Abstract
Background
Pneumoconiosis is associated with pulmonary and cardiovascular diseases; however, the link between pneumoconiosis and sleep disorders is not well understood. This study aimed to investigate the connection between pneumoconiosis and subsequent risk of sleep disorders.
Methods
This population-based retrospective cohort study used data from the National Health Insurance database in Taiwan. The pneumoconiosis cohort consisted of 13,329 patients newly diagnosed between 2000 and 2015. The comparison group included 53,316 age-, sex-, and diagnosis date-matched individuals without pneumoconiosis. The development of sleep disorders was monitored until the end of 2018. Cox proportional hazard regression models were used for risk assessment.
Results
The incidence of sleep disorders was 1.31 times higher in the pneumoconiosis cohort than in the comparison cohort (22.8 vs. 16.2 per 1000 person-years). After controlling for age, sex, comorbidity, and medication, the adjusted hazard ratio (aHR) was 1.24 (95% confidence interval [CI] = 1.17–1.32). Stratified analyses by age group, sex, and comorbidity status showed significant associations between pneumoconiosis and sleep disorders (aHRs, 1.19–1.64). In addition, patients with pneumoconiosis had a significantly increased risk of developing sleep apnea (aHR = 1.71, 95% CI = 1.31–2.22).
Conclusion
This study demonstrates that patients with pneumoconiosis are at a higher risk of developing sleep disorders and sleep apnea. Healthcare professionals should pay close attention to sleep quality and disturbances in patients with pneumoconiosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s44197-024-00225-5.
Keywords: Pneumoconiosis, Interstitial Lung Disease (ILD), Occupational disease, Sleep disorder, Sleep apnea
Introduction
Pneumoconiosis is a group of interstitial lung diseases (ILD) caused by inhaling mineral dust or fiber and is the most common occupational disease. Workers usually come into contact with these harmful substances in their workplace environment [1]. Despite its preventable nature, pneumoconiosis remains prevalent, particularly in developing countries, with over 500,000 living cases and 60,000 new cases reported annually [2]. The disease carries a high mortality rate, with over 20,000 deaths reported annually [3]. Inhaling mineral dust or fiber can lead to various respiratory and cardiovascular disorders, including progressive massive fibrosis, pneumothorax, chronic obstructive pulmonary disease (COPD), atrial fibrillation, congestive heart failure (CHF), coronary artery disease (CAD), peripheral arterial disease, and cerebrovascular disease (CVD) [4–11], which often contribute to premature death [12–14].
Pneumoconiosis may be associated with sleep disorders as a result of combination of respiratory abnormality and alterations in sleep architecture. Chronic cough and breathlessness could contribute to sleep disturbance [15–18]. Interstitial changes and poor pulmonary function, leading to decreased oxygen saturation, could also decrease sleep efficiency [19, 20]. In addition, patients with pneumoconiosis experienced increased stage 1 sleep, decreased slow-wave sleep, decreased rapid eye movement sleep, increased sleep fragmentation, and increased arousal [19, 20].
Evidence showed that sleep disturbance was prevalent in patients with pneumoconiosis. Several studies confirmed that patients with pneumoconiosis have poorer sleep quality than those without it [15, 16, 21–23]. However, previous studies have been limited by their small size, study design, and follow-up duration. Thus, this research aimed to address these limitations using a nationwide, population-based, retrospective cohort study to explore the association between pneumoconiosis and subsequent risk of sleep disorders.
Materials and methods
Data Sources
The National Health Insurance (NHI) program in Taiwan, which covered over 99.5% of its residents since 2010, maintains a comprehensive database managed by the Ministry of Health and Welfare. This database containing the total population in the NHI system between 1998 and 2018, which contains extensive medical data such as demographic information, clinical visit dates, diagnostic codes, prescriptions, and treatments, was used in this study. Data were analyzed in the Health and Welfare Data Science Center, Taiwan Ministry of Health and Welfare (at China Medical University), and the access permit number was H109068. This study was approved by the Research Ethics Committee of China Medical University Hospital (CMUH107-REC2-181).
Study cohorts
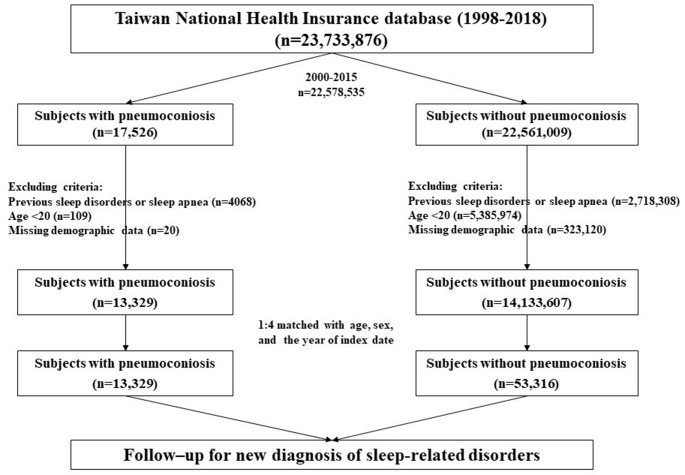
In Taiwan, Occupational Safety and Health Administration of the Ministry of Labor (http://www.osha.gov.tw) had published official guidelines to diagnose pneumoconiosis (supplementary data) based on International Labour Organization International Classification of Radiographs of Pneumoconiosis (https://www.ilo.org), which had updated in 1980, 2000 and 2011. The study enrolled adult patients newly diagnosed with pneumoconiosis (International Classification of Diseases [ICD] codes 500–505 and J60–J65) between 2000 and 2015 in the pneumoconiosis cohort (Fig. 1). Pneumoconiosis was diagnosed when the ICD code was registered in one inpatient discharge record or two outpatient visit records [8, 24, 25]. Using ICD codes for defining diseases in the Taiwan NHI database has been validated, and most of the reported positive predictive values ranged from 80 to 99% [26]. The date of pneumoconiosis diagnosis was defined as the index date, we had excluded those with a previous diagnosis of pneumoconiosis from January 1, 1998, to the index date. Patients with preexisting sleep disorders were excluded. The comparison cohort consisted of adults without pneumoconiosis, 1:4 matching with age, sex, and the index date. Both groups were monitored until the diagnosis of sleep disorders/sleep apnea, insurance system withdrawal, death, or end of 2018.
Fig. 1.
Flowchart of study participants selection
Outcomes and covariates
The primary outcomes were sleep-related disorders (ICD codes 307.4 [specific disorders of sleep of nonorganic origin], 327 [organic sleep disorders], 780.5 [sleep disturbances], F51 [sleep disorders not due to a substance or known physiological condition], and G47 [sleep disorders]), which were classified as sleep apnea (SA, ICD codes 327.2, 780.51, 780.53, 780.57, and G47.3) and sleep disorders (other ICD codes from sleep-related disorders). We used accompanied prescription (Anatomical Therapeutic Chemical Classification System code: N05B and N05C) to validate sleep disorders and accompanied polysomnography (17008B) to confirm SA. Covariates such as age, sex, comorbidities, and medication were considered. Baseline comorbidities including hypertension (ICD codes 401–405 and I10–I16), diabetes mellitus (ICD codes 250 and E08–E13), hyperlipidemia (ICD codes 272 and E78), CVD (ICD 430–438 and I60–I69), asthma/COPD (ICD codes 491, 492, 493, 496, and J41–J45), chronic liver disease and cirrhosis (CLD, ICD codes 571 and K70–K77), chronic kidney disease (CKD, ICD codes 585 and N18), tuberculosis (ICD codes 010–018 and A15–A19), obesity (ICD codes 278 and E66), tobacco use disorders (ICD codes 305.1, 989.84, V15.82, F17, O99.33, P96.81, T65.2, Z57.31, Z71.6, Z72.0, Z77.22, and Z87.891), which may be associated with development of sleep-related disorders. Corticosteroid use (Anatomical Therapeutic Chemical Classification System code: H02) was defined as those using the medication for > 28 days.
Statistical analysis
The chi-squared and Student t-tests were used to compare age groups, sex, comorbidities, and medication. The Kaplan–Meier method was used to estimate the cumulative incidence of sleep disorders in both cohorts, with the log-rank test determining significance. The study used univariate and multivariate Cox proportional hazard regression models to estimate crude and adjusted hazard ratios (cHRs and aHRs) and 95% confidence intervals (CIs). SAS statistical software (version 9.4 for Windows; SAS Institute, Inc., Cary, NC, USA) was used for data analysis, and a p-value of < 0.05 was deemed statistically significant.
Results
A total of 13,329 patients with pneumoconiosis and 53,316 individuals without pneumoconiosis in a comparison cohort were included (Table 1). The mean age values were 70.2 ± 10.9 years and 70.1 ± 11.2 years in the pneumoconiosis and comparison cohorts, respectively, in which 91.4% of individuals in both cohorts were male. The proportions of comorbidities and medication use in the two cohorts were as follows: hypertension, 51.8% vs. 52.3%; diabetes mellitus, 7.07% vs. 4.00%; hyperlipidemia, 20.1% vs. 24.6%; CVD, 15.8% vs. 12.1%; asthma/COPD, 49.9% vs. 12.7%; CLD 12.9% vs. 8.23%; CKD, 3.52% vs. 1.69%; tuberculosis, 3.3% vs. 1.8%; obesity, 0.18% vs. 0.25%; tobacco use disorders, 1.19% vs. 0.65%; and corticosteroid use, 44.7% vs. 31.3%. No significant difference in hypertension and obesity was noticed between the two cohorts. The mean follow-up times were 5.61 ± 5.23 and 9.45 ± 5.25 years in the pneumoconiosis and comparison cohorts, respectively.
Table 1.
Characteristics for individuals with and without pneumoconiosis
| Pneumoconiosis | |||||
|---|---|---|---|---|---|
| No | Yes | ||||
| N = 53,316 | N = 13,329 | ||||
| n | % | n | % | p-value † | |
| Age | 0.99 | ||||
| 20 − 49 | 2664 | 5.0 | 666 | 5.0 | |
| 50 − 64 | 10,472 | 19.6 | 2618 | 19.6 | |
| ≥ 65 | 40,180 | 75.4 | 10,045 | 75.4 | |
| Mean ± SD | 70.1 | ± 11.2 | 70.2 | ± 10.9 | 0.20 |
| Gender | 0.99 | ||||
| Women | 4604 | 8.6 | 1151 | 8.6 | |
| Men | 48,712 | 91.4 | 12,178 | 91.4 | |
| Comorbidity | |||||
| Hypertension | 27,864 | 52.3 | 6898 | 51.8 | 0.29 |
| Diabetes mellitus | 2131 | 4.0 | 943 | 7.1 | < 0.001 |
| Hyperlipidemia | 13,088 | 24.6 | 2683 | 20.1 | < 0.001 |
| CVD | 6472 | 12.1 | 2104 | 15.8 | 0.003 |
| Asthma/COPD | 6743 | 12.7 | 6655 | 49.9 | < 0.001 |
| CLD | 4390 | 8.2 | 1720 | 12.9 | < 0.001 |
| CKD | 902 | 1.7 | 469 | 3.5 | < 0.001 |
| Tuberculosis | 960 | 1.8 | 446 | 3.3 | < 0.001 |
| Obesity | 135 | 0.25 | 24 | 0.18 | 0.12 |
| Tobacco use disorders | 344 | 0.65 | 159 | 1.19 | < 0.001 |
| Medication | |||||
| Corticosteroid | 16,705 | 31.3 | 5954 | 44.7 | < 0.001 |
CKD, chronic kidney disease; CLD, chronic liver disease and cirrhosis; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; SD, standard deviation
† Chi-squired test and t-test
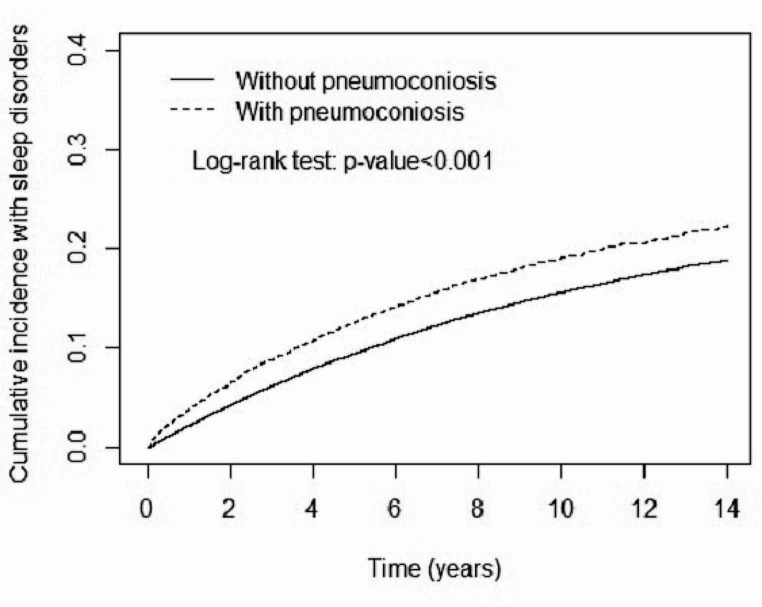
During the follow-up period, the cumulative incidence of sleep disorders was significantly higher in the pneumoconiosis cohort than in the comparison cohort (p < 0.001 in the log-rank test, Fig. 2). The overall incidence of sleep disorders was 1.31-fold higher in the pneumoconiosis cohort than in the comparison cohort (22.8 vs. 16.2 per 1000 person-years, respectively) with an aHR of 1.24 (95% CI 1.17–1.32) after adjusting for age, sex, comorbidity, and medication (Table 2). The aHRs of sleep disorders were 1.26-fold higher in women than in men (95% CI 1.17–1.34). In addition, the risk of sleep disorders was significantly higher in patients with hypertension (aHR 1.31, 95% CI 1.25–1.37), asthma/COPD (aHR 1.55, 95% CI 1.46–1.64), and tuberculosis (aHR 1.34, 95% CI 1.28–1.41).
Fig. 2.
Cumulative incidence of sleep disorders for individuals with and without pneumoconiosis
Table 2.
Risk factor analyses for sleep disorders among all study individuals
| Event | PY | Rate † | Crude HR (95% CI) |
Adjusted HR # (95% CI) |
|
|---|---|---|---|---|---|
| Pneumoconiosis | |||||
| No | 7277 | 448,800 | 16.2 | 1.00 | 1.00 |
| Yes | 1480 | 65,026 | 22.8 | 1.31 (1.24 − 1.38) *** | 1.24 (1.17 − 1.32) *** |
| Age | |||||
| 20 − 49 | 263 | 35,717 | 7.4 | 1.00 | 1.00 |
| 50 − 64 | 1904 | 125,769 | 15.1 | 2.02 (1.78 − 2.30) *** | 2.01 (1.76 − 2.29) *** |
| ≥ 65 | 6590 | 352,339 | 18.7 | 2.31 (2.04 − 2.61) *** | 2.14 (1.89 − 2.43) *** |
| Gender | |||||
| Men | 7812 | 466,977 | 16.7 | 1.00 | 1.00 |
| Women | 945 | 46,849 | 20.2 | 1.23 (1.15 − 1.32) *** | 1.26 (1.17 − 1.34) *** |
| Comorbidity | |||||
| Hypertension | |||||
| No | 3771 | 277,896 | 13.6 | 1.00 | 1.00 |
| Yes | 4986 | 235,930 | 21.1 | 1.46 (1.40 − 1.52) *** | 1.31 (1.25 − 1.37) *** |
| Diabetes mellitus | |||||
| No | 8560 | 500,655 | 17.1 | 1.00 | 1.00 |
| Yes | 197 | 13,171 | 15.0 | 0.77 (0.67 − 0.89) *** | 0.60 (0.52 − 0.69) *** |
| Hyperlipidemia | |||||
| No | 6473 | 401,863 | 16.1 | 1.00 | 1.00 |
| Yes | 2284 | 111,963 | 20.1 | 1.20 (1.15 − 1.26) *** | 1.02 (0.97 − 1.08) |
| CVD | |||||
| No | 7697 | 467,383 | 16.5 | 1.00 | 1.00 |
| Yes | 1060 | 46,443 | 22.8 | 1.26 (1.18 − 1.34) *** | 1.03 (0.97 − 1.11) |
| Asthma/COPD | |||||
| No | 6918 | 441,255 | 15.7 | 1.00 | 1.00 |
| Yes | 1839 | 72,571 | 25.3 | 1.50 (1.42 − 1.58) *** | 1.55 (1.46 − 1.64) *** |
| CLD | |||||
| No | 8115 | 480,082 | 16.9 | 1.00 | 1.00 |
| Yes | 642 | 33,744 | 19.0 | 1.03 (0.95 − 1.12) | 0.93 (0.86 − 1.01) |
| CKD | |||||
| No | 8650 | 508,845 | 17.0 | 1.00 | 1.00 |
| Yes | 107 | 4981 | 21.5 | 1.09 (0.90 − 1.32) | 0.89 (0.73 − 1.08) |
| Tuberculosis | |||||
| No | 8543 | 504,672 | 16.9 | 1.00 | 1.00 |
| Yes | 214 | 9154 | 23.4 | 1.45 (1.37 − 1.52) *** | 1.34 (1.28 − 1.41) *** |
| Obesity | |||||
| No | 8740 | 512,657 | 17.1 | 1.00 | 1.00 |
| Yes | 17 | 1169 | 14.6 | 0.83 (0.51 − 1.33) | 0.75 (0.47 − 1.21) |
| Tobacco use disorders | |||||
| No | 8702 | 511,060 | 17.0 | 1.00 | 1.00 |
| Yes | 55 | 2766 | 19.9 | 1.03 (0.79 − 1.34) | 1.00 (0.76 − 1.30) |
CI, confidence interval; CKD, chronic kidney disease; CLD, chronic liver disease and cirrhosis; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; HR, hazard ratio; PY, person-years
† Incidence rate per 1,000 person-years
# Multivariable analysis including age, gender, comorbidity, and medication
*** p < 0.001
Table 3 presents data on the incidences and aHRs of sleep disorders in the pneumoconiosis and comparison cohorts, stratified by age, sex, and comorbidities. Among individuals aged 20–49, 50–64, and ≥ 65 years, compared with the comparison cohort, the age-specific aHRs in the pneumoconiosis cohort were 1.64 (95% CI 1.21–2.23), 1.35 (95% CI 1.19–1.54), and 1.19 (95% CI 1.10–1.28), respectively. The sex-specific aHRs were 1.24 (95% CI 1.16–1.32) in men and 1.29 (95% CI 1.08–1.53) in women. For comorbidity, the aHRs were 1.38 (95% CI 1.30–1.47) and 1.23 (95% CI = 1.04–1.46) in those with and without comorbidities, respectively.
Table 3.
Incidences and hazard ratios of for individuals with and without pneumoconiosis by age, gender, comorbidity
| Pneumoconiosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Event | PY | Rate † | Event | PY | Rate † |
Crude HR (95% CI) |
Adjusted HR # (95% CI) |
|
| Age | ||||||||
| 20 − 49 | 187 | 30,389 | 6.2 | 76 | 5,329 | 14.3 | 2.22 (1.70 − 2.90) *** | 1.64 (1.21 − 2.23) ** |
| 50 − 64 | 1534 | 109,077 | 14.1 | 370 | 16,691 | 22.2 | 1.47 (1.31 − 1.65) *** | 1.35 (1.19 − 1.54) *** |
| ≥ 65 | 5556 | 309,334 | 18.0 | 1034 | 43,006 | 24.0 | 1.24 (1.16 − 1.33) *** | 1.19 (1.10 − 1.28) *** |
| Gender | ||||||||
| Women | 775 | 40,442 | 19.2 | 170 | 6407 | 26.5 | 1.30 (1.10 − 1.54) ** | 1.29 (1.08 − 1.53) ** |
| Men | 6502 | 408,358 | 15.9 | 1310 | 58,619 | 22.4 | 1.31 (1.23 − 1.39) *** | 1.24 (1.16 − 1.32) *** |
| Comorbidity ‡ | ||||||||
| No | 2010 | 180,748 | 11.1 | 148 | 11,432 | 13.0 | 1.12 (0.95 − 1.33) | 1.23 (1.04 − 1.46) * |
| Yes | 5267 | 268,053 | 19.7 | 1332 | 53,593 | 24.9 | 1.20 (1.13 − 1.27) *** | 1.38 (1.30 − 1.47) *** |
CI, confidence interval; HR, hazard ratio; PY, person-years
† Incidence rate per 1,000 person-years
# Multivariable analysis including age, gender, comorbidity, and medication
‡ Individuals with any comorbidity of hypertension, diabetes mellitus, hyperlipidemia, CVD, asthma/COPD, CLD, CKD, tuberculosis, obesity, and tobacco use disorders were classified into the comorbidity group
* p < 0.1, ** p < 0.01, *** p < 0.001
Table 4 presents information on the risk of SA in the pneumoconiosis and comparison cohorts. The aHRs of SA was 1.71 (95% CI 1.31–2.22) in the pneumoconiosis and comparison cohorts, respectively.
Table 4.
Incidences and hazard ratios of sleep apnea for individuals with and without pneumoconiosis by age, gender, comorbidity
| Pneumoconiosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Event | PY | Rate † | Event | PY | Rate † |
Crude HR (95% CI) |
Adjusted HR # (95% CI) |
|
| Overall | 345 | 448,800 | 0.77 | 86 | 65,026 | 1.32 | 2.17 (1.71 − 2.75) *** | 1.71 (1.31 − 2.22) *** |
| Age | ||||||||
| 20 − 49 | 38 | 30,389 | 1.25 | 6 | 5329 | 1.13 | 1.03 (0.44 − 2.44) | 1.08 (0.43 − 2.70) |
| 50 − 64 | 116 | 109,077 | 1.06 | 17 | 16,691 | 1.02 | 1.31 (0.79 − 2.19) | 0.94 (0.53 − 1.68) |
| ≥ 65 | 191 | 309,334 | 0.62 | 63 | 43,006 | 1.46 | 2.95 (2.21 − 3.92) *** | 2.49 (1.81 − 3.42) *** |
| Gender | ||||||||
| Women | 13 | 40,442 | 0.32 | 6 | 6407 | 0.94 | 3.18 (1.21 − 8.38) * | 2.65 (0.95 − 7.43) |
| Men | 332 | 408,358 | 0.81 | 80 | 58,619 | 1.36 | 2.15 (1.68 − 2.75) *** | 1.65 (1.26 − 2.17) *** |
| Comorbidity ‡ | ||||||||
| No | 90 | 180,748 | 0.50 | 11 | 11,432 | 0.96 | 2.35 (1.26 − 4.40) ** | 2.33 (1.24 − 4.41) ** |
| Yes | 255 | 268,053 | 0.95 | 75 | 53,593 | 1.40 | 1.74 (1.34 − 2.25) *** | 1.71 (1.31 − 2.23) *** |
CI, confidence interval; HR, hazard ratio; PY, person-years
† Incidence rate per 1,000 person-years
# Multivariable analysis including age, gender, comorbidity, and medication
‡ Individuals with any comorbidity of hypertension, diabetes mellitus, hyperlipidemia, CVD, asthma/COPD, CLD, CKD, tuberculosis, obesity, and tobacco use disorders were classified into the comorbidity group
* p < 0.1, ** p < 0.01, *** p < 0.001
Discussion
As the first of its kind, this retrospective cohort study investigated the association between pneumoconiosis and the development of sleep disorders using nationwide population-based data. The study found that the risk of sleep disorders was 31% higher for people with pneumoconiosis than those without pneumoconiosis. This association was observed across all subgroups analyzed, including those stratified by age, sex, and comorbidities. In addition, patients with pneumoconiosis had a significant higher risk of SA than those without pneumoconiosis. The study findings provide important epidemiological insights into the relationship between pneumoconiosis and sleep disorders on a large scale.
The mechanisms between pneumoconiosis and sleep disorders are unconclusive. Respiratory symptoms, oxygen desaturation, and disruption of the normal sleep architecture may play an essential role. This poor sleep quality may further negatively affect the quality of life, daytime functioning, chronic fatigue, stress, depressive mood, disease progression, cardiovascular comorbidity, and even survival [27, 28].
The association between pneumoconiosis and SA should be more emphasized. SA has a link closer to severe cardiovascular comorbidities, such as CHF, CAD, and CVD. Therefore, cumulative evidence has shown that SA worsens disease progression and mortality in patients with ILD [29, 30]. A recent meta-analysis identified a 61% prevalence of obstructive SA among patients with various forms of ILD, with 26% having moderate-to-severe disease [31]. The mechanism may involve decreased respiratory muscle mass, decreased lung volume increased breathing work, decreased gas exchange, and corticosteroid treatments [32]. Regarding pneumoconiosis and SA sharing comparable cardiovascular risk and may have a synergy effect, the existence of SA in patients with pneumoconiosis must be detected early.
A key study strength is the inclusion of a large pneumoconiosis cohort and a well-matched comparison cohort, with a high rate of follow-up completion. Given the high costs of conducting a prospective cohort study, the use of the Taiwan National Health Insurance database for a retrospective cohort study was an appropriate and cost-effective alternative. In addition, the study accurately reflects real-world scenarios in which pneumoconiosis, sleep disorders, and all comorbidities were diagnosed during medical consultations [33–35].
However, this study has some limitations that should be considered. First, the diagnosis of pneumoconiosis, sleep disorders, and comorbidities relied on the accuracy and competence of clinical physicians using ICD codes. Second, important information such as occupational history, smoking habits, physical activity, and family history were not included in the database, which could have influenced the results. Third, clinical variables such as the pneumoconiosis stage, body weight or body mass index, laboratory data, pulmonary function tests, and imaging results were not available for analysis.
Conclusion
Patients with pneumoconiosis have a significantly higher risk of developing sleep disorders than those without pneumoconiosis. Moreover, the risk of SA was also significantly higher in patients with pneumoconiosis. Healthcare professionals should pay more attention to sleep quality and disturbance in such patients.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the MOHW Health and Welfare Data Science Center at China Medical University for providing administrative and technical support.
Abbreviations
- aHR
Adjusted hazard ratio
- CAD
Coronary artery disease
- CHF
Congestive heart failure
- cHR
Crude hazard ratio
- CI
Confidence interval
- CKD
Chronic kidney disease
- CLD
Chronic liver disease and cirrhosis
- COPD
Chronic obstructive pulmonary disease
- CVD
Cerebrovascular disease
- ICD
International Classification of Diseases
- ILD
Interstitial lung diseases
- NHI
National Health Insurance
- SA
Sleep apnea
Author contributions
YSL, TCS, CYT, TCH, and WHH concepted the manuscript; CLL and DYC collected the data; YSL, TCS, CYT, TCH, and WHH made formal analysis; All authors wrote original draft; All authors have read and approved the latest version of the manuscript.
Funding
The study was supported by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW112-TDU-B-212-144004), China Medical University Hospital (DMR-111-105, DMR-112-087, DMR-113-009, DMR-113-156), and An Nan Hospital (ANHRF112-13).
Data availability
All data generated or analyzed during this study are included in this manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of China Medical University Hospital (CMUH107-REC2-181). Informed consent was waived due to the data extracted from the National Health Insurance Database, which provides only comprehensive de-identified healthcare information.
Consent for publication
All authors have read and approved the latest version of the manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Qi XM, Luo Y, Song MY, Liu Y, Shu T, Liu Y, et al. Pneumoconiosis: current status and future prospects. Chin Med J. 2021;134:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet. 2018;392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W, Liang R, Zhang R, Bin Wang S, Cao X, Wang, et al. Prevalence of coal worker’s pneumoconiosis: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2022;29:88690–8. [DOI] [PubMed] [Google Scholar]
- 4.Sun BQ, Zhao HL, Xie Y. Progress in epidemiological studies on pneumoconiosis with comorbidities. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2021;39:389–93. [DOI] [PubMed] [Google Scholar]
- 5.Pan JH, Cheng CH, Wang CL, Dai CY, Sheu CC, Tsai MJ, et al. Risk of pneumothorax in pneumoconiosis patients in Taiwan: a retrospective cohort study. BMJ Open. 2021;11:e054098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu WS, Lin CL. Risk of atrial fibrillation in patients with pneumoconiosis: a nationwide study in Taiwan. Clin Cardiol. 2020;43:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen CM, Lin CL, Lin MC, Chen HY, Lu NH, Kao CH. Pneumoconiosis increases the risk of congestive heart failure: a nationwide population-based cohort study. Medicine. 2016;95:e3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang JH, Shen TC, Chen KW, Lin CL, Hsu CY, Wen YR, et al. Risk of acute myocardial infarction in pneumoconiosis: results from a retrospective cohort study. Biomedicines. 2023;11:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen CH, Lin TY, Huang WY, Chen HJ, Kao CH. Pneumoconiosis increases the risk of peripheral arterial disease: a nationwide population-based study. Medicine. 2015;94:e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng YY, Hsu KH, Chen YH, Lin CH. Increased risk of ischemic stroke in patients with pneumoconiosis. J Clin Neurosci. 2015;22:363–7. [DOI] [PubMed] [Google Scholar]
- 11.Chuang CS, Ho SC, Lin CL, Lin MC, Kao CH. Risk of cerebrovascular events in pneumoconiosis patients: a population-based study, 1996–2011. Medicine. 2016;95:e2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall NB, Blackley DJ, Halldin CN, Laney AS. Current review of pneumoconiosis among US coal miners. Curr Environ Health Rep. 2019;6:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beggs JA, Slavova S, Bunn TL. Patterns of pneumoconiosis mortality in Kentucky: analysis of death certificate data. Am J Ind Med. 2015;58:1075–82. [DOI] [PubMed] [Google Scholar]
- 14.Paul R, Adeyemi O, Arif AA. Estimating mortality from coal workers’ pneumoconiosis among Medicare beneficiaries with pneumoconiosis using binary regressions for spatially sparse data. Am J Ind Med. 2022;65:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang WK, Lum CM, Ungvari GS, Chiu HF. Health-related quality of life in community-dwelling men with pneumoconiosis. Respiration. 2006;73:203–8. [DOI] [PubMed] [Google Scholar]
- 16.Cho S, Cho OH. Depression and quality of life in older adults with pneumoconiosis: the mediating role of death anxiety. Geriatr Nurs. 2022;44:215–20. [DOI] [PubMed] [Google Scholar]
- 17.Leonard R, Zulfikar R, Stansbury R. Coal mining and lung disease in the 21st century. Curr Opin Pulm Med. 2020;26:135–41. [DOI] [PubMed] [Google Scholar]
- 18.Kawaji T, Hasegawa T, Uchiyama Y. Dyspnea and outcome expectations are associated with physical activity in persons with pneumoconiosis: a cross-sectional study. BMC Pulm Med. 2022;22:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal S, Richardson B, Krishnan V, Schneider H, Collop NA, Danoff SK. Interstitial lung disease and sleep: what is known? Sleep Med. 2009;10:947–51. [DOI] [PubMed] [Google Scholar]
- 20.Myall KJ, West A, Kent BD. Sleep and interstitial lung disease. Curr Opin Pulm Med. 2019;25:623–8. [DOI] [PubMed] [Google Scholar]
- 21.Hu XC, Hu ZY, Fu YK, Ma HY, Zhu AA, Zhou YJ, et al. Investigation and analysis of quality of life of some pneumoconiosis patients in Hangzhou. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2019;37:673–7. [DOI] [PubMed] [Google Scholar]
- 22.Jing H, Cui P, Wang WX, Li J, Wang L, Bi BQ, et al. Study on the quality of life and influencing factors of pneumoconiosis in migrant workers. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020;38:682–5. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Wang Q, Wang Y, Tong R. Measuring the health related quality of life and economic burden of illness among migrant workers with pneumoconiosis. J Occup Environ Med. 2023;65:e105–12. [DOI] [PubMed] [Google Scholar]
- 24.Wei CH, Li CH, Shen TC, Hung YT, Tu CY, Hsia TC, et al. Risk of chronic kidney disease in pneumoconiosis: results from a retrospective cohort study (2008–2019). Biomedicines. 2023;11:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HM, Liu DY, Hsu HL, Yu TL, Yu TS, Shen TC, et al. Risk of depression in patients with pneumoconiosis: a population-based retrospective cohort study. J Affect Disord. 2024;352:146–52. [DOI] [PubMed] [Google Scholar]
- 26.Huang YT, Wei T, Huang YL, Wu YP, Chan KA. Validation of diagnosis codes in healthcare databases in Taiwan, a literature review. Pharmacoepidemiol Drug Saf. 2023;32:795–811. [DOI] [PubMed] [Google Scholar]
- 27.Kolilekas L, Manali E, Vlami KA, Lyberopoulos P, Triantafillidou C, Kagouridis K, et al. Sleep oxygen desaturation predicts survival in idiopathic pulmonary fibrosis. J Clin Sleep Med. 2013;9:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gille T, Didier M, Boubaya M, Moya L, Sutton A, Carton Z, et al. Obstructive sleep apnea and related comorbidities in incident idiopathic pulmonary fibrosis. Eur Respir J. 2017;49:1601934. [DOI] [PubMed] [Google Scholar]
- 29.Khor YH, Ryerson CJ, Landry SA, Howard ME, Churchward TJ, Edwards BA, et al. Interstitial lung disease and obstructive sleep apnea. Sleep Med Rev. 2021;58:101442. [DOI] [PubMed] [Google Scholar]
- 30.Khor YH, Ng Y, Sweeney D, Ryerson CJ. Nocturnal hypoxemia in interstitial lung disease: a systematic review. Thorax. 2021;76:1200–8. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Y, Wang Y, Dai L. The prevalence of obstructive sleep apnea in interstitial lung disease: a systematic review and meta-analysis. Sleep Breath. 2021;25:1219–28. [DOI] [PubMed] [Google Scholar]
- 32.Locke BW, Lee JJ, Sundar KM. OSA and chronic respiratory disease: mechanisms and epidemiology. Int J Environ Res Public Health. 2022;19:5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med. 2015;175:1527–9. [DOI] [PubMed] [Google Scholar]
- 34.Lai SW, Lin CL, Liao KF. Risk of contracting pneumonia among patients with pre-dialysis chronic kidney disease: a population-based cohort study in Taiwan. Biomedicine. 2017;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan’s National Health Insurance Research Database: past and future. Clin Epidemiol. 2019;11:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.