Abstract
Drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome (DIHS), is a severe drug-induced hypersensitivity reaction with 10% mortality. To date, there is insufficient evidence regarding the association between DRESS/DIHS and serum levels of vancomycin (VCM). Here, we report the case of a 46-year-old woman undergoing peritoneal dialysis who developed VCM-induced DRESS/DIHS. She was hospitalized for peritonitis with abdominal pain and treated with VCM. On day 10 of hospitalization, her abdominal symptoms improved; however, fever, skin rash, lymphadenopathy, eosinophilia, atypical lymphocytes, and liver and renal dysfunction developed. Based on the clinical course and laboratory findings, we diagnosed the patient with DRESS/DIHS due to VCM. Since her serum VCM concentration was high at 39.8 μg/mL, hemodialysis (HD) was performed to remove VCM, which caused her symptoms to improve. However, serum levels of VCM rebounded and the same symptoms recurred. Therefore, we re-performed HD; no further relapse occurred. This clinical course showed that increased serum VCM levels were associated with DRESS/DIHS onset and severity, suggesting that it is a blood level-dependent disease and that removal of VCM by HD is a potential therapeutic option.
Keywords: Drug reaction with eosinophilia and systemic symptoms, Drug-induced hypersensitivity syndrome, Vancomycin, Dialysis
Introduction
Drug reaction with eosinophilia and systemic symptoms (DRESS), or drug-induced hypersensitivity syndrome (DIHS), is a rare, severe, delayed, T-cell-mediated, drug-induced hypersensitivity reaction that affects multiple organs and has 10% mortality [1]. Its incidence ranges between one in 1,000 and one in 10,000 drug exposures and symptoms include rash, fever, hematological abnormalities (leukocytosis, eosinophilia, and atypical lymphocytosis), lymphadenopathy, and internal organ involvement, most commonly the liver or kidneys. DRESS/DIHS typically develops 2–6 weeks after drug initiation [2]. Although its etiology is not fully understood, it is thought to result from complex interactions between several human herpes viruses (HHVs) and comprehensive (including drug-specific and antiviral) immune responses [3].
Although DRESS/DIHS commonly occurs after treatment with anticonvulsants, it is also induced by other drug types, including antimicrobials and uric acid-lowering compounds [4]. It has been associated with reduced clearance of phenytoin, minocycline, and allopurinol, indicating that its onset and severity depend on blood level or dosage of the drug [5–7]. Although a cross-sectional study suggested an association between blood vancomycin (VCM) levels and DRESS/DIHS, a causal relationship has not been completely clarified [8].
Herein, we describe a case of DRESS/DIHS that was improved by removal of VCM by hemodialysis (HD) in a patient undergoing peritoneal dialysis (PD), in a blood level-dependent manner. To our best knowledge, this is the first demonstration of an association between DRESS/DIHS and serum VCM levels.
Case presentation
A 46-year-old woman with a two-year history of PD, due to secondary focal segmental glomerulosclerosis associated with glycogen storage disease (GSD) type Ia, visited our hospital due to malaise and chills for a day. Her regimen was continuous ambulatory PD therapy, including three dwells of dialysate glucose solution (1.5% with fill volume 1.5 L). There had been no changes in oral medications during the previous three months; her home medications were furosemide, esaxerenone, alfacalcidol, calcium carbonate, lanthanum carbonate, roxadustat, and corn starch. Since esaxerenone is contra-indicated for patients undergoing dialysis in Japan, we discontinued it after hospitalization. She had no history of allergy.
Investigations, diagnosis, and management
The physical findings on admission were: height, 156 cm; weight, 59.6 kg; body mass index, 24.4 kg/m2; body temperature, 37.8 ℃; blood pressure, 108/91 mmHg; pulse, 112 beats/min; and oxygen saturation on room air, 97%. Although the patient reported lower abdominal pain, we observed no evidence of peritoneal irritation. The peritoneal catheter exit site was normal. The laboratory findings on admission were: leukocyte count, 17,600 /μL (neutrophils 90.2%, lymphocytes 6.4%, monocytes 2.1%, eosinophils 1.1%, and basophils 0.2%); absolute eosinophil count, 193/μL; hemoglobin, 11.8 g/dL; total protein, 7.1 g/dL; albumin, 2.9 g/dL; blood urea nitrogen, 59.0 mg/dL; creatinine, 8.8 mg/dL; and C-reactive protein, 1.53 mg/dL (Table 1). The peritoneal effluent was cloudy, with a white-cell count of 235/μL and polymorphonuclear cell fraction of 72%. Electrocardiography and chest radiography revealed no significant abnormalities.
Table 1.
Laboratory data and microbiological tests on and after admission
| Day of admission | Day 11 after admission | ||
|---|---|---|---|
| Blood count | |||
| White blood cells (/μL) | 17,600 | 3100 | |
| Neutrophils (%) | 90.2 | 50 | |
| Lymphocytes (%) | 6.4 | 35 | |
| Atypical Lymphocytes (%) | 0 | 1 | |
| Monocytes (%) | 2.1 | 3 | |
| Eosinophils (%) | 1.1 | 11 | |
| Absolute eosinophil count (/μL) | 193 | 341 | |
| Basophils (%) | 0.2 | 0 | |
| Hemoglobin (g/dL) | 11.8 | 11.4 | |
| Platelets (/μL) | 34.7 × 104 | 21.5 × 104 | |
| Blood chemistry | |||
| Total protein (mg/dL) | 7.1 | 6.3 | |
| Serum albumin (g/dL) | 2.9 | 2.1 | |
| Sodium (mEq/L) | 138 | 130 | |
| Chloride (mEq/L) | 100 | 90 | |
| Potassium (mEq/L) | 4.4 | 3.9 | |
| Calcium (mg/dL) | 8.2 | 6.6 | |
| Phosphorus (mg/dL) | 5.9 | 6.9 | |
| Aspartate aminotransferase (IU/L) | 18 | 194 | |
| Alanine aminotransferase (IU/L) | 13 | 67 | |
| Lactate dehydrogenase (IU/L) | 204 | 404 | |
| Blood urea nitrogen (mg/dL) | 59 | 57.7 | |
| Serum creatinine (mg/dL) | 8.75 | 13.52 | |
| Intact parathyroid hormone (pg/ml) | 58.1 | Not applicable | |
| Iron (μg/dL) | 25 | 57 | |
| Total iron binding capacity (μg/dL) | 380 | 240 | |
| Immunologic test | |||
| C-reactive protein (mg/dL) | 1.53 | 3.96 | |
| Beta 2-microglobulin (mg/L) | 25.2 | Not applicable | |
| Ferritin (ng/mL) | 44 | 166 | |
| Immunoglobulin G (mg/dL) | 1458 | ||
| Immunoglobulin M (mg/dL) | 120 | ||
| Immunoglobulin A (mg/dL) | 242 | ||
| Immunoglobulin E (IU/mL) | 610 | ||
| Soluble interleukin-2 receptor (IU/mL) | 6794 | ||
| Complement C3 (mg/dL) | 138 | ||
| Complement C4 (mg/dL) | 54 | ||
| 50% hemolytic complement activity (U/mL) | > 60.0 | ||
| Anti-nuclear antibody | < 1:40 | ||
| Perinuclear anti-neutrophil cytoplasmic antibody | Negative | ||
| Cytoplasmic anti-neutrophil cytoplasmic antibody | Negative | ||
| HBs antigen | Negative | ||
| HCV antibody | Negative | ||
| Human herpesvirus 6 DNA | Negative | ||
| Lymphocyte transformation test to vancomycin | Negative | ||
| Cytomegalovirus immunoglobulin G | Positive | ||
| Cytomegalovirus immunoglobulin M | Negative | ||
| Residual kidney function and peritoneal dialysis adequacy | |||
| Renal Kt/V(urea) (/week) | 0.44 | ||
| Peritoneal dialysis Kt/V(urea) (/week) | 1.31 | ||
| Total Kt/V(urea) (/week) | 1.76 | ||
| Culture test | |||
| Blood cultures | Negative | ||
| Peritoneal effluent culture | Negative | ||
DNA deoxyribonucleic acid
After admission, the patient was diagnosed with PD catheter-related and/or enteric peritonitis. Therefore, to cover all enteric bacteria, intravenous administration of VCM at a dose of 30 mg/kg and meropenem (MEPM, 0.5 g/day) was initiated. We selected intravenous rather than intraperitoneal administration to more promptly initiate antibiotics. The patient’s clinical course is summarized in Fig. 1. On day 4 of hospitalization, she showed a trend toward fever resolution; her abdominal pain improved and the peritoneal effluent white cell count decreased to 34/µL (polymorphonuclear cell fraction 24%). At the first therapeutic drug monitoring (TDM) for VCM 3 days after a single dose, serum levels were markedly high at 39.8 μg/mL; therefore, she did not receive a second VCM dose. On day 9 of hospitalization, her fever recurred. However, peritoneal effluent and blood cultures to detect bacteria were negative. The patient’s abdominal symptoms did not recur and her PD effluent was clear without an increase in peritoneal effluent cells.
Fig. 1.
Clinical course and response to hemodialysis. VCM vancomycin, MEPM meropenem, HD hemodialysis
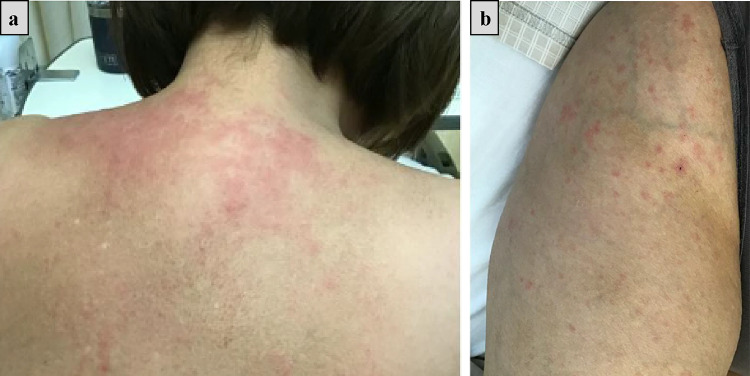
Although MEPM had also been discontinued, on day 10 of hospitalization, she had a fever of 39.2 ℃, erythema appeared on her limbs and body (Fig. 2), and her urine output decreased from 600 mL/day at baseline to 100 mL/day. Whole-body CT revealed lymphadenopathy in both the axillary and inguinal regions. On day 11 of hospitalization, laboratory examination revealed increased eosinophils (11.0%) and elevated liver enzymes (aspartate aminotransferase 194 IU/L and alanine aminotransferase 67 IU/L) (Table 1). Both HHV-6 reactivation and lymphocyte transformation test for VCM were negative (Table 1). HHV-7 testing was not performed. Since serologic tests for cytomegalovirus suggested past infection, we did not perform antigenemia for cytomegalovirus (Table 1). These results led us to consider the possibility of drug allergy rather than aggravation of peritonitis.
Fig. 2.
Erythema on the back (a) and right thigh (b)
Based on the symptoms and laboratory findings, the patient was diagnosed with DRESS/DIHS. Considering the possibility that high serum VCM was the causative agent, we performed HD to remove it. HD conditions were as follows: dialysis membrane material, polysulfone; membrane area, 1.5 m2; dialysate flow rate, 500 ml/min; and blood flow rate, 120 ml/min. After HD, serum VCM levels decreased from 24.4 μg/mL before to 11.4 μg/mL after. On the next day, the fever resolved, urine output increased to 500 mL/day, liver enzyme levels peaked out (aspartate aminotransferase 127 IU/L and alanine aminotransferase 117 IU/L), and skin rash improved.
On day 14 of hospitalization, serum VCM levels rebounded to 15.3 μg/mL. On day 18 of hospitalization, fever, decreased urine output (200 mL/day), and skin rash were again observed with the rebound of serum VCM levels. However, these clinical symptoms improved again after a second HD session, after which there was no rebound in VCM levels. On day 36 of hospitalization, serum VCM levels had maintained a low level of 3.3 μg/mL. The patient was discharged on day 38 of hospitalization following our confirmation of no recurrence of DRESS/DIHS -related symptoms or PD-related peritonitis. During hospitalization, we did not detect any increase in the peritoneal effluent white cell count, and the final peritoneal effluent culture test was negative.
Discussion
We present a case of VCM-induced DRESS/DIHS that improved after VCM removal by HD in a patient undergoing PD, in a blood level-dependent manner. We first inferred that the abdominal pain and peritoneal effluent findings on admission had been caused by peritonitis. However, subsequent recurrent fever, rash, elevated eosinophils, and lymphadenopathy, as well as liver and kidney injury led us to revise our diagnosis to VCM-induced DRESS/DIHS.
Due to its diverse clinical manifestations, a diagnostic scoring system, the European Registry of Severe Cutaneous Adverse Reaction Criteria (RegiSCAR), has been proposed for DRESS [9]; our patient’s score was seven, corresponding to a DRESS probability of “Definite.” This score has components for fever; eosinophilia greater than 10% with leukopenia; lymphadenopathy; atypical lymphocytes (all one point each); renal and liver involvement (two points); and negative tests for antinuclear antibody, blood culture, and hepatitis (one point). It has been reported that 41% of DRESS/DIHS cases exhibit HHV-6 reactivation [2]. However, to our knowledge the most recent article reviewing antibacterial drug-induced DRESS/DIHS reported viral reactivation only in 13% of cases [10]. In addition, it has been reported that the lymphocyte transformation test has a sensitivity of 60–70% [11]. Although our patient was negative for both HHV-6 reactivation and lymphocyte transformation, the diagnostic criteria for DRESS were met. We could not exclude the possibility that MEPM had caused DRESS/DIHS in our patient; however, MEPM has rarely been reported as a cause [10]. Indeed, our patient experienced fever again when serum VCM levels rebounded, which resolved after the second HD session. These observations support the hypothesis that VCM causes DRESS/DIHS in a dose-dependent manner.
Although a VCM trough level > 15 μg/mL is an independent predictor of nephrotoxicity, the association with DRESS/DIHS was not examined. A review of 254 cases of antimicrobial induced DRESS/DIHS reported that 18% were due to VCM. Of note, VCM trough values were reported for 13 cases, three of which were above the recommended range (23%), suggesting no association with DRESS/DIHS incidence [10]. In contrast, a recent case–control study found an association between high trough VCM and DRESS/DIHS. A trough of 25 μg/mL was associated with approximately twofold increased risk, while a trough of 30 μg/mL was associated with an approximately ninefold increased risk [8]. However, this study included patients whose DRESS RegiSCAR scores were “Probable” or higher, thus not limited to "Definite.” Importantly, it has also been reported that a patient undergoing PD developed DRESS/DIHS due to elevated blood VCM levels [12]. Patients on PD had low residual kidney function and poor clearance, as the weekly renal Kt/V(urea) was 0.44 in our case (Table 1). Therefore, VCM dosage and timing of TDM should be carefully planned in patients undergoing PD. Notably, our case report might fill a gap in the literature, as it shows a longitudinal association between blood VCM levels and DRESS/DIHS.
Even after improvement in DRESS/DIHS, patients remain at risk for autoimmune sequelae, including autoimmune thyroiditis, fulminant type 1 diabetes, autoimmune hemolytic anemia, and alopecia [4]. Importantly, impairment in the glycolytic system in patients with GSD Ib may cause CD4 + T-cell dysfunction, leading to autoimmune disease [13]. To the best of our knowledge, this is the first report showing the occurrence of DRESS/DIHS in a patient with GSD Ia. However, considering that some GSD Ia cases resemble GSD Ib clinically [14], it is possible that the underlying immune abnormality of GSD was associated with the onset of DRESS/DIHS in our patient.
The prompt discontinuation of high-risk medications is the initial and most important step in DRESS/DIHS treatment. High-dose systemic corticosteroid treatment also has been reported [15]; however, there are no randomized trials supporting its use. The decision to administer systemic steroids is usually based on the severity of the patient’s reaction, particularly in cases of organ damage, in which their efficacy has been suggested. Acute kidney injury is a common DRESS/DIHS manifestation in more than one-third of cases, with at least 10% progressing to acute kidney injury requiring renal replacement therapy [16]. Some reports have suggested the efficacy of plasma exchange. Although no studies have directly demonstrated the efficacy of drug removal using dialysis [17, 18], VCM half-lives in the systemic circulation range between 66 and 115 h in patients on continuous ambulatory PD [19]. On the other hand, one HD session has been shown to remove 33–50% of plasma VCM [20, 21]; hence, it is a more efficient removal method than PD that is likely to provide more rapid improvement. However, it has been shown that whole-body VCM only decreased 17% with one HD session; a high permeability membrane, such as polysulfone, is an important factor in post-dialysis rebound of VCM serum levels due to redistribution [21]. As plasma VCM levels are reported to rebound by balancing the slow transfer of VCM from tissue to blood and the rapid elimination of VCM by HD [20], continuous monitoring is important. Consistent with these data, VCM levels decreased to less than 15 μg/mL, accompanied by improvements in clinical symptoms after first HD session in our case, but we observed the rebound of serum VCM levels with DRESS/DIHS-related symptoms. Therefore, continuous regular HD sessions (three times per week), or combining HD with plasma exchange are considered to be more effective in DRESS/DIHS than intermittent HD [22].
As we discussed earlier, we cannot exclude the possibility that MEPM had caused DRESS/DIHS in our patient, although this has been reported as an unlikely cause [10]. Importantly, MEPM has a protein binding rate of 2% and a volume distribution (Vd) of 0.30–0.48 L/kg. Its protein binding rate is lower than that of VCM (10–50%) and its Vd is equal to or lower than that of VCM which is known to be 0.4–1.0 L/kg [23]. Therefore, the removal of MEPM is more efficient than that of VCM, suggesting that MEPM is unlikely to cause DRESS/DIHS. In addition, since our patient had a low level of albumin and mild obesity, both of which decrease the protein binding rate and Vd, respectively, VCM should have been removed efficiently with only one rebound.
To the best of our knowledge, this is the first case report of high blood VCM levels associated with DRESS/DIHS onset, with their reduction significantly improving DRESS/DIHS symptoms. We successfully treated DRESS/DIHS involving organ damage by drug removal using HD without immunosuppressive therapy. In our opinion, for DRESS/DIHS patients with severe renal dysfunction drug removal via HD should be considered. Taking into account the high prevalence of methicillin resistant bacteria in our hospital, VCM was a logical choice for our patient`s initial treatment of PD-associated peritonitis [24]; however, VCM dosage and timing of TDM should be carefully planned in patients undergoing PD.
Acknowledgements
We would like to thank the medical staff for their skillful care while treating this patient and Editage (www.editage.com) for English language editing.
Author contributions
RM, TN and SY wrote the manuscript. RM, TN, KU, NY, EK, KM, TY, TK, SY, and KH took clinical care of the patient. JY and KH supervised the manuscript. All authors have read and approved the final manuscript.
Funding
The authors have no financial sources to declare.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
All the authors have declared no competing interest.
Human and/or animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the patient for publication of this case report and all accompanying images.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ryunosuke Mitsuno and Takashin Nakayama contributed equally to this work.
References
- 1.Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutan Med Surg. 1996;15:250–7. 10.1016/s1085-5629(96)80038-1. [DOI] [PubMed] [Google Scholar]
- 2.Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588–97. 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Miyagawa F, Asada H. Current perspective regarding the immunopathogenesis of drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS). Int J Mol Sci. 2021;22:2417. 10.3390/ijms22042147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hama N, Abe R, Gibson A, Phillips EJ. Drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): clinical features and pathogenesis. J Allergy Clin Immunol Pract. 2022;10:1155-1167.e5. 10.1016/j.jaip.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng CY, Yeh YT, Wang CW, Hung SI, Yang CH, Chang YC, et al. Impact of the HLA-B(*)58:01 allele and renal impairment on allopurinol-induced cutaneous adverse reactions. J Invest Dermatol. 2016;136:1373–81. 10.1016/j.jid.2016.02.808. [DOI] [PubMed] [Google Scholar]
- 6.Chung WH, Chang WC, Lee YS, Wu YY, Yang CH, Ho HC, et al. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA. 2014;312:525–34. 10.1001/jama.2014.7859. [DOI] [PubMed] [Google Scholar]
- 7.Maubec E, Wolkenstein P, Loriot MA, Wechsler J, Mulot C, Beaune P, et al. Minocycline-induced DRESS: evidence for accumulation of the culprit drug. Dermatology. 2008;216:200–4. 10.1159/000112926. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal KG, Alvarez-Arango S, Fu X, Kroshinsky D, Choi H, Phillips E, et al. Risk factors for vancomycin drug reaction with eosinophilia and systemic symptoms syndrome. JAMA Dermatol. 2022;158:1449–53. 10.1001/jamadermatol.2022.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80. 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 10.Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77:275–89. 10.1007/s00228-020-03005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809–20. 10.1111/j.1398-9995.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 12.Kumar T, Teo I, McCormick BB. Systemic toxicity of intraperitoneal vancomycin. Case Rep Nephrol. 2016;2016:3968690. 10.1155/2016/3968690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melis D, Carbone F, Minopoli G, La Rocca C, Perna F, De Rosa V, et al. Cutting edge: increased autoimmunity risk in glycogen storage disease type 1b Is associated with a reduced engagement of glycolysis in T cells and an impaired regulatory T cell function. J Immunol. 2017;198:3803–8. 10.1177/08968608221080586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishnani PS, Austin SL, Abdenur JE, Arn P, Bali DS, Boney A, et al. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16: e1. 10.1038/gim.2014.128. [DOI] [PubMed] [Google Scholar]
- 15.Kirchhof MG, Wong A, Dutz JP. Cyclosporine treatment of drug-induced hypersensitivity syndrome. JAMA Dermatol. 2016;152:1254–7. 10.1001/jamadermatol.2016.2220. [DOI] [PubMed] [Google Scholar]
- 16.Madigan LM, Fox LP. Vancomycin-associated drug-induced hypersensitivity syndrome. J Am Acad Dermatol. 2019;81:123–8. 10.1016/j.jaad.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Higuchi M, Agatsuma T, Iizima M, Yamazaki Y, Saita T, Ichikawa T, et al. A case of drug-induced hypersensitivity syndrome with multiple organ involvement treated with plasma exchange. Ther Apher Dial. 2005;9:412–6. 10.1111/j.1744-9987.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- 18.Oiwa H, Yoshida S, Okada H, Yasunishi M, Kamidani R, Suzuki K, et al. Atypical drug-induced hypersensitivity syndrome with multiple organ failure rescued by combined acute blood purification therapy: a case report. Int J Emerg Med. 2023;16:33. 10.1186/s12245-023-00511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam E, Lien Y, Kraft W, Kraft W, Piraino B, Vozmediano V, et al. Vancomycin in peritoneal dialysis: clinical pharmacology considerations in therapy. Perit Dial Int. 2020;40:384–93. 10.1177/0896860819889774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Böhler J, Reetze-Bonorden P, Keller E, Kramer A, Schollmeyer PJ. Rebound of plasma vancomycin levels after haemodialysis with highly permeable membranes. Eur J Clin Pharmacol. 1992;42:635–9. 10.1007/BF00265928. [DOI] [PubMed] [Google Scholar]
- 21.Pollard TA, Lampasona V, Akkerman S, Tom K, Hooks MA, Mullins RE, et al. Vancomycin redistribution: dosing recommendations following high-flux hemodialysis. Kidney Int. 1994;45:232–7. 10.1038/ki.1994.28. [DOI] [PubMed] [Google Scholar]
- 22.Waldman RA, Grant-Kels JM. Thinking outside the box: is there a role for extracorporeal blood purification in DRESS syndrome complicated by acute kidney injury? Clin Dermatol. 2020;38:580–3. 10.1016/j.clindermatol.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35–9. 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 24.Li PD, Chow KM, Cho Y, Fan S, Figueiredo AE, Harris T, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;42:110–53. 10.1177/08968608221080586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.