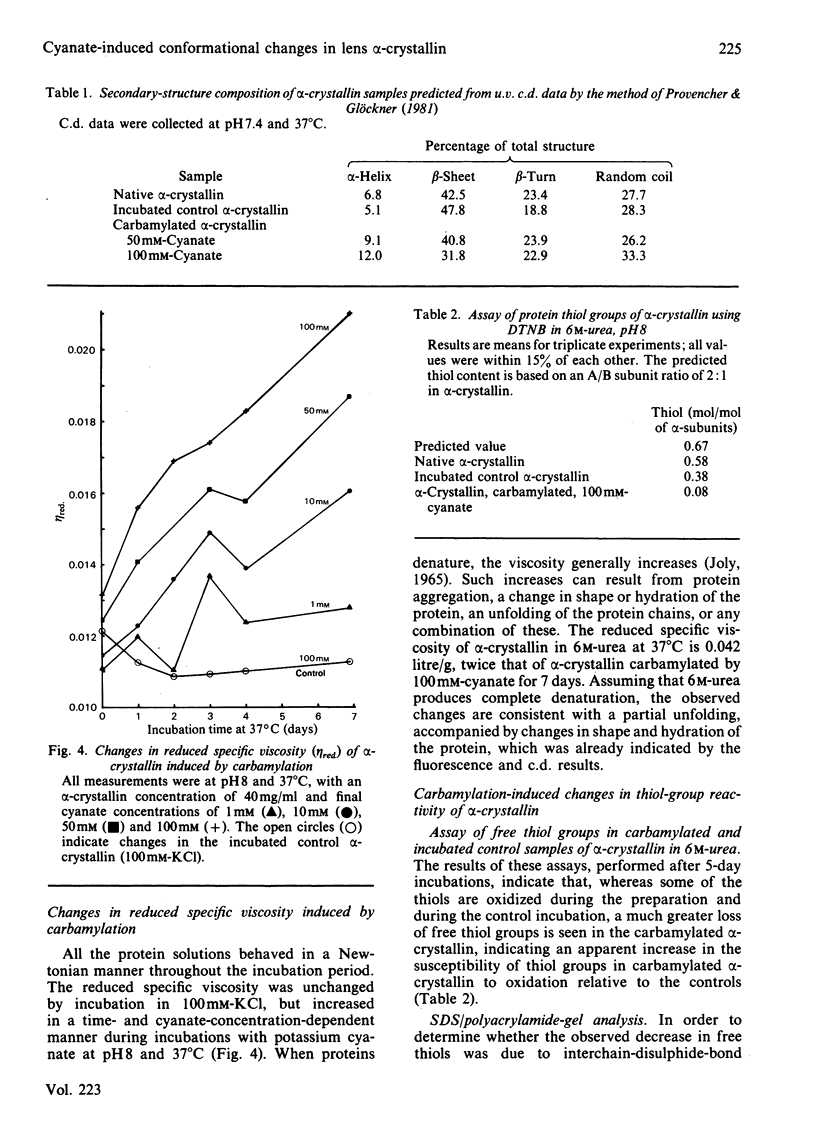
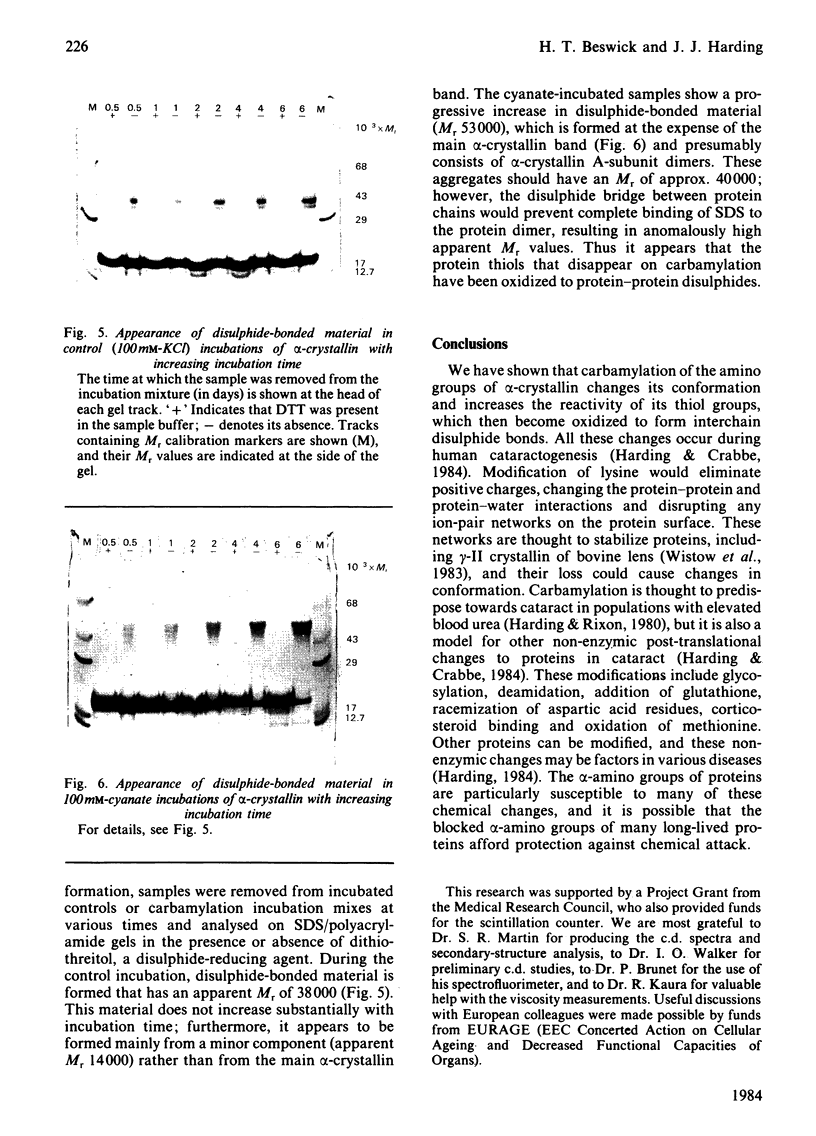

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Harding J. J. Conformational changes in human lens proteins in cataract. Biochem J. 1972 Aug;129(1):97–100. doi: 10.1042/bj1290097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J. J., Rixon K. C. Carbamylation of lens proteins: a possible factor in cataractogenesis in some tropical countries. Exp Eye Res. 1980 Nov;31(5):567–571. doi: 10.1016/s0014-4835(80)80015-7. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Pohlmann R., von Figura K. Inhibition by cyanate of the processing of lysosomal enzymes. Biochem J. 1983 Mar 15;210(3):795–802. doi: 10.1042/bj2100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Some properties of the low molecular weight alpha-crystallin from normal human lens: comparison with bovine lens. Exp Eye Res. 1976 Nov;23(5):471–481. doi: 10.1016/0014-4835(76)90156-1. [DOI] [PubMed] [Google Scholar]
- Kern H. L., Bellhorn R. W., Peterson C. M. Sodium cyanate-induced ocular lesions in the beagle. J Pharmacol Exp Ther. 1977 Jan;200(1):10–16. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. K., Manning J. M. Kinetics of the carbamylation of the amino groups of sickle cell hemoglobin by cyanate. J Biol Chem. 1973 Aug 25;248(16):5861–5865. [PubMed] [Google Scholar]
- Li L. K., Spector A. The optical rotatory dispersion and circular dichroism of calf lens alpha-crystallin. J Biol Chem. 1967 Jul 10;242(13):3234–3236. [PubMed] [Google Scholar]
- Liang J. N., Chakrabarti B. Spectroscopic investigations of bovine lens crystallins. 1. Circular dichroism and intrinsic fluorescence. Biochemistry. 1982 Apr 13;21(8):1847–1852. doi: 10.1021/bi00537a022. [DOI] [PubMed] [Google Scholar]
- Mellado W., Slebe J. C., Maccioni R. B. Tubulin carbamoylation. Functional amino groups in microtubule assembly. Biochem J. 1982 Jun 1;203(3):675–681. doi: 10.1042/bj2030675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian D. C., Mehra V., Jones B. R. Dehydrational crises from severe diarrhoea or heatstroke and risk of cataract. Lancet. 1984 Apr 7;1(8380):751–753. doi: 10.1016/s0140-6736(84)91274-1. [DOI] [PubMed] [Google Scholar]
- Nicholson D. H., Harkness D. R., Benson W. E., Peterson C. M. Cyanate-induced cataracts in patients with sickle-cell hemoglobinopathies. Arch Ophthalmol. 1976 Jun;94(6):927–930. doi: 10.1001/archopht.1976.03910030465005. [DOI] [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- STARK G. R. ON THE REVERSIBLE REACTION OF CYANATE WITH SULFHYDRYL GROUPS AND THE DETERMINATION OF NH2-TERMINAL CYSTEINE AND CYSTINE IN PROTEINS. J Biol Chem. 1964 May;239:1411–1414. [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968 Oct 24;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Argos P. Structural homology of lens crystallins. III. Secondary structure estimation from circular dichroism and prediction from amino acid sequences. Biochim Biophys Acta. 1983 Oct 17;748(1):56–67. doi: 10.1016/0167-4838(83)90027-4. [DOI] [PubMed] [Google Scholar]
- Stark G. R. Reactions of cyanate with functional groups of proteins. 3. Reactions with amino and carboxyl groups. Biochemistry. 1965 Jun;4(6):1030–1036. doi: 10.1021/bi00882a008. [DOI] [PubMed] [Google Scholar]
- Strickland E. H. Aromatic contributions to circular dichroism spectra of proteins. CRC Crit Rev Biochem. 1974 Jan;2(1):113–175. doi: 10.3109/10409237409105445. [DOI] [PubMed] [Google Scholar]
- Wistow G., Turnell B., Summers L., Slingsby C., Moss D., Miller L., Lindley P., Blundell T. X-ray analysis of the eye lens protein gamma-II crystallin at 1.9 A resolution. J Mol Biol. 1983 Oct 15;170(1):175–202. doi: 10.1016/s0022-2836(83)80232-0. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., van Amelsvoort F. M., van der Ouderaa F. J., Bloemendal H. Slow rate of evolution of alpha A chain of alpha-crystallin. Nat New Biol. 1973 Dec 19;246(155):233–236. doi: 10.1038/newbio246233a0. [DOI] [PubMed] [Google Scholar]
- van Dam A. F., ten Cate G. Isolation and some properties of bovine alpha-crystallin. Biochim Biophys Acta. 1966 May 26;121(1):183–186. doi: 10.1016/0304-4165(66)90369-2. [DOI] [PubMed] [Google Scholar]
- van der Ouderaa F. J., de Jong W. W., Bloemendal H. The amino-acid sequence of the alphaA2 chain of bovine alpha-crystallin. Eur J Biochem. 1973 Nov 1;39(1):207–222. doi: 10.1111/j.1432-1033.1973.tb03119.x. [DOI] [PubMed] [Google Scholar]