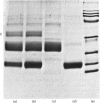
Abstract
The interactions between egg-white cystatin and the cysteine proteinases papain, human cathepsin B and bovine dipeptidyl peptidase I were studied. Cystatin was shown to be a competitive reversible inhibitor of cathepsin B (Ki 1.7 nM, k-1 about 2.3 X 10(-3) s-1). The inhibition of dipeptidyl peptidase I was shown to be reversible (Ki(app.) 0.22 nM, k-1 about 2.2 X 10(-3) s-1). Cystatin bound papain too tightly for Ki to be determined, but an upper limit of 5 pM was estimated. The association was a second-order process, with k+1 1.0 X 10(7) M-1 X s-1. Papain was shown to form equimolar complexes with cystatin. Sodium dodecyl sulphate/polyacrylamide-gel electrophoresis of complexes formed between papain or cathepsin B and an excess of cystatin showed no peptide bond cleavage after incubation for 72 h. The reaction of the active-site thiol group of papain with 5,5'-dithiobis-(2-nitrobenzoic acid) at pH 8 and 2,2'-dithiobispyridine at pH 4 was blocked by complex-formation. Dipeptidyl peptidase I and papain were found to compete for binding to cystatin, contrary to a previous report. The two major isoelectric forms of cystatin were found to have similar specific inhibitory activities for papain, and similar affinities for papain, cathepsin B and dipeptidyl peptidase I. This, together with specific oxidation of the N-terminal serine residue with periodate, showed the N-terminal amino group of cystatin 1 to be unimportant for inhibition. General citraconylation of amino groups resulted in a large decrease in the affinity of cystatin for dipeptidyl peptidase I. It is concluded that the interaction of cystatin with cysteine proteinases has many characteristics similar to those of an inhibitor such as aprotinin with serine proteinases.
Full text
PDF




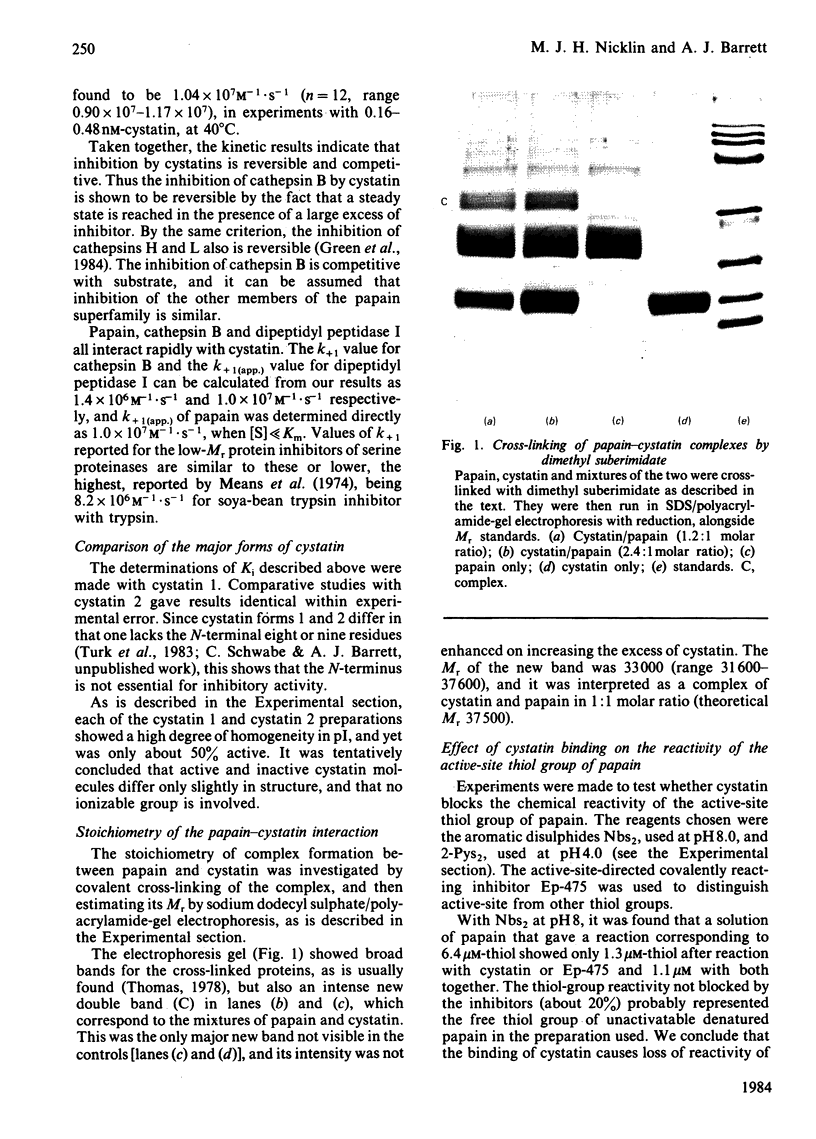
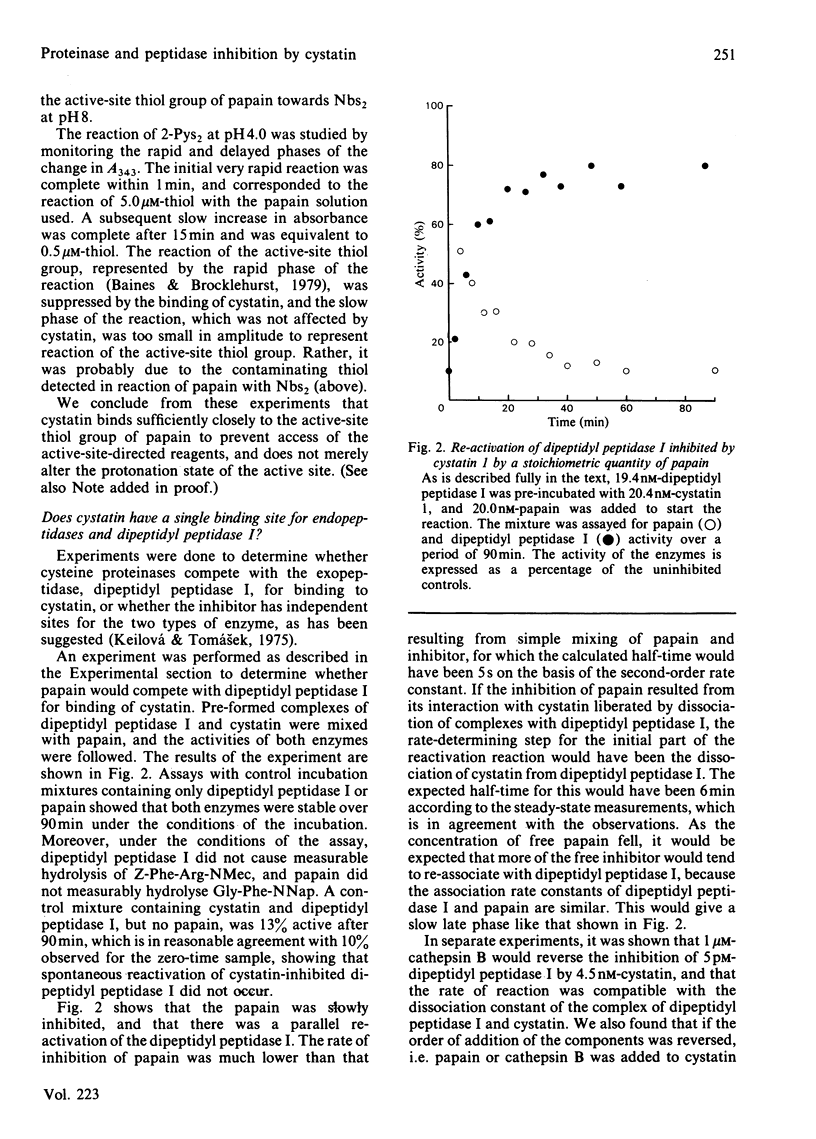


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasi A., Brown M. A., Kembhavi A. A., Nicklin M. J., Sayers C. A., Sunter D. C., Barrett A. J. Cystatin, a protein inhibitor of cysteine proteinases. Improved purification from egg white, characterization, and detection in chicken serum. Biochem J. 1983 Apr 1;211(1):129–138. doi: 10.1042/bj2110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baici A., Gyger-Marazzi M. The slow, tight-binding inhibition of cathepsin B by leupeptin. A hysteretic effect. Eur J Biochem. 1982 Dec;129(1):33–41. doi: 10.1111/j.1432-1033.1982.tb07017.x. [DOI] [PubMed] [Google Scholar]
- Baines B. S., Brocklehurst K. A necessary modification to the preparation of papain from any high-quality latex of Carica papaya and evidence for the structural integrity of the enzyme produced by traditional methods. Biochem J. 1979 Feb 1;177(2):541–548. doi: 10.1042/bj1770541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J. 1980 Jun 1;187(3):909–912. doi: 10.1042/bj1870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S. Tight-binding inhibitors--III. A new approach for the determination of competition between tight-binding inhibitors and substrates--inhibition of adenosine deaminase by coformycin. Biochem Pharmacol. 1976 Dec 15;25(24):2695–2702. doi: 10.1016/0006-2952(76)90259-8. [DOI] [PubMed] [Google Scholar]
- Croft L. R. The amino acid sequence of -crystallin (fraction II) from calf lens. Biochem J. 1972 Jul;128(4):961–970. doi: 10.1042/bj1280961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields R., Dixon H. B. A spectrophotometric method for the microdetermination of periodate. Biochem J. 1968 Aug;108(5):883–887. doi: 10.1042/bj1080883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossum K., Whitaker J. R. Ficin and papain inhibitor from chicken egg white. Arch Biochem Biophys. 1968 Apr;125(1):367–375. doi: 10.1016/0003-9861(68)90672-3. [DOI] [PubMed] [Google Scholar]
- Green G. D., Kembhavi A. A., Davies M. E., Barrett A. J. Cystatin-like cysteine proteinase inhibitors from human liver. Biochem J. 1984 Mar 15;218(3):939–946. doi: 10.1042/bj2180939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. K., Zeitman B. B., Reilly T. J., Ellis S. New observations on the substrate specificity of cathepsin C (dipeptidyl aminopeptidase I). Including the degradation of beta-corticotropin and other peptide hormones. J Biol Chem. 1969 May 25;244(10):2693–2709. [PubMed] [Google Scholar]
- Schwabe C., Anastasi A., Crow H., McDonald J. K., Barrett A. J. Cystatin. Amino acid sequence and possible secondary structure. Biochem J. 1984 Feb 1;217(3):813–817. doi: 10.1042/bj2170813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz W. N., Barrett A. J. Human cathepsin H. Biochem J. 1980 Nov 1;191(2):487–497. doi: 10.1042/bj1910487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk V., Brzin J., Longer M., Ritonja A., Eropkin M., Borchart U., Machleidt W. Protein inhibitors of cysteine proteinases. III. Amino-acid sequence of cystatin from chicken egg white. Hoppe Seylers Z Physiol Chem. 1983 Nov;364(11):1487–1496. doi: 10.1515/bchm2.1983.364.2.1487. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]