Abstract
Background
Liver cancer stem cells (LCSCs) significantly impact chemo-resistance and recurrence in liver cancer. Dopamine receptor D4 (DRD4) is known to enhance the cancer stem cell (CSC) phenotype in glioblastoma and correlates with poor prognosis in some non-central nervous system tumors; however, its influence on LCSCs remains uncertain.
Methods
To investigate the gene and protein expression profiles of DRD4 in LCSCs and non-LCSCs, we utilized transcriptome sequencing and Western blotting analysis. Bioinformatics analysis and immunohistochemistry were employed to assess the correlation between DRD4 expression levels and the pathological characteristics of liver cancer patients. The impact of DRD4 on LCSC phenotypes and signaling pathways were explored using pharmacological or gene-editing techniques. Additionally, the effect of DRD4 on the protein expression and intracellular localization of β-catenin were examined using Western blotting and immunofluorescence.
Results
DRD4 expression is significantly elevated in LCSCs and correlates with short survival in liver cancer. The expression and activity of DRD4 are positive to resistance, self renewal and tumorigenicity in HCC. Mechanistically, DRD4 stabilizes β-catenin and promotes its entry into the nucleus via activating the PI3K/Akt/GSK-3β pathway, thereby enhancing LCSC phenotypes.
Conclusions
Inhibiting DRD4 expression and activation offers a promising targeted therapy for eradicating LCSCs and relieve chemo-resistance.
Subject terms: Prognostic markers, Phosphoinositol signalling
Introduction
Primary liver cancer ranks as the sixth most prevalent malignant neoplasm and the third most fatal cancer globally, with projections indicating a projected increase of over 55% in its incidence and mortality rates by the year 2040 [1]. Approximately 80% of primary liver cancer tissue subtypes are attributed to hepatocellular carcinoma (HCC). The pronounced heterogeneity of HCC limits the efficacy of targeted therapy and immunotherapy to less than 30% of advanced patients, resulting in a mere 10% 5-year survival rate [2]. Currently, cytotoxic chemotherapy drugs remain the primary treatment for advanced HCC. Nevertheless, the prolonged administration of these drugs often induces severe multidrug resistance (MDR) in patients, rendering the drugs clinically ineffective and contributing to tumor recurrence. Chemotherapy can enrich cancer stem cells (CSCs), which possess strong self-renewal capabilities, unique DNA repair mechanisms, resistance to radiation, and anti-apoptotic properties [3]. In particular, liver cancer stem cells (LCSCs) constitute the CSC subset within liver cancer and are believed to be accountable for the observed heterogeneity, resistance, and recurrence in HCC [4]. Despite notable advancements in CSC treatment, the absence of tumor specificity in current therapeutic targets frequently results in severe toxicities during clinical trials.
Dopamine receptors (DRs) are the primary receptors for neurotransmitters such as dopamine (DA). DRs are mainly present in brain tissue and have a small expression in peripheral tissues. Upon activation, DRs can form polymers and transmit signals through both G protein coupling and non-G protein coupling mechanisms. Currently, there are five known subtypes of DRs, with the DRD1 and DRD5 subtypes classified as D1-type receptors, and the DRD2, DRD3, and DRD4 subtypes classified as D2-type receptors [5]. Recently, thioridazine has been identified as a highly effective anticancer drug in various non-targeted high-throughput small-molecule compound libraries [6–8]. The inhibitory effect of thioridazine on the growth of CSCs has been demonstrated by its ability to block D2-type DRs, while not affecting the activity of human pluripotent stem cells (hPSCs). Moreover, the tumorigenic CSCs express all five subtypes of DRs, whereas the expression level in normal hPSCs is significantly low [6]. These findings suggest that D2-type DRs may play a selective role in regulating the characteristics of CSCs. The existing literature primarily focuses on the role of DRD2 in cancer development, with limited research available on the involvement of DRD4 and DRD3 in tumorigenesis.
It has been proved that high expression of DRD4 is an independent predictor of poor survival in glioblastoma (GBM) [9]. A report suggests that DRD4 expression levels in peripheral blood of breast cancer patients are increased and significantly related to tumor classification and poor prognosis [10]. The polymorphism of DRD4 is associated with an increased risk of non-small cell lung cancer [11]. It has also been shown that DRD4 activation can lead to the reduction of inflammatory response induced by tumor-associated macrophages (TAMs), thus enhancing the chemosensitivity of pancreatic cancer mouse models [12]. Additionally, DRD4 was identified as one of the survival-related candidate genes in colon cancer and colorectal cancer patients by machine learning [13, 14]. These results demonstrate the potential of DRD4 as a therapeutic target in tumors of the central and noncentral nervous systems. However, research on DRD4 in noncentral nervous system tumors generally lacks a specific mechanism explanation. So far, there have been no reports on the relationship between DRD4 and liver cancer.
Here, we found that DRD4 high expression is significantly correlated with adverse pathological characteristics and poor prognosis of liver cancer patients. Targeting DRD4 can reverse multidrug resistance, self-renewal, and tumorigenicity by inhibiting PI3K/Akt/GSK3β mediated the entry of β-catenin into the nucleus of LCSCs. This study suggests that DRD4 is a potential target for alleviating chemotherapy resistance and recurrences caused by LCSCs in advanced liver cancer.
Materials and methods
Cells and chemicals
HepG2 and HuH-7 cells were purchased from the ATCC. HepG2-R and HuH-7-R cells were maintained in a medium with 250 nM adriamycin (Sigma, USA) as described previously [15]. All cell lines have been authenticated using STR profiling and confirmed Mycoplasma-negative periodically. The thioridazine, propionylpromazine-d6 hydrochloride, GSK598809, L745870, A412997, Epirubicin, Vincristine sulfate, Toxoflavin, and GSK-3β inhibitor 1 were purchased from MCE (New Jersey, USA). GDC0941 were provided by APExBIO (Houston, USA).
Quantitative real-time PCR
The experiments were performed as described in our previous study [16]. The total RNA was isolated from cells using TRIzol reagent (Invitrogen). cDNAs were synthesized by reverse transcription kit (Promega) in accordance with the manufacturer’s instructions. The primers used were as follows: 5ʹ-ATGCCCCAATCTTGTCCATCT-3ʹ and 5ʹ--3ʹ for DRD2; 5ʹ- CCAGTTCACTATCAGCATGGC-3ʹ and 5ʹ-CCCCTGTTGTGTTGAAACCAA-3ʹ for DRD3; 5ʹ-CTGCCGCTCTTCGTCTACTC-3ʹ and 5ʹ-ATGGCGCACAGGTTGAAGAT-3ʹ for DRD4; 5ʹ-TTGCTGCTTACATTCAGGTTTCA-3ʹ and 5ʹ-AGCCTATCTCCTGTCGCATTA-3ʹ for ABCB1; 5ʹ-GTGGGGCGCCCCAGGCACCA-3ʹ and 5ʹ-CTTCCTTAATGTCACGCACGATTTC-3ʹ for β-actin.
Vectors and transfection
The small guide RNAs (sgRNAs) for DRD4 were synthesized by BGI (Beijing, China). The primers for sgRNA1# were: 5ʹ-CACCGAACCGCAGCACCGCGGACG-3ʹ and 5ʹ-AAACGTCCGCGGTGCTGCGGTTC-3ʹ; The primers for sgRNA2# were: 5ʹ-CACCGCCGGCCGCGGGGGCATCTG-3ʹ and 5ʹ-AAACAGATGCCCCCGCGGCCGGC-3ʹ. The two sgRNA duplexes were transfected into HepG2-R cells using Lipofectamine 2000 (Invitrogen, USA). A 96-well plate was then used to separate cells by limiting dilution, followed by Western blotting to identify the cells. Stable DRD4-knockout cells were obtained and designed as DRD4-KO 1#. The plasmids DRD4-pCDH-MCS-CMV-EF1-puro were ordered from BGI (Beijing, China). The vectors were transfected into HepG2 cells and selected with puromycin. The DRD4 stable overexpressed single subclone cell lines were named HepG2-D4-7#. The vectors of WT-AKT1-pcDNA3.1-FLAG with a PCR-amplified human AKT1 cDNA and MUT-AKT1-pcDNA3.1-FLAG with mutagenesis of Thr308D/Ser473D were obtained from TranSheep (Shanghai, China).
Western blotting
Whole cellular proteins were extracted using RIPA lysis buffer (No. P0013B, Beyotime, China) containing 500 mM NaCl, 1 mM EDTA, 1% NP-40, 50 mM Tris pH 8.0, and 1×cocktail of protease inhibitors (Roche, Lewes, UK). The protein concentration was examined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). Equal amounts of proteins per sample were isolated by SDS-PAGE and then blotted onto polyvinylidene difluoride (PVDF) membrane and blocked with 5% nonfat milk for 1 h at room temperature. Blots were probed with the indicated primary antibodies overnight at 4 °C and followed by incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody. Protein bands were visualized using the enhanced chemiluminescence (ECL) detection kit (GE Healthcare Biosciences). These antibodies were used for immunoblotting: anti-DRD4 (Thermo Fisher, #PA5-77947), anti-β-catenin (#8480), anti-P-gp (#13978), anti-p-GSK3β(Ser9) (#9322), anti-GSK3β (#12456), anti-p-AKT (#4060), anti-p-β-catenin(Ser552) (#9566), and anti-GAPDH (#5174) were produced by Cell Signaling Technology (CST, USA). Anti-DRD2 (#ab85367), anti-DRD3 (#ab155098), anti-CD133 (#ab216323), anti-BRG1 (#ab110641), and anti-CD24 (#ab179821) antibodies were obtained from Abcam (Cambridge, UK). The antibody against Flag-tag (#66008-2-Ig) was provided by Proteintech Group (Wuhan, China). Isolation of membrane, cytosol, and nuclei of cells was using the Nucl-Cyto-Mem Preparation Kit (P1201) from Applygen (Beijing, China).
RNA-sequencing analysis
In total, 2 × 106 HepG2, HuH-7, HepG2-R, and HuH-7-R cells were plated and cultured for 48 h. Cells were collected with Trizol reagent. The total RNA was processed by NEBNext® Poly(A) mRNA Magnetic Isolation Module to enrich mRNA, and the product RNA was used for construction Library, via KAPA Stranded RNA-Seq Library Prep Kit (Illumina). Sequencing libraries, denatured by 0.1 M NaOH to generate single-stranded DNA, as amplified in situ Illumina cBot (TruSeq SR Cluster Kit v3-cBot-HS (# GD-401-3001, Illumina)). The ends of the generated fragments were used to run 150 Cycles by the Illumina HiSeq 4000 Sequencer. All the experimental steps after the RNA extraction were conducted in Shanghai Biotechnology Co., Ltd., China. RNA sequencing was performed three times. RNA-Seq data may view at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE272591.
MTS assay
CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS, Promega) was used to assess cell proliferation. Briefly, plates with 96 wells were seeded with cell suspensions (3 × 103/well) in triplicate and treated with different doses of agents, then incubated for 48 h. Each well was filled with 10 µl of MTS assay solution and incubated for 3 h. Microplate readers were used to measure optical density at 490 nm.
Sphere-forming assay
1000 cells per well were seeded in ultra-low attachment 24-well plates (Corning, Tewksbury, MA, USA) containing serum-free DMEM/F12 supplemented with B27 (2%, Invitrogen), 20 ng/ml recombinant human EGF (100 ng/ml, PeproTech, USA), recombinant human basic FGF (20 ng/ml, PeproTech, USA), and N2 supplement (1%, Invitrogen). After 10 days of incubation, the number of spheroid colonies was measured under an inverted phase-contrast microscope (CKX41, Japan).
Immunohistochemistry
The 69 Human liver cancer tissue sections were collected from Yihe Hospital Affiliated to Henan University (Zhengzhou, China). All patients provided written informed consent. The study protocol was approved by the Institutional Review Board of Henan University (ID: HUSOM2023-222) and complied with the Helsinki Declaration. The primary antibodies of DRD4 (DF4866, Affinity Biosciences, Australia), p-Akt (#4060, CST, USA), β-catenin (#8480, CST, USA), and P-gp (#13978, CST, USA) were incubated to slides at a dilution of 1:100-1:400 at 4 °C overnight. Nuclei were counterstained with hematoxylin. After washing the primary antibodies with PBS, incubated the slides at room temperature for 1 h with the secondary antibodies from the two-step immunohistochemistry detection kit (PV-9000, ZSGB-Bio, China). The signals were developed by the solutions of a DAB chromogenic kit (ZLI-9017, ZSGB-Bio, China) for about 5 minutes and stained with Mayer’s Hematoxylin Stain Solution (G1080, Solarbio, China) for 1 minute. Then, the tissues were dehydrated sequentially with anhydrous ethanol at a concentration ranging from 75% to 100%, with each concentration lasting for 3 minutes. After being transparent with xylene for about 15 minutes, it was sealed with neutral gum. The slides were photographed with a microscope system (Olympus BX53, Japan). Quantification analysis of the images was using Image-Pro Plus 6.0.
Bioinformatics analysis
Transcriptome and clinical data were downloaded from the Cancer Genome Atlas (TCGA) (https://portal.gdc.cancer.gov/) and the Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/). Utilizing online software Sangerbox 3.0 to analyze the mRNA expression levels of DRD4 in different stages of liver cancer tissues. The gene sets files (.GMT) of adriamycin_RESISTANCE and LIVER_CANCER_ STEM_ CELL_UP were downloaded from MSigdb (http://software.broadinstitute.org/gsea/msigdb/index.jsp) and analyzed by the Gene set enrichment analysis (GSEA) (http://software.broadinstitute.org/gsea). The correlation between DRD4 expression levels and survival time in liver cancer patinets (n = 365) was analyzed online by the Human Protein Atlas (HPA) (https://www.proteinatlas.org/).
In vivo studies
The animal procedures conducted in this study were ethically approved by the Henan University institutional Experimental Animal Care and Use Committee (ID: HUSOM2023-221). All experiments adhered to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines, the UK Animals (Scientific Procedures) Act of 1986, and its associated guidelines, as well as the European Union (EU) Directive 2010/63/EU for animal experiments. The temperature for animal feeding is 21–25 °C, the relative humidity is 40–60%, natural light is used, and regular feed is used. During the experiment, mice were free to drink and eat, with 12 h of light exposure and 12 h of no light exposure per day. Five weeks old male athymic BALB/c nude mice grade of SPF were purchased from Peking Vital River Laboratory Animal Technology. To determine the impact of knocking-out DRD4 to tumorigenesis of HepG2-R cells in vivo, the mice were randomly blinded divided into two groups by a random number table and identified by ear tags. Each group contained five mice. A total of 0.1 ml HepG2-R-scramble and HepG2-R-DRD4-KO 1# cell suspension (5 × 106 cells/ml) was injected subcutaneously in the right upper flank of the mice. Forty days following tumor implantation, mice were sacrificed. Animal license number SCXK (Beijing) 2021-0006. Primary tumors were dissected, weighted, photographed, and fixed in formalin.
Statistical analysis
The data were presented as Mean ± SD/SEM. A T test was employed to compare the results between two groups. For the analysis of samples consisting of three or more groups, a one-way ANOVA and the Bonferroni post hoc test were utilized. Statistical significance was determined as a p value less than 0.05.
Results
DRD4 is significantly expressed in resistant hepatocellular carcinoma cells
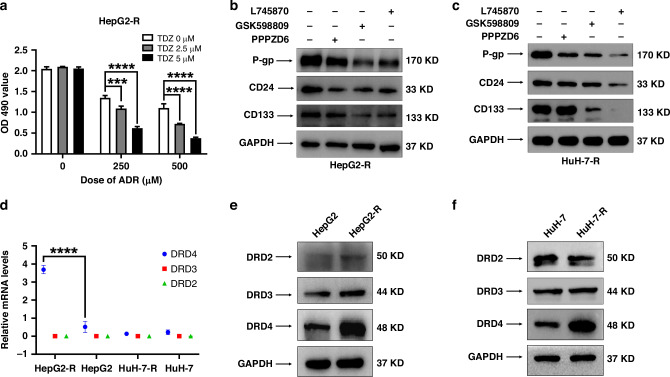
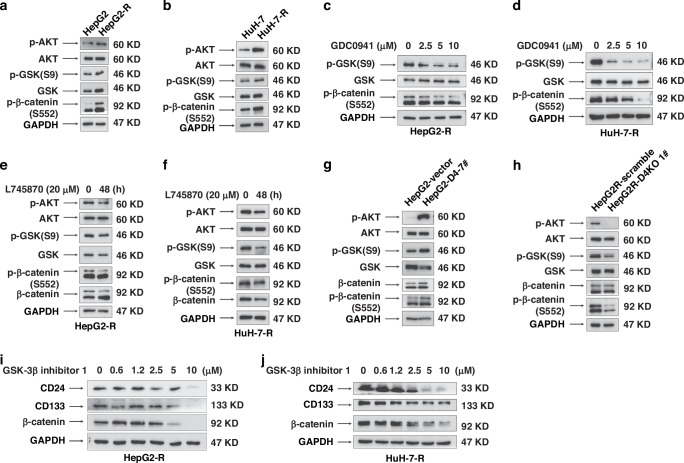
In the previous study, we acquired two HCC cell lines, namely HepG2-R and HuH-7-R, which exhibited heightened levels of multidrug resistance and displayed more pronounced attributes of CSCs when compared to their corresponding parental cell lines, HepG2 and HuH-7 [15]. To explore whether D2-type DRs contribute significantly to HCC drug resistance, we employed thioridazine, a known antagonist of D2-type DRs, as well as adriamycin, either individually or in combination, to treat HepG2-R cells. The administration of thioridazine alone did not impede cell proliferation. However, it notably augmented the susceptibility of HepG2-R cells towards adriamycin (Fig. 1a). To further detect which subtype of D2-type DRs plays a primary role in regulating the expression of CSC biomarkers in HepG2-R cells. HepG2-R cells were treated with propionylpromazine-d6 hydrochloride (a selective agonist of DRD2), GSK598809 (a selective agonist of DRD3), and L745870 (a selective agonist of DRD4) for 48 h, respectively. The Western blotting analysis demonstrates that the protein expression of P-gp, CD24, and CD133 in HepG2-R cells (Fig. 1b) is significantly reduced by treatment with 20 μM GSK598809 and 20 μM L745870 for 48 h. Comparable results were observed in HuH-7-R cells (Fig. 1c).
Fig. 1. DRD4 expression was significantly increased in HepG2-R and HuH-7-R cells.
a HepG2-R cells were seeded at a density of 3 × 103 cells per well in 96-well plates and cultured overnight. The cells were exposed to 250 nM and 500 nM adriamycin (ADR) and 20 μM thioridazine (TDZ) either individually or in combination for a duration of 48 h. The viability of these cells was assessed using an MTS assay, measuring the optical density at 490 nm (OD490 value). Compare the proliferative ability of each group using Graphpad Prism 8.0. by one-way ANOVA. ****p < 0.0001. b, c HepG2-R and HuH-7-R cells were treated with 20 μM of propionylpromazine-d6 hydrochloride(PPPZD6), GSK598809, and L745870 for 48 h, respectively and then analyzed by Western blotting. d The data of relative mRNA levels of DRD4, DRD3, and DRD2 in HepG2-R, HepG2, HuH-7-R, and HuH-7 cells from RNA-seq was analyzed by Graphpad Prism 8.0. ****p < 0.0001. Western blotting was employed to analyze the protein expression levels of DRD2, DRD3, DRD4, and GAPDH in HepG2-R (e) and HuH-7-R (f) cells, in comparison to their respective parental cells.
The gene expression profiles of HepG2-R, HepG2, HuH-7-R, and HuH-7 cells were analyzed by transcriptome sequencing. As the RNA-seq dataset showed that DRD4 mRNA expression levels were dramatically higher in HepG2-R cells than in HepG2. However, DRD3 and DRD2 mRNA expression were almost undetectable in both HepG2-R, HepG2, HuH-7-R, and HuH-7 cells (Fig. 1d). Then we examined the protein expression of D2-type DRs in the parental and resistant HCC cell lines. Compared with HepG2 cells, HepG2-R cells showed markedly elevated protein levels of DRD4 but not DRD2 and DRD3 (Fig. 1e). Similarly, only DRD4 was observed higher expressed in HuH-7-R than in HuH-7 cells (Fig. 1f). These data suggest a critical role of DRD4 in regulating chemotherapy resistance and CSC phenotypes in HCC.
DRD4 is overexpressed and associated with poor outcomes in liver cancer patients
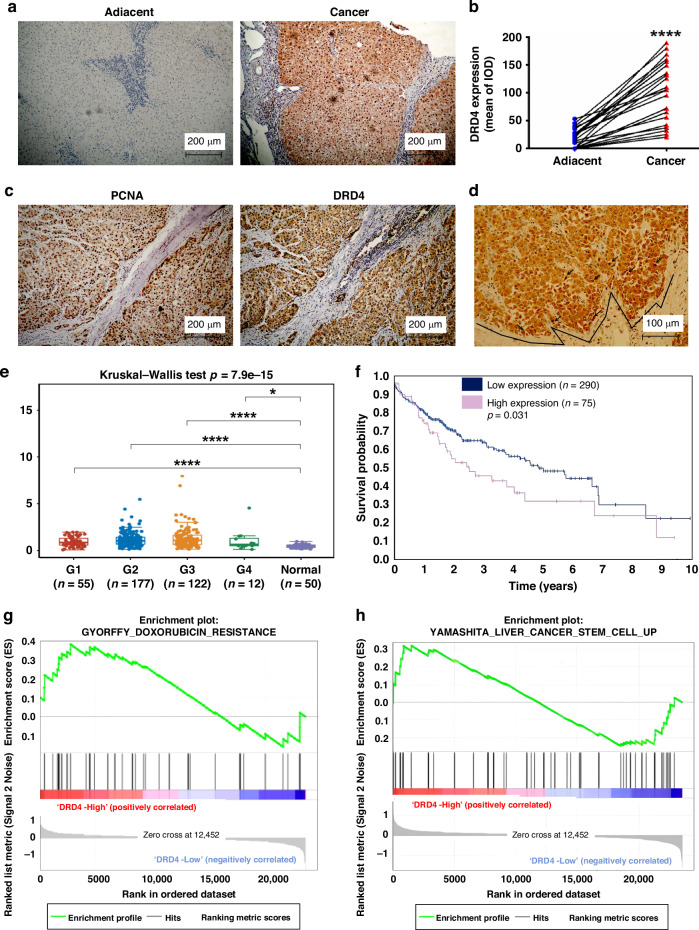
The expression levels of DRD4 in 21 paired liver cancer samples were analyzed using immunohistochemical methods. There was no detectable or low expression of DRD4 in relatively normal liver tissue. In contrast, moderate strong staining of DRD4 was observed in 13 (61.9%) of liver cancer tissues (n = 69) (Fig. 2a). The statistical results showed that there was a significant difference in DRD4 expression between liver cancer and adjacent tissues (p < 0.0001) (Fig. 2b). Staining of serial sections of liver cancer specimens revealed that the positive expression regions of DRD4 (Fig. 2c) are similar to the positive stained area of proliferating cell nuclear antigen (PCNA) (Fig. 2d). Moreover, it was observed that cells overexpressing DRD4 exhibited signs of mitotic activity, as depicted in Fig. 2d. Notably, the expression of DRD4 was specifically localized at the invasive margin of cancerous tissues, indicating a potential association between DRD4 expression and invasiveness. These findings provide evidence suggesting a potential role of DRD4 expression in the oncogenic progression of liver cancer.
Fig. 2. DRD4 is highly expressed in liver cancer specimens and associated with poor prognosis.
a The representative images of immunohistochemistry staining with DRD4 antibody from the adjacent non-cancerous tissues (left) or cancer tissues (right) of HCC patients. b Quantitative analysis of Fig. 2A by Image Pro Plus and t tests. ****p < 0.0001. c PCNA and DRD4 was examined by IHC on serial sections of liver cancer tissues. d The overexpressing region of DRD4 in mitosis (arrow) and the invasive front of the cancer tissue. e DRD4 expression in different histological grades of liver cancer and normal liver tissues from the TCGA database were analyzed by software Sangerbox 3.0 (http://vip.sangerbox.com/), *p < 0.05, ****p < 0.0001. f Correlation between DRD4 protein expression and the 5-year survival analysis of liver cancer. Data were from The Human Protein Atlas (https://www.proteinatlas.org/ENSG00000069696-DRD4). g, h The application of Gene Set Enrichment Analysis (GSEA) on a cohort of liver cancer patients, specifically those found in the GSE112790 database from the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO), revealed a significant enrichment of signals associated with chemotherapy resistance and stem cell characteristics. This enrichment was observed due to the elevated expression levels of DRD4.
The gene expression matrix of liver cancer patients (n = 366) and matched normal data (n = 50) in the Cancer Genome Atlas (TCGA) database was analyzed using the online software Sangerbox 3.0. The data showed that DRD4 mRNA expression levels in pathological grades 1, 2, 3, and 4 of liver cancer tissues were significantly higher than that in normal liver tissues (Fig. 2e). Patients with liver cancer were categorized into groups with high or low DRD4 mRNA expression levels. In terms of 5-year survival rates, the group with high DRD4 expression had a significantly lower survival rate than the group with low DRD4 expression (p < 0.05) (Fig. 2f). Furthermore, the GSEA results of GSE112790 (n = 183) from the GEO database revealed that liver cancer patients in the DRD4 high expression group exhibited an abundance of genes associated with adriamycin resistance and liver cancer stem cells, in contrast to the DRD4 low expression group (Fig. 2g, h). These findings suggest a strong correlation between elevated DRD4 expression and unfavorable outcomes, resistance to treatment, and the transformation of CSCs in liver cancer.
A subsequent analysis of DRD4 expression and clinical characteristics of LIHC (n = 366) in TCGA was conducted using SPASS 20.0 software. The findings presented in Table 1 indicate a significant association between high expression of DRD4 and various patient characteristics, including age, gender, tumor histological classification, pathological stage, vascular tumor cell type, and tissue prospective collection indicator. These results provide strong evidence supporting a positive correlation between DRD4 expression and adverse pathological features of liver cancer, suggesting its potential as a clinical prognostic biomarker for this disease.
Table 1.
Clinicopathologic characteristics of liver cancer patients with high and low DRD4 expression.
| Characteristics | Number | DRD4 Low cases (%) | DRD4 High cases (%) | P value |
|---|---|---|---|---|
| Age(year) | ||||
| ≤60 | 172 | 141 (82.0) | 31 (18.0) | 0.013 |
| >60 | 205 | 186 (91) | 19 (9.3) | |
| Gender | ||||
| Male | 255 | 231 (90.6) | 24 (9.4) | 0.001 |
| Female | 122 | 96 (78.7) | 26 (21.3) | |
| T classification | ||||
| T1 | 185 | 168 (90.8) | 17 (9.2) | 0.033 |
| T2 | 95 | 82 (86.3) | 13 (13.7) | |
| T3 | 81 | 62 (76.5) | 19 (23.5) | |
| T4 | 13 | 12 (92.3) | 1 (7.7) | |
| N classification | ||||
| N0 | 257 | 221 (86.0) | 36 (14.0) | 0.611 |
| N1 | 4 | 3 (75.0) | 1 (25.0) | |
| NX | 115 | 102 (88.7) | 13 (11.3) | |
| Metastasis | ||||
| M0 | 272 | 232 (85.3) | 40 (14.7) | 0.268 |
| M1 | 4 | 3 (75.0) | 1 (25.0) | |
| Mx | 101 | 92 (91.1) | 9 (8.9) | |
| Vital_status | ||||
| live | 286 | 245 (85.7) | 41 (14.3) | 0.276 |
| dead | 91 | 82 (90.1) | 9 (9.9) | |
| Neoplasm_histologic_grade | ||||
| G1 | 55 | 53 (96.4) | 2 (3.6) | 0.075 |
| G2 | 180 | 155 (86.1) | 25 (13.9) | |
| G3 | 124 | 102 (82.3) | 22 (17.7) | |
| G4 | 13 | 12 (92.3) | 1 (7.7) | |
| Pathologic_stage | ||||
| Stage I | 175 | 159 (90.9) | 16 (9.1) | 0.038 |
| Stage II | 87 | 75 (86.2) | 12 (13.8) | |
| Stage III | 86 | 67 (77.9) | 19 (22.1) | |
| Stage IV | 5 | 4 (20.0) | 1 (20.0) | |
| Vascular_tumor_cell_type | ||||
| None | 210 | 194 (92.4) | 16 (7.6) | 0.003 |
| Micro | 94 | 75 (79.8) | 19 (20.2) | |
| Macro | 17 | 13 (76.5) | 4 (23.5) | |
| Tissue_prospective_collection_indicator | ||||
| NO | 249 | 224 (90.0) | 25 (10.0) | 0.01 |
| YES | 128 | 103 (80.5) | 25 (19.5) | |
Activation or overexpression of DRD4 promotes multiple drug resistance and self-renewal of liver cancer cells
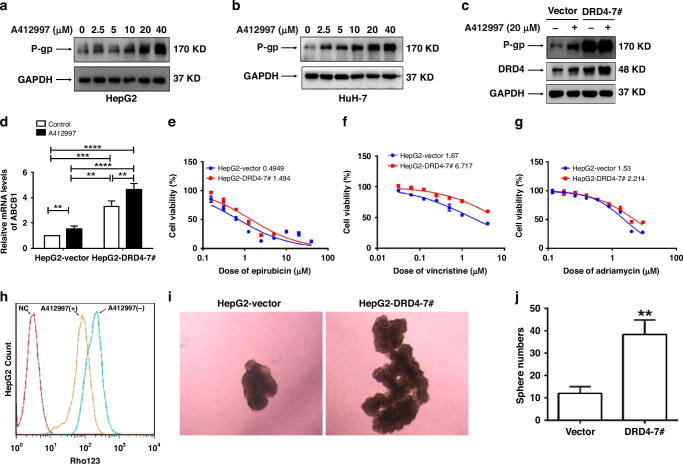
To determine the role of DRD4 in the chemotherapy resistance of HCC cells, we stimulated HepG2 and HuH-7 cells with different doses (0, 2.5, 5, 10, 20, and 40 µM) of A412997 (a specific agonist of DRD4). After 48 h, the whole cell lysate was collected. As the data presented in Fig. 3a, b, it is evident that the upregulation of P-gp expression is notably observed with the escalation of A412997 stimulation dosage. To further investigate the regulatory effect of DRD4 on P-gp expression, we constructed monoclonal HepG2-D4-7# cell lines exogenously overexpressed DRD4 and HepG2-Vector cells expressing an empty vector as a control. As shown in Fig. 3c, overexpression of DRD4 can significantly enhance the protein expression of P-gp. After treatment with A412997 20 µM for 48 h, the expression levels of P-gp in HepG2-DRD4-7# cells were further enhanced. These results fully demonstrate that the activation or over-expression of DRD4 can positively regulate P-gp expression.
Fig. 3. DRD4 activation and overexpression promote multidrug resistance and self-renewal in hepatocellular carcinoma cells.
a, b HepG2 and HuH-7 cells were treated with a gradient doses of A412997 for 48 h, the expression levels of P-gp and GAPDH were detected by western blotting. c Western blotting was used to detect the expression levels of P-gp, DRD4, and GAPDH proteins in HepG2-Vector and HepG2-DRD4-7 # cells. d HepG2-Vector and HepG2-DRD4-7 # cells were treated with or without 20 μM A412997 for 48 h. The relative mRNA levels of ABCB1 were analyzed using quantitative real-time PCR. e–g MTS were used to detect the IC50 of HepG2-Vector and HepG2-DRD4-7 # cells against epirubicin, vincristine, and adriamycin. h HepG2 cells were treated with or without 10 μM A412997 for 48 h.Then incubation with 5 μM Rho123 for 0.5 h. Flow cytometry was used to assess Rho123 accumulation. HepG2 null cells served as negative controls. i A total of 2 × 103 HepG2-Vector and HepG2-DRD4-7 # cells were cultured in serum-free media on ultra-low attachment plates for a duration of 10 days, and photographs were captured using a phase contrast microscope. j Counting results of cell microspheres in Fig. 3H. **p < 0.01, ***p <0.001, ****p<0.0001.
Accordingly, the MTS assay displayed that compared to HepG2-Vector cells, HepG2-D4-7 # cells showed approximately 2.14-3.90-fold, 4.02-6.25-fold, and 1.46-1.71-fold increases in the 50% inhibitory concentrations (IC50) values for doxorubicin, sorafenib, and epirubicin, respectively (Fig. 3e–g). These results indicate that DRD4 over-expression can reduce the sensitivity of HCC cells to some chemotherapy drugs. After treatment with 10 µM or 20 µM A412997 for 48 h, the average fluorescence intensity of Rho123 solution in HepG2 cells was significantly reduced, indicating that A412997 stimulation can remarkably enhance the drug efflux ability, which is consistent with the result of increased P-gp expression caused by DRD4 activation (Fig. 3h).
To investigate the impact of DRD4 on the self-renewal capacity of HCC cells in vitro, we conducted spheroid colony formation assays by culturing HepG2-Vector and HepG2-D4-7# cells in nonadherent conditions with serum-free media. After two weeks of culturing, the growth of spherical colonies, which is considered indicative of self-renewal ability, was observed. Consistent with expectations, HepG2-D4-7# cells exhibited significantly larger and more spheroid colonies compared to HepG2-Vector cells (Fig. 3i, j). These results demonstrate that an elevated level of DRD4 expression or activation can result in robust multi-drug resistance and self-renewal capabilities in HCC cells.
Knocking out or inhibiting DRD4 activation can reverse the chemotherapy resistance and tumorigenicity of hepatocellular carcinoma cells
To verify the significance of DRD4 activity and expression in the drug resistance and CSC phenotypes of HCC cells, we applied different concentrations of L745870 to treat HepG2-R and HuH-7-R cells for 48 h. Western blotting analysis showed that L745870 significantly decreased the protein expression of P-gp, CD24, and CD133. The expression of DRD4 was reduced, too (Fig. 4a, b). We obtained a DRD4 knockout cell line named DRD4-KO 1# by CRISPR/cas 9 that targeted the first exon sequence of DRD4 in HepG2-R cells, followed by screening with antibiotic and single cell subcloning. Compared with the scramble cells which were transfected with only empty plasmids, the levels of P-gp, CD24, and CD133 in DRD4-KO 1 # cells were decreased with the knockout of DRD4 (Fig. 4c).
Fig. 4. DRD4 knockout or inhibition reverses drug resistance and CSC-like phenotypes in HCC cells.
a HepG2-R cells were treated with 20 μM L745870 for 48 h. The protein levels of DRD4, P-gp, CD24, CD133, and GAPDH were determined by immunoblotting. b HuH-7-R cells were treated with 20 μM L745870 for 48 h. The protein levels of DRD4, P-gp, CD24, CD133, and GAPDH were determined by immunoblotting. c The levels of DRD4, P-gp, CD24, CD133, and GAPDH in HepG2-R-Scramble and HepG2-R-DRD4-KO-1# cells were determined by immunoblotting. d–f Using MTS to measure the IC50 of HepG2-R-Scramble and HepG2-R-DRD4-KO-1# cells against adriamycin, vincristine, and epirubicin. g HepG2-R cells were treated with or without L745870 (10 μM) for 48 h. Flow cytometry was used to exame the drug efflux ability of these cells. The null HepG2-R cells were used as a negative control. h 0.5 × 103 HepG2-R-Scramble and HepG2-R-DRD4-KO-1# cells were cultured for 14 days and take photos thougth phase contrast microscope. i Counting results of cell microspheres in Fig. 4H. ***p < 0.001. j, k Balb/c nude mice were subcutaneously grafted with 5 × 106 HepG2-R-Scramble and HepG2-R-DRD4-KO-1# cells (n = 5/group), respectivelly. Tumor growth was monitored (j). On the 40th day, tumors were photographed (k).
As shown in Fig. 4d–f, the IC50 of DRD4-KO 1# cells to adriamycin, vincristine, and epirubicin was significantly reduced compared to scramble cells, with sensitivity increased by 8.19-16.46 folds, 13.53-37.57 folds, and 3.45-4.025 folds, respectively. (P < 0.01, **, P < 0.01, **, P < 0.01, **, Two-tailed) Correspondingly, after 48 h of treatment with L745870 at 20 µM, the average fluorescence intensity of Rho123 in HepG2-R cells was significantly elevated, indicating that L745870 can significantly promote drug accumulation in drug-resistant cells by inhibiting the activity of DRD4 (Fig. 4g). The microsphere formation ability of DRD4-KO 1# cells was significantly reduced compared to scramble cells (Fig. 4h, i). We implanted HepG2-R-scramble cells as well as HepG2-R-DRD4-KO 1# cells into nude mice, at a number of 5 × 106 cells per mouse. Forty days after implantation, all mice in the HepG2-R-scramble group showed obvious tumor formation with the average tumor volume of 1073.33 mm3, while the other group did not have subcutaneous tumor nodules formed in the mice. There is a significant difference in tumor volume between the two groups (p < 0.001) (Fig. 4J, K). These data evidenced that DRD4 is a potential target for reversing chemotherapy resistance and tumorigenicity in HCC.
DRD4 promotes β-catenin accumulation in the nucleus via PI3K/Akt signaling pathway
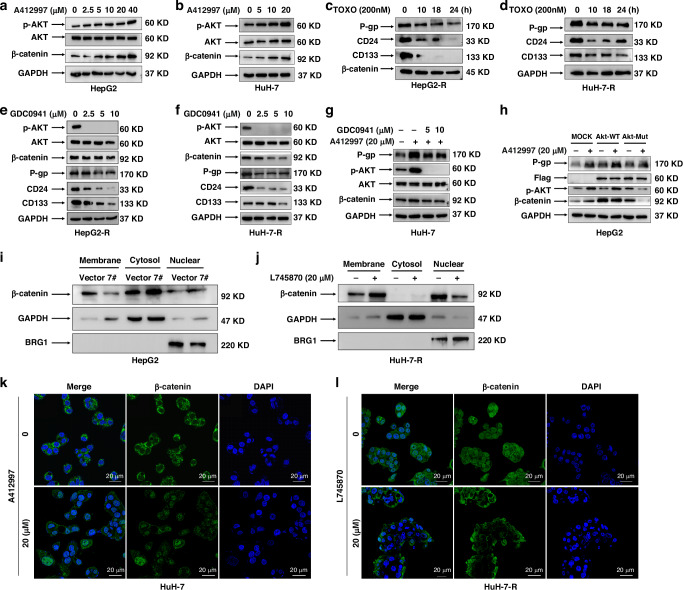
DRD4 is a member of the G protein-coupled receptor (GPCR) family, which mediates a series of complex signal cascades to achieve extracellular signal transmission. To elucidate the molecular mechanism by which DRD4 regulates the MDR and CSC-like phenotypes of liver cancer, we analyzed the phosphorylation status of PI3K/Akt, Wnt/β-catenin, JAK/STAT3, MAPK/ERK, and NF-kB are all known to play a key role in chemotherapy resistance and tumorigenesis [16]. Intriguingly, stably elevated expression of p-Akt and β-catenin was seen posttreatment with various concentrations of DRD4 agonist A412997 (0, 2.5, 5, 10, 20 or 40 μM) for 48 h in HepG2 cells (Fig. 5a). While the phosphorylation status of other signals was not obviously changed (the data was not shown). Similar data was obtained in HuH-7 cells (Fig. 5b). Therefore, we focus on clarifying the relationship between DRD4, p-Akt, and β-catenin in the preservation of the CSC phenotypes in HCC cells.
Fig. 5. DRD4 enhances β-catenin expression in the nucleus via PI3K/Akt signaling pathway.
a, b HepG2 and HuH-7 cells were treated with the indicated concentrations of A412997 for 48 h and detected using immunoblotting. c, d A dose of 200 nM Toxoflavin was applied for three hours to HepG2-R and HuH-7-R cells and detected by western blotting. e, f HepG2-R and HuH-7-R cells were treated with the indicated concentration of GDC0941 for 48 h. The protein expression levels of p-AKT, AKT, β-catenin, P-gp, CD24, CD133, and GAPDH were detected. g HuH-7 cells were treated with or without GDC0941 (5 and 10 µM) in the presence or absence of 20 µM A412997 and then analysed by Western blotting. h HuH-7 cells were transfected with empty plasmids, AKT wild-type plasmids, and AKT mutant plasmids respectively and cultured for 60 h. Cells were treated with 20 µM A412997 and GDC0941 (0, 5, and 10 µM) for 24 h. The protein expression of P-gp, Flag, p-AKT, β-catenin and GAPDH was detected by Western blotting. h The membrane, cytoplasm, and nuclear proteins of HepG2-Vector and HepG2-DRD4-7 # cells were extracted, and the expression of β-catenin was detected by Western blotting. Using GAPDH as the internal reference for cytoplasmic proteins and BRG1B as the internal reference for nuclear proteins. i, j The membrane, cytoplasm, and nuclear proteins of HepG2-Vector, HepG2-D4-7# cells and HuH-R cells which were treated with 20 µM L745870 for 48 h were extracted. The detection of β-catenin expression was performed using Western blotting analysis. Using GAPDH as the internal reference for cytoplasmic proteins and BRG1B as the internal reference for nuclear proteins. k, l HuH-7 cells were subjected to a 48-h treatment with 20 µM A412997, while HuH-7-R cells underwent a similar 48-h treatment with 20 µM L745870. The localization of β-catenin within the cells was assessed through immunofluorescence. Bar = 20 µm.
As Fig. 5c, d shows that 200 nM of toxoflavin could remarkably decrease the expression level of P-gp, CD24, and CD133 in a time-dependent manner (Fig. 5c, d). Dose course analyses revealed that the administration of GDC0941, an inhibitor of PI3Kα/δ, for 48 h resulted in the suppression of p-Akt, β-catenin, P-gp, CD24, and CD133 levels. This observation indicates the inhibitory impact of PI3K on Akt activation and β-catenin expression (Fig. 5e, f). These data demonstrate that both PI3K/Akt and β-catenin signalings are vital to the CSC biomarkers expression of HCC cells. Furthermore, the up-regulation effect of A412997 on P-gp, p-Akt, and β-catenin expression could be effectively blocked by 5 μM and 10 μM GDC0941 pretreatment for 2 h (Fig. 5g), implying that the DRD4 signal transmission is mediated by PI3K/Akt/β-catenin pathway.
β-catenin is the principal mediator of intercellular adhesion and Wnt signaling, which is integral to cell proliferation, differentiation, and apoptosis [17]. Based on the investigation conducted by Fang et al., it is posited that the regulation of AKT is dependent on the participation of β-catenin, thereby exerting a crucial influence on tumor development [18]. To verify the effect of AKT activation induced by A412997 on β-catenin expression, HepG2 cells were transfected with WT- or MUT- AKT plasmids for 60 h and then stimulated with A412997 20 µM for 24 h. Compared with cells transfected with empty plasmids of pcDNA3.1, the exogenous expression of Akt (Flag-tagged) was obviously enhanced in cells transfected with WT-AKT and MUT-AKT plasmids (Fig. 5h). Treatment with A412997 20 µM for 24 h can increase P-gp, p-Akt, and β-catenin expression by the mock and WT-AKT, but not the AKT Thr308D/Ser473D mutant (Fig. 5h). According to these results, Akt activation mediates DRD4 activation-induced LCSC biomarker expression.
It is known that AKT kinase mediates β-catenin (Ser552) phosphorylation and activation, leading to β-catenin separates from the cell membrane complex and accumulates in the cytoplasm and nucleus, and enhances its binding with 14-3-3ƫ through a binding motif containing Ser552. The interaction between them plays a regulatory role in transcription factors and promotes the malignant transformation of tumor cells [19]. To examine whether DRD4 affected β-catenin translocation through activating AKT, detection of β-catenin subcellular distribution in HepG2-Vector and HepG2-D4-7 # cells was performed. Overexpression of DRD4 resulted in translocation of a portion of β-catenin from cell-cell contacts into the cytosol and nucleus. Compared to control cells, β-catenin is less expressed on the membrane and more expressed in the cytoplasm and nucleus of HepG2-D4-7 # cells (Fig. 5i). Similarly, inhibition of DRD4 with L745870 20 µM for 48 h was capable of maintaining β-catenin expression on the membrane and reduce its expression in the nucleus (Fig. 5j). Immunofluorescence was used to investigate the relationship between DRD4 activation and cellular localizations of β-catenin. The treatment with 20 μM A412997 for 48 h resulted in a notable increase in the cytosolic and nuclear localization of β-catenin in HuH-7 cells, whereas in the absence of treatment, β-catenin was primarily localized at cell-cell contacts (Fig. 5k). Similarly, L745870 20 µM administration for 48 h led to an increase in cytosolic and nuclear β-catenin, while reducing its nuclear accumulation in HuH-7-R cells (Fig. 5l). These findings provide evidence that DRD4 can facilitate AKT phosphorylation, thereby causing the dissociation of β-catenin from the cell membrane and its subsequent entry into the nucleus.
DRD4 upregulates p-β-catenin(Ser552) and p-GSK3β(Ser9) expression to promote the nucleation of β-catenin
According to reports, PI3K/Akt can activate glycogen synthesis kinase 3β (GSK3β) when Wnt signaling is in its inactive state [20]. Activation of GSK3β promotes phosphorylation of the N-terminal domain of β-catenin and then degrades through the ubiquitin/proteasome pathway. The activation of Wnt or Akt signals can promote GSK3β phosphorylation at the Ser9 site which leads to the activity of GSK3β being reduced, thereby inhibiting the phosphorylation of Ser33, Ser37, and Thr41 sites of β-catenin dependent by GSK3β. The stable and low-phosphorylated β-catenin is released from the Axin complex, translocated to the nucleus, and interacts with transcription factors of the TCF/LEF-1 family, leading to an increase in target gene transcription, inducing dormant CSCs to transform into active CSCs.
In accordance with Fig. 6a, b, high expression of p-GSK3β (Ser9) and p-β-catenin(Ser552) are present in HepG2-R and HuH-7-R cells. We treated HepG2-R and HuH7-R cells with 20 µM L745870 for 48 h, respectively. The results showed that compared to untreated cells, the application of GDC0941 treatment can reduce the expression of p-β-catenin(Ser552) and p-GSK3β (Ser9) in a dose-dependent manner (Fig. 6c, d). The addition of L745870 can achieve the same effect as GDC0941(Fig. 6e, f). Thus, DRD4 can positively regulate PI3K/Akt mediate the phosphorylation of GSK3β(Ser9) and β-catenin (Ser552) in HepG2-R and HuH-7-R cells.
Fig. 6. DRD4 upregulates p-β-catenin(Ser552) and p-GSK3β(Ser9) expression to promote the nucleation of β-catenin by activating PI3K/Akt.
a, b The protein of HepG2, HepG2-R, HuH-7, and HuH-7-R cells were extracted and detected by Western blotting. c, d Western blotting detection of protein expression in HepG2-R and HuH-7-R cells treated with different concentrations of GDC0941. e, f HepG2-R and HuH-7-R cells were treated with 20 µM L745870 followed by detected with Western blotting. g, h The protein expression in HepG2-Vector, HepG2-DRD4-7#, HepG2-R-Scramble, and HepG2-R-DRD4-KO-1# cells was determined by Western blotting. i, j HepG2-R and HuH-7-R cells were incubating with specified amounts of GSK-3β inhibitor1 for 48 h, they were subsequently assessed using Western blotting.
The data from Fig. 6g, h demonstrate a significant increase in the expression of p-GSK (Ser9) and p-β-catenin (Ser552). Notably, the expression of these proteins exhibited an inverse relationship in HepG2-R-Scramble and HepG2-R-DRD4-KO-1 # cells. These results suggest a positive correlation between the expression level of DRD4 and GSK (Ser9) and β-catenin (Ser552). We further investigate the role of GSK3βin the CSC phenotypes of HepG2-R and HuH-7-R cells. Apply different concentrations of GSK3β Inhibitor1 treated the two cell lines for 48 h, respectively. Then, collect the whole cell protein lysate for immunoblotting detection. In the following Fig. 6i, j, with the dosage of GSK3β Inhibitor1 increased, the expression of P-gp, CD24, and CD133 was continuously downregulated. Therefore, GSK3β May play an important role in the maintenance of LCSC phenotypes induced by DRD4.
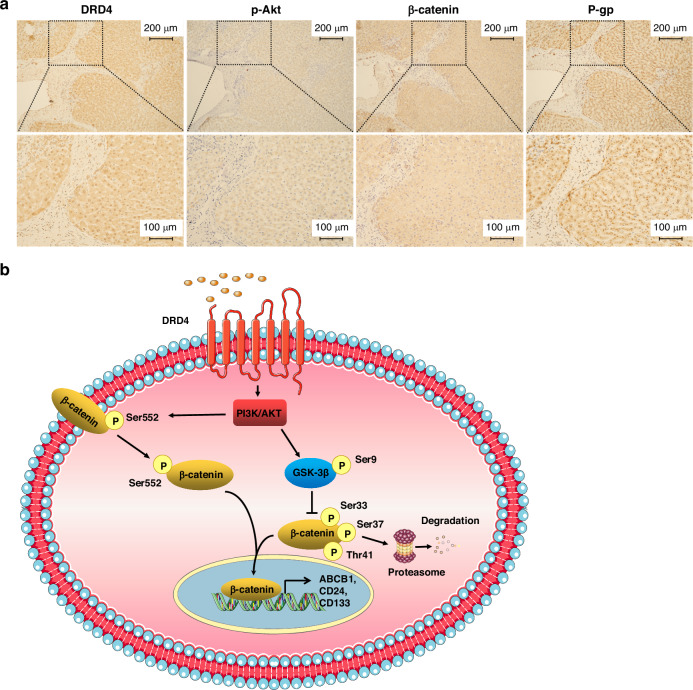
In light of the cellular-level findings discussed above, we speculate that Akt/β-catenin mediate the downstream signals of DRD4 and promote MDR and CSC phenotypes in HCC. To validate this hypothesis in vivo, immunohistochemical staining was performed on liver cancer clinical samples. In the region of liver cancer tissue where DRD4 staining is positive, p-Akt, β-catenin, and P-gp are co-expressed (Fig. 7a). This result suggests that DRD4/Akt/β-catenin signaling axis is also present in human liver cancer tissues.
Fig. 7. DRD4, p-Akt, β-catenin, and P-gp are coexpressed in human liver cancer tissues.
a Immunohistochemical detection the expression of DRD4, p-Akt, β-catenin, and P-gp in human liver cancer patients samples. Bar = 200 μM (upper) and Bar = 100 μM (lower). b Schematic diagram of the mechanism of DRD4 regulates cancer stem-like phenotypes in human liver cancers: the activation of DRD4 improves protein stability of β-catenin and facilitates the translocation of β-catenin into the nucleus via activating p-GSK-3β(Ser9)and p-β-catenin (Ser552) through PI3K/Akt signaling pathway, leading to overtranscriptional expression of CSC markers and consequent chemotherapy resistance.
Discussion
The presence of CSCs has been established as significantly associated with the advancement of chemotherapy resistance, radiation resistance, recurrence, and metastasis in various tumor types, including liver cancer. Consequently, CSCs have been recognized as a crucial focus for tumor treatment. Currently, the primary clinical drugs employed for tumor treatment primarily target rapidly dividing and differentiated tumor cells, exhibiting limited sensitivity towards CSCs. However, efforts have been made to develop small molecule drugs, vaccines, antibodies, and CAR-T cells (chimeric antigen receptor T cells) specifically designed to target CSCs. Some of these therapeutic interventions have already reached the stage of clinical testing and implementation [21–23]. The occurrence of serious adverse events and treatment-related toxicity in clinical trials is mainly due to the presence of known biomarkers of CSCs in normal cells within the body. Consequently, the identification of specific biomarkers is crucial in effectively targeting CSCs.
The peripheral nerves serve as a vital constituent of the cellular microenvironment, facilitating the transmission of local environmental cues to the central nervous system. Moreover, the growth of local nerve terminals assumes a significant role in fostering tissue development, repair, and regeneration [24]. These processes exhibit comparable mechanisms to the initiation and progression of malignant cells. Within the tumor microenvironment, growth factors and neuropeptides can elicit the proliferation and aberrant activation of nerve terminals within tumors [25, 26]. The atypical activation of sympathetic and parasympathetic nerves can prompt the generation of CSCs [27, 28]. The role of neurotransmitters and their receptors in tumors is receiving increasing attention, but it is far from being fully studied.
DA serves as the predominant catecholamine neurotransmitter within the brain, being synthesized by nerve cells or synapses. A seminal investigation demonstrated the capacity of dopamine decarboxylase inhibitors to impede the proliferation of melanoma cells, thereby establishing the initial evidence for the therapeutic potential of DA in peripheral tissue tumors [29]. DRs are recognized targets for psychiatric disorders, and their roles in central nervous system tumors such as pituitary tumors and gliomas have been reported. The roles of DRs in noncentral tumors exhibit tissue selectivity and complexity, as evidenced by various reports.
Dolma et al. found that DRD4 antagonists L-741742 and PNU-96415E can selectively impede the stem cell viability and clonality of GBM without affecting normal neural stem cells by inhibiting the downstream effectors PDGFRβ, ERK1/2, and mTOR, thereby disrupting the activation of the autophagy lysosome pathway and apoptosis resistance of cells [9]. Wen et al. reported that the novel small molecule compound LCC-09 reverses the stemness of GBM by inhibiting DRD4/Akt/mTOR signaling [30]. In this study, we initially discovered that DRD4 shows promise as a potential therapeutic target for HCC. We observed a significant correlation between high expression of DRD4 and an unfavorable prognosis in patients with liver cancer. Additionally, our findings suggest that the activation or excessive expression of DRD4 can lead to an augmented drug efflux and the formation of microspheres within HCC cells. In contrast, the inhibition of DRD4 can result in a substantial enhancement of chemotherapeutic efficacy.
Similarly to Wen et al.‘s findings in GBM, our study demonstrates that the DRD4/PI3K/Akt pathway is also activated in HCC cells. However, our study elucidates a discernible mechanism involving GSK-3β/β-catenin, rather than mTOR signaling. As depicted in Fig. 7B, the activation of DRD4 promotes the phosphorylation of GSK-3β (Ser9), which increasing β-catenin nuclear translocation. Our result showed that the overexpression or activation of DRD4 in HCC cells leads to an increase in the phosphorylation of β-catenin at Ser552, which in turn enhances its expression in both the cytoplasm and nucleus. Consequently, this study confirms a previously unidentified β-catenin signaling pathway, independent of Wnt, which effectively governs through the involvement of DRD4. However, it remains unclear whether Wnt signaling is involved in this process, as well as whether there is any crosstalk between DRD4 and Wnt/β-catenin signaling pathways. The focus of this study is primarily on the regulatory signals that occur after the activation of DRD4, while the mechanism responsible for the upregulation and activation of DRD4 upstream has not yet been revealed. The in vivo experimental approach in this study is limited because immunocompromised mice have been used, therefore the role of the immune system cannot be ruled out, and only subcutaneous implantation of HCC cells has been performed. The application of NCG mice with severe immunodeficiency and other implantation methods such as orthotopic implantation for tumorigenesis experiments will provide better support for the conclusions of this study.
In summary, our research substantiates the potential of DRD4 as a viable therapeutic target in LCSCs. Additionally, we present experimental evidence supporting the feasibility of pharmacological intervention targeting DRD4, thereby offering valuable insights for the advancement of resistance-attenuating strategies in liver cancer.
Acknowledgements
We sincerely appreciate the contributions from the TCGA, GEO, GSEA, and HPA databases.
Author contributions
ZY, PZ, YZ, RG, JH, QW, and HL participated in the experimental data collection; ZZ provided technical assistance; ZR designed experiments and drafted the manuscript; SL, YH, and DC helped revise the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Youth Fund of National Natural Science Foundation of China (Nos. 81803575 and 31902287), Key R&D and Promotion Projects of Henan Province (No.242102310467), Key Specialized Research and Promotion Project of Henan Province in 2023 (No. 232102311205), Henan Medical Science and Technology Research Program Project (No. LHGJ20210801), Program for Innovative Talents of Science and Technology in Henan Province (No. 23HASTIT043), College Students Innovation and Entrepreneurship Training Program of Henan University (No. 20231022007).
Data availability
Almost all data generated or analysed during this study are included in this manuscript and additional files, while others are available from the corresponding author on reasonable request, except for the information related to patient privacy. RNA-Seq data generated in this study are available on Gene Expression Omnibus (GEO) with accession number GSE272591 at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE272591.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The experiments involving animal studies and patient specimens were reviewed and approved by the Ethics Committee of the Medical School of Henan University (HUSOM2023-221 and HUSOM2023-222). Informed consent was obtained from all participants involved in the study. All authors agree with the content of the manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zhengyan Yang, Pai Zhang, Yiwei Zhao.
Contributor Information
Zhiguang Ren, Email: Renzhiguang66@outlook.com.
Yanzhong Hu, Email: hyz@henu.edu.cn.
Daxiang Cui, Email: daxiangcui@126.com.
References
- 1.Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peitzsch C, Tyutyunnykova A, Pantel K, Dubrovska A. Cancer stem cells: the root of tumor recurrence and metastases. Semin Cancer Biol. 2017;44:10–24. [DOI] [PubMed] [Google Scholar]
- 4.Liu YC, Yeh CT, Lin KH. Cancer stem cell functions in hepatocellular carcinoma and comprehensive therapeutic strategies. Cells. 2020;9:1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garau L, Govoni S, Stefanini E, Trabucchi M, Spano PF. Dopamine receptors: pharmacological and anatomical evidences indicate that two distinct dopamine receptor populations are present in rat striatum. Life Sci. 1978;23:1745–50. [DOI] [PubMed] [Google Scholar]
- 6.Sachlos E, Risueno RM, Laronde S, Shapovalova Z, Lee JH, Russell J, et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149:1284–97. [DOI] [PubMed] [Google Scholar]
- 7.Rho SB, Kim BR, Kang S. A gene signature-based approach identifies thioridazine as an inhibitor of phosphatidylinositol-3’-kinase (PI3K)/AKT pathway in ovarian cancer cells. Gynecol Oncol. 2011;120:121–7. [DOI] [PubMed] [Google Scholar]
- 8.Cheng HW, Liang YH, Kuo YL, Chuu CP, Lin CY, Lee MH, et al. Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Dis. 2015;6:e1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolma S, Selvadurai HJ, Lan X, Lee L, Kushida M, Voisin V, et al. Inhibition of dopamine receptor d4 impedes autophagic flux, proliferation, and survival of glioblastoma stem cells. Cancer Cell. 2016;29:859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akbari ME, Kashani FL, Ahangari G, Pornour M, Hejazi H, Nooshinfar E, et al. The effects of spiritual intervention and changes in dopamine receptor gene expression in breast cancer patients. Breast Cancer. 2016;23:893–900. [DOI] [PubMed] [Google Scholar]
- 11.Campa D, Zienolddiny S, Lind H, Ryberg D, Skaug V, Canzian F, et al. Polymorphisms of dopamine receptor/transporter genes and risk of non-small cell lung cancer. Lung Cancer. 2007;56:17–23. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Zhang R, Zhang X, Liu J, Wu H, Li Y, et al. Dopamine improves chemotherapeutic efficacy for pancreatic cancer by regulating macrophage-derived inflammations. Cancer Immunol Immunother. 2021;70:2165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CJ, Baek B, Cho SH, Jang TY, Jeon SE, Lee S, et al. Machine learning with in silico analysis markedly improves survival prediction modeling in colon cancer patients. Cancer Med. 2023;12:7603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Wu S, Peng Y, Zhao Y, Dong Y, Ran F, et al. Constructing a cancer stem cell related prognostic model for predicting immune landscape and drug sensitivity in colorectal cancer. Front Pharm. 2023;14:1200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, Wan W, Zhang P, Wang S, Zhao Z, Xue J, et al. Crosstalk between heat shock factor 1 and signal transducer and activator of transcription 3 mediated by interleukin-8 autocrine signaling maintains the cancer stem cell phenotype in liver cancer. J Gastroenterol Hepatol. 2023;38:138–52. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanying W, Lei H, Ying D, Pingping Z, Guanling H, Jianjun L, et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. 2015;16:413–25. [DOI] [PubMed] [Google Scholar]
- 18.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amable G, Martinez-Leon E, Picco ME, Di Siervi N, Davio C, Rozengurt E, et al. Metformin inhibits beta-catenin phosphorylation on Ser-552 through an AMPK/PI3K/Akt pathway in colorectal cancer cells. Int J Biochem Cell Biol. 2019;112:88–94. [DOI] [PubMed] [Google Scholar]
- 20.Soutto M, Peng D, Katsha A, Chen Z, Piazuelo MB, Washington MK, et al. Activation of beta-catenin signalling by TFF1 loss promotes cell proliferation and gastric tumorigenesis. Gut. 2015;64:1028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekaii-Saab T, El-Rayes B. Identifying and targeting cancer stem cells in the treatment of gastric cancer. Cancer Am Cancer Soc. 2017;123:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Li W, Huang K, Zhang Y, Kupfer G, Zhao Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J Hematol Oncol. 2018;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng KC, Guo YL, Liu Y, Dai HR, Wang Y, Lv HY, et al. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol. 2017;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boilly B, Faulkner S, Jobling P, Hondermarck H. Nerve dependence: from regeneration to cancer. Cancer Cell. 2017;31:342–54. [DOI] [PubMed] [Google Scholar]
- 25.Faulkner S, Jobling P, March B, Jiang CC, Hondermarck H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 2019;9:702–10. [DOI] [PubMed] [Google Scholar]
- 26.Zahalka AH, Estape AA, Maryanovich M, Nakahara F, Cruz CD, Finley L, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358:321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, Verhaegen ME, et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16:400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wick MM, Kramer RA, Gorman M. Enhancement of L-dopa incorporation into melanoma by dopa decarboxylase inhibition. J Invest Dermatol. 1978;70:358–60. [DOI] [PubMed] [Google Scholar]
- 30.Wen YT, Wu AT, Bamodu OA, Wei L, Lin CM, Yen Y, et al. A novel multi-target small molecule, LCC-09, inhibits stemness and therapy-resistant phenotypes of glioblastoma cells by increasing miR-34a and deregulating the DRD4/Akt/mTOR signaling axis. Cancers (Basel). 2019;11:1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Almost all data generated or analysed during this study are included in this manuscript and additional files, while others are available from the corresponding author on reasonable request, except for the information related to patient privacy. RNA-Seq data generated in this study are available on Gene Expression Omnibus (GEO) with accession number GSE272591 at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE272591.