Abstract
A 46-year-old Japanese man was referred to our hospital because of a marked increase in his eosinophil count (22,870 /μL) and elevated liver enzyme levels. Computed tomography (CT) showed thrombi measuring approximately 8 cm in both femoral veins. A liver biopsy revealed eosinophilic infiltration, hepatocyte necrosis, fibrosis, and multiple thrombi. We suspected acute liver injury and deep vein thrombosis associated with hypereosinophilic syndrome and initiated steroids and heparin treatment. Four days after starting treatment, the patient experienced sudden chest pain and cardiopulmonary arrest. CT revealed bilateral pulmonary artery thrombosis, and despite administration of a tissue plasminogen activator, the patient died.
Keywords: hypereosinophilic syndrome, acute liver injury, pulmonary embolism
Introduction
Hypereosinophilic syndrome (HES) is a leukoproliferative disorder characterized by marked eosinophilia of unknown origin and organ damage due to tissue eosinophilia (1). According to the World Health Organization (WHO) diagnostic criteria, HES is considered a diagnosis of exclusion, and it is necessary to exclude diseases that cause secondary eosinophilia, such as allergies, parasites, and malignancies. HES is diagnosed when the peripheral blood eosinophil count is >1.5×109/L, there is no increase in peripheral blood or bone marrow blasts, and eosinophil clonality is not demonstrated (2). The organ infiltration of eosinophils includes the lymph nodes, skin, gastrointestinal tract, lungs, and liver (3), and acute hepatitis may occur if the liver is infiltrated (4).
Venous thromboembolism (VTE) is a complication of HES, affecting approximately 25% of patients, with a mortality rate of 5-10% (5). To date, many types of thrombosis have been reported, including pulmonary embolism (PE), deep vein thrombosis (DVT), intracardiac thrombosis, inferior vena cava thrombosis, portal vein thrombosis, and cerebral vein thrombosis. However, PE, especially DVT, is a rare but fatal complication (6-10).
We herein report a case of HES with acute liver injury and bilateral PE associated with DVT.
Case Report
A 46-year-old man was referred to our hospital because of nausea and anorexia for the previous 5 days. Upon admission, he had a slight fever (37°C) and pain in both thighs, in addition to gastrointestinal symptoms. He was 175.4 cm tall, weighed 82.4 kg, and had a body mass index (BMI) of 26.78 kg/m2 but was muscular rather than obese. His blood pressure, pulse rate, respiratory rate, and oxygen saturation were normal. The patient had a history of cholecystectomy for gallbladder stones four years previously. Preoperative laboratory data at that time showed an increase in the number of eosinophils [white blood cells 13,700 /μL, eosinophils 3,068 /μL (22.4%)], but the FIP1-like-1 (FIP1L1)-platelet-derived growth factor receptor a (PDGFRa) fusion gene, which is associated with chronic eosinophilic leukemia, was negative. The patient received no specific treatment for eosinophilia and was followed up. He had drug allergies to bepotastine besylate and mequitazine but no bronchial asthma or atopic dermatitis. He had not recently taken any medications and had no family history of liver or blood disorders.
The laboratory data at admission are shown in Table. The white blood cell count and eosinophil count were markedly increased, and liver enzyme levels were elevated. A decrease in platelet count and prothrombin time (PT, %) and an increase in fibrin were noted. Hepatitis B surface antigen, hepatitis C virus antibody, antinuclear antibody, antimitochondrial antibody, parasite eggs, and antiparasitic antibody were not detected. Computed tomography (CT) revealed atrophy of the left hepatic lobe, enlargement of the right hepatic lobe, and an extensive irregular area with contrast enhancement in S4/8 of the liver (Fig. 1a). No bile duct obstruction or periportal collar sign was observed. High-density areas of approximately 8 cm, which appeared to be thrombi, were observed in both femoral veins (Fig. 1b). Lymphadenopathy from the periportal and bilateral iliac regions to the groin. No abnormalities were observed in the lung field.
Table.
Laboratory Data on Admission.
| Peripheral blood | Biochemistry | ||||||||||||
| WBC | 31,700 | /μL | TP | 7.4 | g/dL | NH3 | 50 | µg/dL | |||||
| Neu | 18.1 | % | Alb | 2.9 | g/dL | HbA1c | 5.4 | % | |||||
| Eo | 72.3 | % | T-Bil | 2.7 | mg/dL | FBS | 89 | mg/dL | |||||
| Ba | 0.7 | % | D-Bil | 1.3 | mg/dL | ||||||||
| Lym | 5.7 | % | AST | 146 | U/L | Immunological markers | |||||||
| Mon | 3.2 | % | ALT | 181 | U/L | ANA | <40 | ||||||
| RBC | 4.56×106 | /μL | LDH | 499 | U/L | AMA-M2 | <5.0 | index | |||||
| Hb | 14.2 | g/dL | ALP | 538 | U/L | IgG | 2,060 | mg/dL | |||||
| Plt | 6.7×104 | /μL | γ-GTP | 381 | U/L | IgA | 454 | mg/dL | |||||
| Amy | 31 | U/L | IgM | 600 | µg/dL | ||||||||
| Coagulation | CK | 48 | U/L | IgE | 8,857 | U/L | |||||||
| PT | 53 | % | BUN | 10.5 | mg/dL | sIL-2R | 2,999 | U/mL | |||||
| APTT | 47.4 | s | Cr | 0.68 | mg/dL | ||||||||
| FDP | 25.3 | μg/mL | Na | 135 | mEq/L | Infection markers | |||||||
| D-dimer | 8.6 | μg/mL | K | 4.6 | mEq/L | HBsAg | (-) | ||||||
| Fib | 90 | mg/dL | Cl | 102 | mEq/L | Anti-HCV | (-) | ||||||
| Ca | 8.2 | mg/dL | Parasite eggs | (-) | |||||||||
| CRP | 11.7 | mg/dL | |||||||||||
WBC: white blood cell, Neu: neutrophil, Eo: eosinophil, Ba: basophil, Lym: lymphocyte, Mon: monocyte, RBC: red blood cell, Hb: hemoglobin, Plt: platelet, PT: prothrombin time, APTT: activated partial thromboplastin time, FDP: fibrinogen/fibrin degradation product, Fib: fibrinogen, TP: total protein, Alb: albumin, T-Bil: total bilirubin, D-Bil: direct bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyltransferase, Amy: amylase, CK: creatine kinase, BUN: blood urea nitrogen, Cr: creatinine, CRP: C-reactive protein, FBS: fasting blood sugar, ANA: anti-nuclear antibody, AMA: anti-mitochondrial antibody, Ig: immunoglobulin, sIL-2R: soluble interleukin-2 receptor, HBsAg: hepatitis B surface antigen, Anti-HCV: antibody against hepatitis C virus
Figure 1.
Images of abdominal contrast-enhanced computed tomography (a, b), esophagogastroduodenoscopy (c, d) and Hematoxylin and Eosin staining from a bone marrow puncture (e, f) on admission. (a) Atrophy of the left hepatic lobe, enlargement of the right hepatic lobe, and an extensive irregular area with contrast enhancement in the liver were found. No bile duct obstruction or periportal collar sign was observed. (b) High-density thrombi approximately 8 cm in length in both femoral veins were found (arrows). Lymphadenopathy at the hilum of liver and that from bilateral iliac regions to the groin were found. (c) No esophagitis was observed. (d) Redness and erosion of the mucosa throughout the stomach were found. A biopsy from the stomach revealed few eosinophils. (e, f) The bone marrow was slightly hyperplastic, and the morphology of the eosinophilia was normal. No numerical or morphological findings in megakaryocytes and no chromosomal abnormalities were found. (e) Low magnification. (f) High magnification.
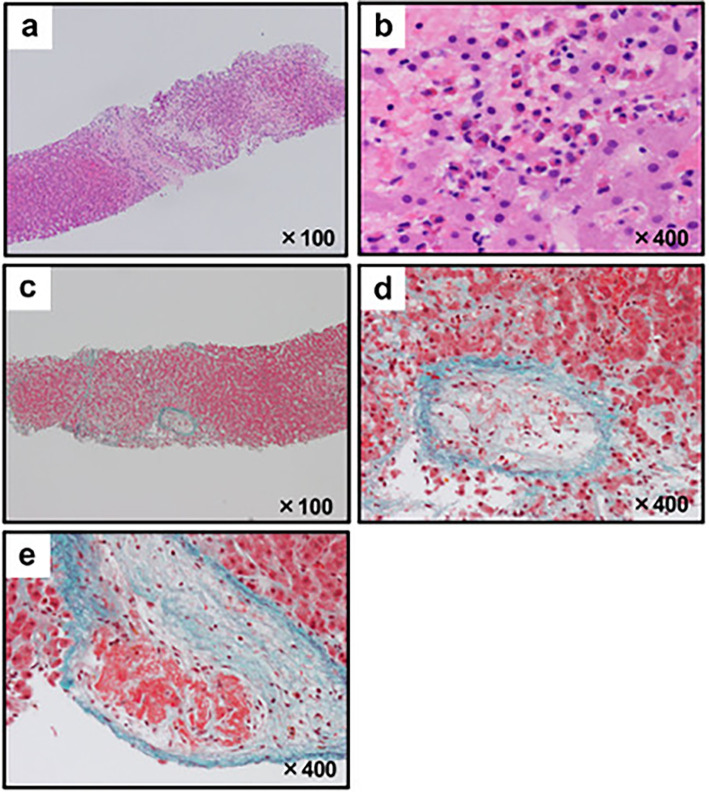
Esophagogastroduodenoscopy revealed no esophagitis (Fig. 1c); however, redness and erosion of the mucosa were observed throughout the stomach (Fig. 1d). Eosinophilic gastritis was suspected, but a biopsy revealed a few eosinophils. A bone marrow puncture examination revealed normal eosinophilia morphology with slightly hyperplastic bone marrow (Fig. 1e, f). There were no numerical or morphological findings in the megakaryocytes, and no chromosomal abnormalities were observed. A liver biopsy revealed infiltration of eosinophils, ischemic changes in hepatocytes, fibrosis, and loss of hepatocytes due to necrosis (Fig. 2a, b). In addition, thrombus formation was observed in several central veins (Fig. 2c, d). Laboratory data and bone marrow findings ruled out eosinophilic leukemia, granulomatous eosinophilic polyangiitis, and parasitic diseases.
Figure 2.
Microphotographs from the liver biopsy. (a, b) Hematoxylin and Eosin staining showed the infiltration of eosinophils, ischemic changes in hepatocytes, fibrosis and loss of hepatocytes due to necrosis. (a) Low magnification. (b) High magnification. (c-e) Elastica-Masson staining with high magnification showed fibrosis and thrombus formation in several central veins. (c) Low magnification. (d, e) High magnification.
One day after hospitalization, intravenous steroid administration was initiated to treat HES with acute liver injury due to eosinophilic infiltration. In general, HES is treated mainly with steroids, but there is no standard dose, with some studies reporting prednisolone at 1 mg/kg and others reporting a median maximum dose of 40 mg/day (11). Considering that the eosinophil count was high, but the liver damage was not severe, prednisolone was started at 40 mg/day. Heparin (20,000 U/day) was intravenously administered for femoral vein thrombosis associated with HES. Because the activated partial thromboplastin time (APTT) was greater than 40 s (42.7-47.4 s), heparin was continued at the same dose. After starting steroids, eosinophils and liver enzymes tended to improve, but 6 days after the start of heparin (9 days after hospitalization), sudden chest pain and dyspnea developed, and cardiopulmonary arrest occurred within a few minutes. CT revealed thrombi in both pulmonary arteries (Fig. 3a, b), and we diagnosed him with acute PE associated with DVT. Cardiopulmonary resuscitation and tissue plasminogen activator (t-PA) as thrombolytic therapy were initiated, but resuscitation was not possible, and the patient unfortunately died.
Figure 3.
Computed tomography images at the onset of pulmonary embolism. Arrows indicate thrombi in both pulmonary arteries. (a) Right pulmonary artery. (b) Left pulmonary artery.
The clinical course of the patient from admission is shown in Fig. 4.
Figure 4.
Clinical course during hospitalization. WBC: white blood cells, Eo: eosinophils, ALT: alanine transaminase, PSL: prednisolone
Discussion
We encountered a rare case of HES, acute liver injury, and PE associated with DVT in a patient who died soon after hospitalization. Liu et al. reported that of 63 HES patients with VTE, 59 (93.7%) had DVT, 27 of whom had PE. All cases were mild, and remission was achieved with heparin therapy alone. In addition, the BMI (cutoff value 24.1 kg/m2), eosinophil count (cutoff value 6.2×103/μL), and disease duration (cutoff value 13.9 months) were listed as risk factors for DVT associated with HES (3). The higher the risk factors, the more likely thrombus formation is to occur. Assuming that eosinophilia had persisted for 4 years without scrutiny and treatment, the disease duration was longer than 13.9 months, and the eosinophil count was also applicable, suggesting that the risk of DVT was high. DVT in this case was approximately 8 cm long on both sides and was fatal because it embolized the pulmonary arteries on both sides.
HES is frequently associated with multiple organ damage, and 30-32% of cases are complicated by liver injury (12,13). Although Budd-Chiari syndrome may be associated with HES in some cases and requires a differential diagnosis, no definite mechanism of liver injury has been proposed (14). Several cases of HES leading to chronic liver damage have been reported, and eosinophil control with steroid therapy is recommended in such cases (4,15). Budd-Chiari syndrome was ruled out on CT in this case. Liver injury and atrophy of the left lobe had not been observed four years previously, but a liver biopsy revealed necrosis and fibrosis, suggesting the possibility of progression to chronic liver injury. Multiple thrombi, ischemic changes, and necrosis in the liver tissue suggest that thrombi might be involved in liver injury. CT did not reveal a large thrombus in the portal vein or inferior vena cava, but it was suspected that there might have been microthrombi, which might have been the cause of uneven enhancement in the liver. Furthermore, it is possible that the atrophy of the left lobe might have been caused by thrombi.
Several mechanisms for thrombus formation by HES have been reported. A cationic protein contained in eosinophils is thought to stimulate thrombus formation by binding to heparin generated in the body, neutralizing its anticoagulant effect, and reducing the clotting time by interacting with factor XII (16). Direct microvascular endothelial cell injury by eosinophil basic proteins has also been reported to cause thrombus formation (17). Activated eosinophils are known to induce inflammation by releasing various substances such as cytokines, chemokines, lipid mediators, and cytotoxic granule proteins (18). As described above, various possible mechanisms of thrombus formation have been reported, which are considered to be high-risk and require appropriate follow-up. In addition, it would be useful to search for other thrombogenic factors, such as protein C/S, lupus anticoagulant, and anti-cardiolipin antibody, which have not been investigated in this case. VTE associated with HES is rare and mild; however, it can sometimes be severe and progress rapidly, as in this case. We considered an inferior vena cava (IVC) filter for DVT, but the patient developed a pulmonary embolus prior to the procedure. Although t-PA was administered as thrombolytic therapy, it was not effective and did not save the patient's life. When a patient is diagnosed with DVT, immediate placement of an IVC filter or transportation to an advanced critical care center should be considered.
In conclusion, we described a rare case of HES with acute liver injury and PE associated with DVT. In the treatment of HES, it is important to check for liver injury and DVT, and it has been suggested that thrombi may lead to PE and should be evaluated and treated immediately and appropriately.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol 97: 129-148, 2022. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Ed. In: Leukemia. The 5(th) Edition of the World Health Organization Classification of Hematolymphoid Tumors. Exon Publications, Brisbane, 2022 [Internet]. [cited 2023 Sep 29]. Available from: https://doi.org/10.36255/exon-publications-leukemia. [PubMed]
- 3.Liu Y, Meng X, Feng J, Zhou X, Zhu H. Hypereosinophilia with concurrent venous thromboembolism: clinical features, potential risk factors, and short-term outcomes in a Chinese cohort. Sci Rep 10: 8359, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawamura T, Hiraoka A, Toshimori A, et al. A possible case of hepatitis due to hypereosinophilic syndrome. Intern Med 55: 1453-1458, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Ogbogu PU, Rosing DR, Horne MK. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin 27: 457-475, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallardo C, Mancinetti M, Périard D, Hayoz D. Thrombosis of palmar and interdigital arteries in the hypereosinophilic syndrome. Vasa 43: 222-224, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Fujita K, Ishimaru H, Hatta K, Kobashi Y. Hypereosinophilic syndrome as a cause of fatal thrombosis: two case reports with histological study. J Thromb Thrombolysis 40: 255-259, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Gao SJ, Wei W, Chen JT, et al. Hypereosinophilic syndrome presenting with multiple organ infiltration and deep venous thrombosis: a case report and literature review. Medicine (Baltimore) 95: e4658, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boussir H, Ghalem A, Ismaili N, El Ouafi N. Eosinophilic myocarditis and hypereosinophilic syndrome. J Saudi Heart Assoc 29: 211-213, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J, Huang X, Zhou W, et al. Thrombosis in the portal venous system caused by hypereosinophilic syndrome: a case report. Medicine (Baltimore) 97: e13425, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol 124: 1319-1325. e3, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fauci AS, Harley JB, Roberts WC, Ferrans VJ, Gralnick HR, Bjornson BH. NIH conference. The idiopathic hypereosinophilic syndrome. Clinical, pathophysiologic, and therapeutic considerations. Ann Intern Med 97: 78-92, 1982. [DOI] [PubMed] [Google Scholar]
- 13.Weller PF, Budley GJ. The idiopathic hypereosinophilic syndrome. Blood 83: 2759-2779, 1994. [PubMed] [Google Scholar]
- 14.Inoue A, Michitaka K, Shigematsu S, et al. Budd-Chiari syndrome associated with hypereosinophilic syndrome; a case report. Intern Med 46: 1095-1100, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Minola E, Sonozogni A. Chronic hepatitis in hypereosinophilic syndrome: report of an usual case. Infez Med 13: 182-186, 2005. [PubMed] [Google Scholar]
- 16.Spry CJ, Davies J, Tai PC, Olsen EG, Oakley CM, Goodwin JF. Clinical features of fifteen patients with the hypereosinophilic syndrome. Q J Med 52: 1-22, 1983. [PubMed] [Google Scholar]
- 17.Narayan S, Ezughah F, Standen GR, Pawade J, Kennedy CT. Idiopathic hypereosinophilic syndrome associated with cutaneous infarction and deep venous thrombosis. Br J Dermatol 148: 817-820, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Ohnishi H, Miyahara N, Gelfand EW. The role of leukotriene B4 in allergic diseases. Allergol Int 57: 291-298, 2008. [DOI] [PubMed] [Google Scholar]