Abstract
Stress can lead to gut dysbiosis in brain-gut axis disordered diseases as irritable bowel syndrome (IBS), yet the mechanisms how stress transfer from the brain to the gut and disrupt gut microbiota remain elusive. Here we describe a stress-responsive brain-to-gut axis which impairs colonocytes’ mitochondria to trigger gut dysbiosis. Patients with IBS exhibit significantly increased facultative anaerobes and decreased obligate anaerobes, related to increased serum corticotropin-releasing hormone (CRH) level and defected colonocytes’ mitochondria ultrastructure. Mice exposed to acute stress experienced enhanced CRH-CRH receptor type 1 (CRHR1) signaling, which impaired mitochondria and epithelium hypoxia in the colon, subsequently triggered gut dysbiosis. Antagonizing CRHR1 expression to inhibit cAMP/Ras/MAPK signaling or activating mitochondria respiration conferred resilience against stress-induced mitochondria damaging and epithelium hypoxia impairment, ultimately improving gut dysbiosis. These results suggest that the CRH-CRHR1-mitochondria pathway plays a pivotal role in stress-induced gut dysbiosis that could be therapeutically targeted for stress-induced gastrointestinal diseases.

Yiming Zhang et.al report that psychological stress activated Corticotropin-releasing hormone (CRH)-CRH receptor type 1 (CRHR1)-mitochondria pathway to trigger gut dysbiosis and reveal CRHR1 upregulation damages mitochondria via cAMP/Ras/MAPK signaling in colonocytes.
Subject terms: Next-generation sequencing, Cellular microbiology
Introduction
Psychological stress triggers brain-gut communication disorder and gut dysbiosis, which are associated with the development and progression of gastrointestinal diseases, such as irritable bowel syndrome (IBS)1,2. However, the mechanism of how stress transfers from the brain to the gut remains elusive3–5. Corticotropin-releasing hormone (CRH), primarily secreted from neurons in the paraventricular nucleus (PVN), is a well-known mediator of the neuroendocrine and visceral responses to stress. CRH exerts its effects through the activation CRH receptor type 1 and 2 (CRHR1/2), altering colonic motility and inducing visceral hypersensitivity6,7. Notably, one experiment reported that stress-induced CRHR1 activation can leads to strong but reversible modification of mitochondria dynamics and morphology, these changes which are associated with bioenergetic defects and reduction of neuronal activity8. Mitochondria is a dynamic organelle that easily responds to environmental stimuli and cellular energy requirements9. Recent insights suggest that colonocytes’ mitochondria respiration serves as a control switch, mediating the shift between gut homeostasis and dysbiosis10. During homeostasis, colonocytes’ mitochondria oxidative phosphorylation (OXPHOS) results in epithelial hypoxia, which maintains a microbiota dominated by obligate anaerobes. Conditions that damage mitochondria increase epithelial oxygenation, driving an expansion of facultative anaerobes, and increasing the risks of intestinal inflammation11–13. Emerging studies reveal the involvement of colonocyte mitochondria dysfunction in the pathophysiology of intestinal diseases14. Accurately, damaged mitochondria could signal inflammasome activation, leading to the production of pro-inflammatory cytokine in IBD. Moreover, mitochondria mutation enhances proliferation and metastatic potential while decreasing apoptosis, driving colorectal cancer (CRC) tumorigenesis15–17.
In this study, we unveil the CRH-CRHR1-mitochondria pathway as a crucial mechanism that conveys psychological stress from the brain to the gut and triggers gut dysbiosis. Utilizing metagenomics, transcriptomics, Oxygeaph-2k oroboros (O2K) technology, Seahorse Xfe24 technology, and non-invasive micro-test technology (NMT), we identify mitochondria respiration as a stress-responsive relay signal that shifts strains from obligate to facultative anaerobes. We confirm this correlation in patients with IBS and an acute stress mouse model, and provide sufficient evidence from both in vitro and in vivo experiments that decreasing CRHR1 expression and activating mitochondria can both improve stress-induced gut dysbiosis. Together, these findings cooperatively reveal a gut microbiota regulatory pathway involving mitochondria decision-making that underlies stress-driven brain-gut comorbidity, such as IBS.
Methods
Human studies
All subjects participated in this study did so voluntarily and provided written informed consent. The study adhered to the principles outlined in the Declaration of Helsinki and received approval from the Peking University Third Hospital Medical Science Research Ethics Committee (protocol number IRB00006761-M2021081). Subjects meeting the ROME IV criteria for IBS were recruited from the Department of Gastroenterology, Peking University Third Hospital, Beijing, China. Written informed consent was obtained from all patients prior to study enrollment. Subjects with a history of any types of cancer, severe heart, liver, lung, kidney, blood, endocrine, nervous system, or autoimmune diseases, multiple peripheral vascular lesions, abdominal surgery (exclude of appendicitis), schizophrenia and inflammatory bowel disease were excluded from the study. Additionally, pregnant or lactating women were not included. Patients who had taken probiotics or antibiotics within the past two weeks or antidepressants within the past month were also excluded. Healthy control subjects, free from any gastrointestinal (GI) symptoms, were recruited through advertising. Each healthy control subject completed a questionnaire to confirm the absence of GI symptoms. Subjects with a history of any diseases mentioned above were excluded, following the same exclusion criteria as for the patients with IBS. All subjects underwent a screening blood test, abdominal ultrasound scan and colonoscopy, and those with abnormal physical examination indicators were excluded from the study. Demographic information about the participants, including age and gender, is provided in the Supplementary Table 1.
Questionnaire in human subjects
All subjects participating in the study completed a comprehensive questionnaire to gather information on various lifestyle factors and health conditions. The questionnaire included questions on exercise, physical activity levels, cigarette smoking habits, alcohol consumption, underlying medical conditions, current medications, as well as the sections to measure the severity of bowel symptom, the quality of life related to IBS, and mental health status at the baseline assessment. Additionally, the frequency of antibiotic treatment within the past year was recorded. Participants were also queried about any worsening of IBS symptoms due to stress. The Daily bowel habits and frequency were recorded using the Bristol Stool Form18. The Gastrointestinal symptom severity was evaluated by the Gastrointestinal Symptom Rating Scale (GSRS)19. The Bowel Symptom Severity Scales (IBS-SSS) were used to measure the severity of bowel symptom20. The IBS-QOL questionnaire was used to assess the quality of life for patients with IBS21. The Hamilton Depression Scale (HAMD) and Hamilton Anxiety Scale (HAMA) were used to assess the psychological statues22.
Colonoscopy and biopsy of colonic mucosa
Before colonoscopy, all participants underwent routine cleansing procedures. During the colonoscopy, mucosal biopsy was taken from sigmoid colon and distal ileum, and then fixed with 4% paraformaldehyde and 2.5% glutaraldehyde for immunohistochemical staining and transmission electron microscopy separately.
Mice experiments
The Department of Laboratory Animal Science at the Peking University Health Science Center approved for all animal experiments conducted in this study. Male C57BL/6 J mice, aged 5–6 weeks were acquired from The SpePharm (Beijing) Biotechnology Co., Ltd. Mice were reared and sustained on Maintain Diet (Xietong Pharmaceutical Bio-engineering, #1010088) throughout the duration of the experiment. The study complied with the Peking University Health Science Center Animal Research Ethics Committee (protocol number PUIRB-LA2023484).
Psychological stress and stereotactic injection in vivo
To assess the effects of psychological stress, the mice were randomly divided into the water avoidance stress (WAS) group and the Sham group. In the WAS group, the mice were placed on a platform (150 × 150 × 80 mm) affixed to the bottom of a plastic tank (45 cm length × 25 cm width × 25 cm height). The tank was filled with warm water (25 °C) up to 10 mm below the top of the platform. The mice were placed on the platform to avoid the water stimulus for one hour. In contrast, the Sham group mice were placed in the same setup but without water. Immediately following the stress exposure, samples were collected from both groups.
For stereotactic injection of CRH in Paraventricular nucleus (PVN), the mice were randomly divided into four groups: Control group, Corticotropin-releasing hormone (CRH) group, CRH + NBI (CRH receptor blocker) group, and CRH + 5-aminosalicylic acid (5-ASA) group. Mice were anesthetized using tribromoethanol and then were placed in a stereotactic frame with sustained anesthesia during and post injection. 300 nl of 100 nM CRH was injected at a rate of 10 nl/sec into the PVN of mice in the CRH group using a Nanoject II Auto-Nanoliter Injector (Drummond). In the CRH + NBI group, 2 ml of 1um NBI30775 (CRH receptor blocker) was injected to block the CRH receptors. In the CRH + 5-ASA group, 2 ml of 1650 mg/kg 5-ASA was injected. Mice were kept under anesthesia until sample collection.
Tissue mitochondria isolation
For measuring mitochondria high-resolution respirometry, colon epithelium was obtained and then mitochondria were isolated using Tissue mitochondria isolation Kit (Beyotime, C3606) following manufacturer’s instructions.
High-resolution respirometry
Mitochondria function was estimated as oxygen consumption by high-resolution respirometry (Oxygraph-2k, Oroboros Instruments). Briefly, mitochondria were isolated from the fresh colon epithelium using tissue mitochondria isolation kit and mechanically homogenized and permeabilized in respiration media Mir05 (0.5 mM EGTA, 3 mM MgCl2, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose and 0.1% [w/v] bovine serum albumin [pH 7.1]). The tissue homogenate was added to a 2 ml chamber to assess oxygen flux. Leak respiration was measured by adding glutamic (10 mM), malate (2 mM) and pyruvate (5 mM) to the chamber in the absence of ADP. The contribution of the NADH-derived pathway to oxidative phosphorylation was measured by adding ADP (1 mM, Oxphos I). Subsequently, added succinate (10 mM) was added to assess ADP-stimulated respiration when NADH and succinate-linked pathways are simultaneously transferring electrons to the Q junction (Oxphos I + II). Next, Trifluoromethoxy carbonylcyanide phenylhydrazone (FCCP; 0.5 mM) was titrated to achieve maximum flux through the electron transfer system (ETS I + II) under this experimental condition. Respiration was inhibited by the sequential addition of rotenone (0.5 mM) and antimycin A 2.5 uM). The remaining O2 flux after inhibition with antimycin A (O2 flux independent of the electron transfer system) was subtracted to calculate the different respiratory states. Finally, TMPD (0.5 mM) and Ascorbate(2 mM) were added to assess Complex IV activity.
Measurement of net flux of oxygen alongside the colon epithelial
The net flux of oxygen across the colon epithelial of mice was measured utilizing the Non-invasive Micro-test technology (NMT). The fresh colon epithelium was obtained from the mice, mounted horizontally in the measuring chamber, loosely fixed in position using dental wax, and then was submerged in a solution containing 120 mM NaCl, 4.5 mM KCl, 1.0 mM MgSO4, 1.8 mM Na2HPO4, 0.2 mM NaH2PO4, 1.25 mM CaCl2, 25 mM NaHCO3 (pH 7.4-7.5), which was equilibrated for 30 min. Then discarded waste liquid and added 5-10 ml fresh test liquid to the sample and started testing. The detection point of proximal or distal epithelium was found under microscope, and the O2 flow sensor was positioned approximately 10 um away from the detection point, each site underwent 5-6 replicate measurements. The real time flow data of O2 were captured and analyzed using the imFluxes V2.0 software (YoungerUSA LLC, Amherst, USA). Positive values indicated an efflux of oxygen, whereas negative values signified absorption.
Primary colon epithelium cells Respiration assays
The oxygen consumption rate (OCR) was measured using the Seahorse XFe24 analyzer (Seahorse Bioscience). To assess the basal metabolic rate, the analysis was carried out in XF Assay DEME Medium (Seahorse Bioscience) supplemented with 4mM L-glutamine, 25 mM glucose, and 10% FBS. To investigate ATP-Linked respiration, maximal respiration and non-mitochondria respiration, analysis was performed in XF Assay DEME Medium under basal conditions and following the addition of Oligomycin (2.5 uM; Seahorse Bioscience), Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP; 2 uM; Seahorse Bioscience), Rotenone (0.5 uM; Seahorse Bioscience) and Antimycin A (0.5 uM; Seahorse Bioscience).
TEM sample preparation and quantitative analysis
Fix the fresh samples in 1% osmium tetroxide in 0.1 M phosphate buffer solution (PBS, pH 7.4) at room temperature (20°C) for 2 hours. Rinse the samples with 0.1 M phosphate buffer solution (PBS, pH 7.4) three times, 15 minutes each. Dehydrate the tissue samples through a series of graded ethanol solutions: 50%-70%-80%-90%-95%-100%-100%, 15 minutes for each step. Infiltrate the samples with a 1:1 mixture of propylene oxide and 812 embedding agents overnight, followed by pure 812 embedding agent overnight. Polymerize the embedded samples at 60°C for 48 hours. Cut ultrathin sections of 60-80 nm thickness using an ultramicrotome.
TEM images were acquired in a Jeol JEM 1400 transmission electron microscope at 120 kV. A magnification level of ×20000 was chosen to study the mitochondria ultrastructure within an image. ImageJ software was used to assess the area of mitochondria. Additionally, the number of mitochondria was manually counted (field of view: 12.77 um2). Mitochondria were classified regarding their morphological characteristic as regular or irregular membrane. Moreover, the mitochondria cristae were investigated and distinguished in well-defined cristae and aberrant cristae.
Corticosterone measurement
Inhumans, the measurement of corticosterone in serum was conducted using the Human Corticosterone ELISA Kit (Abmart, AB-J0695B) according to the manufacturer’s instructions. Similarly, for mice, corticosterone levels in serum were determined using a Mice Corticosterone ELISA Kit (Abmart, AB-W30880), also following the manufacturer’s specified protocol. In brief, the CRH extracted from the serum was added in 96-well plates and then incubated with the detection antibody for 120 min in 25°C. Subsequently, TMB solution was added to each well for 15-20 min in 25°C, and the absorbance was read in 450 nm using a I-control™ System (Tecan).
CRH measurement
For human, the quqntification of CRH in serum was performed using the Human CRH ELISA Kit (Abmart, AB-J0454B) according to the manufacturer’s instructions. Analogously, in mice, CRH levels in serum were measured with the Mice CRH ELISA Kit (Abmart, AB-J0210A) adhering to the recommended protocol. In brief, the CRH extracted from the serum was added in 96-well plates and then incubated with the detection antibody for 50 min in 37°C. Subsequently, Substrate A and Substrate B was added to each well for 15 min in 37°C, and the absorbance was read in 450 nm using a I-control™ System (Tecan).
Lactate measurements
To assess intracellular lactate levels, colon epithelium was isolated and then deproteinized using the Deproteinizing Sample Preparation Kit (Abcam, ab204708) following the manufacturer’s instructions. Lactate measurement in the colon epithelium lysates was performed using the Lactate Assay Kit (Sigma, MAK064) according to the manufacturer’s instructions. In brief, the lactate extracted from the colon epithelium was added in 96-well plates and then incubated with the Lactate Reaction Mixes for 30 min in 37°C, and the absorbance was read in 570 nm using a I-control™ System (Tecan).
ATP measurements
For measuring intracellular ATP levels, colon epithelium was isolated and then deproteinized using Deproteinizing Sample Preparation Kit (Abcam, ab204708) following manufacturer’s instructions. ATP measurement in colon epithelium lysates was performed using the ATP Colorimetric/Fluorometric Assay Kit (Sigma, MAK190) according to the manufacturer’s instructions. In brief, the ATP extracted from the colon epithelium was added in 96-well plates and then incubated with the ATP Reaction Mixes for 30 min in 37°C, and the absorbance was read in 570 nm using a I-control™ System (Tecan).
Pyruvate dehydrogenase activity measurements
To measure Pyruvate Dehydrogenase activity, colon epithelium was isolated and then lysed with Pyruvate Dehydrogenase assay buffer on ice for 10 min and insoluble material was removed by centrifugation for 5 min at 10,000 g at 4°C. Pyruvate Dehydrogenase activity was measured with supernatants using the PDH activity assay kit (Sigma, MAK183) according to the manufacturer’s instructions. In brief, the Pyruvate Dehydrogenase extracted from the colon epithelium was added in 96-well plates and then incubated with the Pyruvate Dehydrogenase Reaction Mixes for 30 min in 37°C, and the absorbance was read in 450 nm using a I-control™ System (Tecan).
Citrate synthase activity measurements
To measure Citrate Synthase activity, colon epithelium was isolated and then lysed with Citrate Synthase assay buffer on ice for 10 min and insoluble material were removed by centrifugation for 5 min at 10,000 g at 4°C. Citrate Synthase activity was measured with supernatants using the Citrate Synthase activity assay kit (Sigma, MAK193) according to the manufacturer’s instructions. In brief, the Citrate Synthase extracted from the colon epithelium was added in 96-well plates and then incubated with the Citrate Synthase Reaction Mixes for 30 min in 37°C, and the absorbance was read in 412 nm using a I-control™ System (Tecan).
Histopathology
The tissue of the proximal colon was fixed in 10% phosphate-buffered formalin and 5 um thick sections of the fixed tissue were stained with hematoxylin and eosin. Scoring of the stained tissue sections were performed by a veterinary pathologist based on the criteria listed (Table). Representative images were taken using an Olympus BX41 microscope.
| Histologic feature | Score | Criteria |
|---|---|---|
| Epithelial injury | 0 | No mucosal damage |
| 1 | Focal lymphoepithelial lesion | |
| 2 | 25-50% mucosa erosion/ulcer formation | |
| 3 | Over 50% mucosa erosion/ulcer formation. | |
| Infiltration by inflammatory cells | 0 | No inflammatory cell infiltration in lamina propria |
| 1 | Less inflammatory cells infiltrate the lamina propria | |
| 2 | Medium inflammatory cells in the lamina propria and in the submucosa | |
| 3 | Numerous inflammatory cells infiltrated the lamina propria and infiltrated the entire intestinal wall | |
| Mucus layer damage | 0 | None |
| 1 | Slight mucus layer damage | |
| 2 | Moderate mucus layer damage | |
| 3 | Severe mucus layer damage | |
| Reduction of goblet cells | 0 | No reduction of goblet cells |
| 1 | less 10% reduction of goblet cells | |
| 2 | 10-50% reduction of goblet cells | |
| 3 | Over 50% reduction of goblet cells |
Hypoxia staining
For detection of hypoxia, mice were treated with 60 mg/kg of pimonidazole HCl i.p. (HypoxyprobeTM-1 kit, Hypoxyprobe #HP1-200kit) one hour prior to euthanasia. Colon samples were fixed in 10% buffered formalin and paraffin-embedded tissue was probed with mice anti- pimonidazole monoclonal IgG1 (MAb 4.3.11.3) and then stained with Alexa fluor 546-conjugated goat anti- mice IgG antibody (Life Technologies #A1103). Samples were counterstained with DAPI using SlowFade Gold mountant (Invitrogen #S36936). Samples were scored based on the degree of colonic epithelial hypoxia (0: no hypoxia; 1: mild focal hypoxia; 2: moderate multifocal hypoxia; 3: intense diffuse hypoxia) Representative images were obtained using either a Zeiss Axiovert 200 M fluorescent microscope or a Zeiss LSM 880 confocal microscope.
Real-time PCR
Total RNA of Mice colon epithelium was extracted using Kit (TIANGEN, Beijign, China). Reverse transcription of 1 mg total RNA was performed using the All-In-One 5X MaserMix (ABM, Richmond, Canada) following the manufacturer’s instructions. The forward and reverse primers of selected genes are shown in Table. Real-time PCR was performed using SYBR premix Ex Taq (Takara, Shiga, Japan). Reactions were prepared following the manufacturer’s protocol. All reactions were carried out in triplicate (QuantStudio™ 5, Thermo, MA, USA). The cDNA of each gene was amplified through 60 cycles of 15 s at 95°C, 30 s at 58°C, and 30 s at 72°C. Data analysis was carried out using QuantStudio 5 Real-time PCR Software (Thermo) and Microsoft Excel. Expression values are presented relative to B2M values in the corresponding samples.
| Forward sequence (5’–3’) | Reverse sequence (5’-3’) | |
|---|---|---|
| mGAPDH | GGTGCTGAGTATGTCGTGGA | CCTTCCACAATGCCAAAGTT |
| CRHR1 | CAGTACAGGAAGGCTGTGAA | CTGAAAGGACTCCAGGAAAGAG |
| CRHR2 | ACGTCCGAGACAATCCAATAC- | CTCGCCAGGATTGACAAAGA |
| Drp1 | CTGGATCACGGGACAAGG | GTTGCCTGTTGTTGGTTCCT |
| Fis1 | CCTGATTGATAAGGCCATGAA | ACAGCCAGTCCAATGAGTCC |
| OPA1 | TGACAAACTTAAGGAGGCTGTG | CATTGTGCTGAATAACCCTCAA |
| MFN2 | CATTCTTGTGGTCGGAGGAG | AAGGAGAGGGCGATGAGTCT |
| CYB | CCTAGCAATCGTTCACCTCC | TATGGGGTCGGGTGTTTAGT |
| PHB | TCCTGCCTTCTATCACCACA | CCACAAATCTGGCTCTCTCC |
| TUFM | CTATGCCCACACAGACTGTC | ACCATCTCTGAGTCCTGGAC |
| TFB1M | GCTGAGAGACTTGTAGCCAC | TCCTTCGAAACTGAAACGCA |
| TFB2M | GTTCGATCTGTACTCCTGCG | ATTCCCCGTGCTTTGACTTT |
| ZO-1 | AATGAATGATGGTTGGTATGG | TGACAGGTAGGACAGACG |
| Occludin | ATGGCAAGCGATCATACCC | TTCCTGCTTTCCCCTTCG |
RNA isolation and library preparation
Total RNA was extracted using the TRIzol reagent (Invitrogen, CA, USA) according to the manufacturer’s protocol. RNA purity and quantification were evaluated using the NanoDrop 2000 spectrophotometer (Thermo Scientific, USA). RNA integrity was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Then the libraries were constructed using VAHTS Universal V6 RNA-seq Library Prep Kit according to the manufacturer’s instructions. The transcriptome sequencing and analysis were conducted by OE Biotech Co., Ltd. (Shanghai, China).
RNA sequencing and differentially expressed genes analysis
The libraries were sequenced on an llumina Novaseq 6000 platform and 150 bp paired-end reads were generated. About 46 raw reads for each sample were generated. Raw reads of fastq format were firstly processed using fastp and the low quality reads were removed to obtain the clean reads. Then about 46 clean reads for each sample were retained for subsequent analyses. The clean reads were mapped to the reference genome using HISAT2. FPKM of each gene was calculated and the read counts of each gene were obtained by HTSeq-count. PCA analysis were performed using R (v 3.2.0) to evaluate the biological duplication of samples. Differential expression analysis was performed using the DESeq2. Q value < 0.05 and foldchange > 2 or foldchange < 0.5 was set as the threshold for significantly differential expression gene (DEGs). Hierarchical cluster analysis of DEGs was performed using R (v 3.2.0) to demonstrate the expression pattern of genes in different groups and samples. The radar map of top 30 genes was drew to show the expression of up-regulated or down-regulated DEGs using R packet ggradar.
RNA differentially expressed functions analysis
Based on the hypergeometric distribution, GO6, KEGG7 pathway, Reactome and WikiPathways enrichment analysis of DEGs were performed to screen the significant enriched term using R (v 3.2.0), respectively. R (v 3.2.0) was used to draw the column diagram, the chord diagram and bubble diagram of the significant enrichment term.
Gene Set Enrichment Analysis (GSEA) was performed using GSEA software. The analysis was www.oebiotech.com used a predefined gene set, and the genes were ranked according to the degree of differential expression in the two types of samples. Then it is tested whether the predefined gene set was enriched at the top or bottom of the ranking list.
DNA isolation and library construction
Total DNA was isolated from sample using a QIAamp® Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. DNA concentration and integrity were assessed by a NanoDrop2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis, respectively. DNA was fragmented by S220 Focused-ultrasonicators (Covaris, USA) and cleaned up by Agencourt AMPure XP beads (Beckman Coulter Co., USA). Then the libraries were constructed using TruSeq Nano DNA LT Sample Prepararion Kit (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. The Metagenome sequencing and analysis were conducted by OE Biotech Co., Ltd. (Shanghai, China).
Bioinformatic analysis
The Metagenome sequencing and analysis were conducted by OE biotech Co., Ltd. (Shanghai, China). The raw data was in FASTQ format. Reads were trimmed and filtered using fastp (v 0.20.1). Host pollution control was needed if the DNA was extracted from host-related environment. The post-filtered pair-end reads were aligned against the host genome using bbmap (v38.93-0) and the aligned reads were discarded. Metagenome assembly was performed using MEGAHIT (v 1.2.9) after getting valid reads. ORF prediction of assembled contigs (longer than 500 nt) using prodigal (v 2.6.3) was performed and translated into amino acid sequences. The non-redundant gene sets were built for all predicted genes using MMSeqs2 (v 13.45111). The clustering parameters were 95% identity and 90% coverage.
Microbial KEGG function forecasting analysis
The longest gene was selected as representative sequence of each gene set. clean reads of each sample were aligned against the non-redundant gene set (95% identity) use salmon (v 1.8.0), and the abundant information of the gene in the corresponding sample was counted. The gene set representative sequence (amino acid sequence) was annotated with KEGG database with an e-value of 1e-5 using DIAMOND (v 0.9.10.111). The taxonomy of the species was obtained as a result of the corresponding taxonomy database of the NR Library, and the abundance of the species was calculated using the corresponding abundance of the genes. In order to construct the abundance profile on the corresponding taxonomy level, abundance statistics were performed at each level of Domain, Kingdom, Phylum, Class, Order, Family, Genus, Species. The PCA analysis and plotting of the abundance spectrum of the species or functional abundance spectrum were carried out using R software (v 4.1.2), and the results of the equidistant matrix of PCoA and NMDS were calculated and analyzed.
Quantification and statistical analysis
For human studies, normally distributed continuous data are expressed as the Mean ± Standard error (SEM) and non-normally distributed data are expressed as median and interquartile range. Categorical data are represented as number (%). The characteristics of HC and patients with IBS were compared with a one-way ANOVA, followed by Bonferroni multiple comparison test for normally distributed data, by one-tailed non-parametric test (Mann-Whitney) or unpaired Student’s t test.
For cell and mice experiments, an unpaired Student’s t test was used on the transformed data to determine whether differences between groups were statistically significant (p < 0.05). When more than two treatments were used, statistically significant differences between groups were determined by one-way ANOVA followed by Tukey’s HSD test (between > 2 groups). Significance of differences in histopathology or hypoxia scores was determined by a one-tailed non-parametric test (Mann-Whitney) in two groups, and one-tailed non-parametric test (Kruskal Wallis test) followed by Dunn’s multiple-comparison test were performed in more than two groups. We performed all statistical analyses using the Statistical Package for Social Science version 26.0 (SPSS INC., Chicago, IL, USA) and GraphPad Prism 8.0 Software. Statistical significance was defined as p < 0.05.
Result
Patients with IBS have aberrant mitochondria and dysbiosis
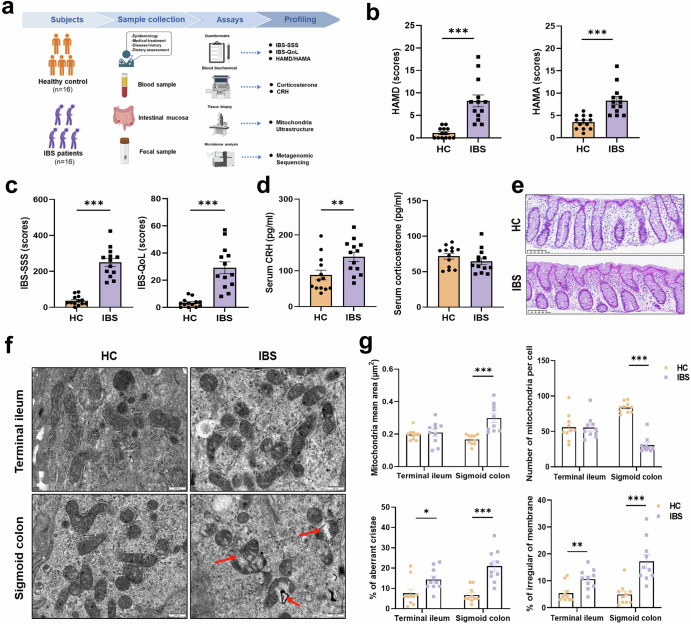
Patients with IBS fulfilling the Rome IV criteria and healthy controls (HC) were investigated. All study participants had blood and stool samples and underwent screening colonoscopy, with mucosal biopsies obtained from the colon and/or terminal ileum (Fig. 1a). Demographic information about the participants, including mean age, gender ratio and body mass index (BMI) were matched between the two groups (Supplementary Table 1). In turn, statistical analysis of clinical scales23 found that patients with IBS exhibited significantly more severe mental and physical symptoms compared with HC (Fig. 1b, c and Supplementary Fig. 1 and Table 2). Afterward, we measured serum corticosterone and CRH concentrations to assess the activity status of the HPA axis and found patients with IBS showed significantly higher CRH concentration than HC (Fig. 1d). Histologically examination revealed that both patients with IBS and HC had barely intestinal inflammation (Fig. 1e). However, patients with IBS exhibited significant mitochondria ultrastructure damage compared to HC. This damage included decreased numbers of mitochondria per cell, swollen mitochondria, irregular mitochondria membrane, and aberrant mitochondria cristae (Fig. 4f, g).
Fig. 1. Damaged colonocytes’ mitochondria and dysbiosis in patients with IBS.
a Graphic of HC and patients with IBS clinical trial. b Scores of depressions and anxiety were measured using HAMD and HAMA in HC and patients with IBS (n = 13). c Scores of IBS symptom severity (IBS-SSS) and IBS quality of life (IBS-QoL) were measured in participators (n = 13). d Corticosterone and CRH concentration of serum were measured in participators by ELISA (n = 13). e Representative images of histopathological changes in hematoxylin and eosin (H&E)-stained sections of the colon mucosa in patients with IBS and HC (scale bar = 250 um). f TEM images of colon epithelial cells mitochondria in HC and IBS group, red arrow aims to damaged mitochondrion (scale bar = 500 nm). g Mitochondria mean area; Number of mitochondria per cells; Percentage of aberrant cristae mitochondria; Percentage of irregular (n = 10). Data are displayed as Mean ± SEM; Independent-Sample t test were performed; Data non normally distributed were analyzed by Mann-Whitney U test. (*p < 0.05, **p < 0.01, ***p < 0.001).
Fig. 4. Gut dysbiosis and correlation analysis in C57BL/6 J mice.
a, b, c, d The Wilcoxon rank-sum test demonstrates significant differences in abundance at the family (a, b) and genus (c, d) levels based on microbiota profiling of feces from sham and WAS (n = 10 and 18). The violin plot displays the median (center line inside the violin), interquartile range (IQR, dotted line inside the violin), minimums and maximums within 1.5 times the IQR (bound of the violin); Independent-Sample t test was performed. (*p < 0.05, **p < 0.01). e, f Heatmap shows correlation analysis between abundance of microbiota and host stress indicators in Sham and WAS group mice at family (e) and genus (f) levels. g, h Correlation analysis between abundance of microbiota and mitochondria oxphos level in Sham and WAS group mice at family (g) and genus (h) level. i, j Correlation analysis between abundance of microbiota and net colon epithelium oxygen absorption in Sham and WAS group mice at family (i) and genus (j) level. The black line shows the best linear fit. The bule of the dots represent the density of the connectivity values. The gray shadow shows the 95% confidence interval. Correlation analysis was performed based on Sham and WAS group mice (n = 10 and 18). Symbols indicate significance: *p < 0.05, **p < 0.01, ***p < 0.001.
According to the results of metagenomic sequencing, we found that difference microbiota between patients with IBS and HC are primarily distributed in the family to species level of bacterial classification (Supplementary Fig. 2 and Fig. 3a, b). Lefse analysis exhibited the LDA scores of these different microbiota contributions (Supplementary Fig. 3c). Meta analysis exhibited a shift in the microbial strains present in patients with IBS compared to HC (Fig. 2a–d and Supplementary Fig. 4). Specifically, patients with IBS exhibited a decrease in obligate anaerobes, such as Methanobacteriaceae at family level and Ruminococcus at genus level (Fig. 2a, c). Conversely, there was an increase in facultative anaerobes, including Pasteurellaceae at family level and Haemophilus at genus level (Fig. 2b, d). Correspondingly, microbiota aerobic reaction increased while anaerobic reaction decreased in patients with IBS compared to HC (Supplementary Fig. 5). Moreover, KEGG function forecasting analysis revealed that the function of microbiota pathogenicity and starch metabolism increased while capacity of amino acid metabolism decreased in patients with IBS compared with HC (Supplementary Fig. 5).
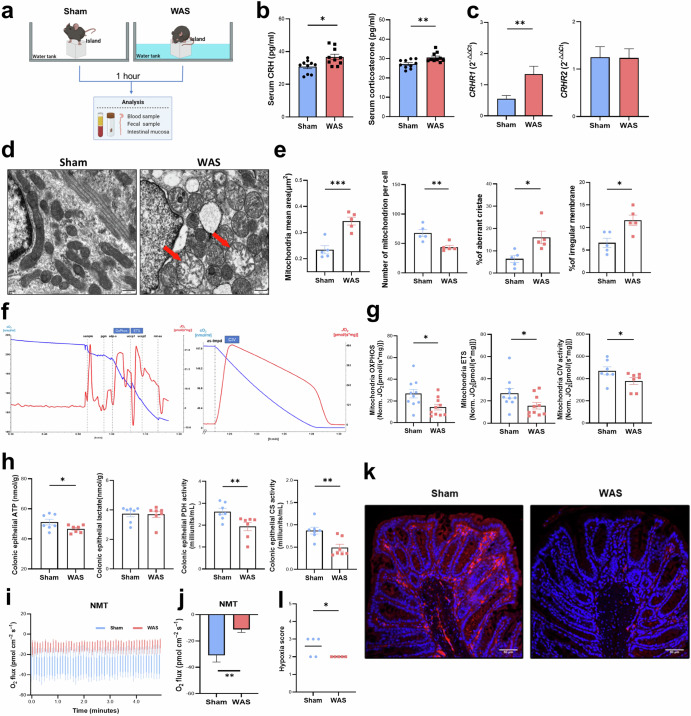
Fig. 3. Water avoidance stress in mice activates the HPA axis and damages the colonocytes’ mitochondria ultrastructure and function.
a Graphic of sham stress and acute water avoidance stress mice model. b Corticosterone and CRH concentration of serum were determined by ELISA (n = 10). c CRHR1 and CRHR2 genes expression level were determined by RT-PCR (n = 10). d TEM images of colon epithelial cells mitochondria in mice, red arrow aims to damaged mitochondrion (scale bar = 500 nm). e Mitochondria quantitative and morphological changes: Mitochondria mean area; Number of mitochondria per cells; Percentage of aberrant cristae mitochondria; Percentage of irregular (n = 5). f Representative high-resolution respirometry recordings of colonocytes’ mitochondria by using O2K: The oxygen flux (JO2, red line) is calculated as the negative slope of the oxygen concentration (cO2, blue line) with time (x-axis), character marks indicate times of injection of substrates and inhibitors. g OXPHOS, maximum oxidative phosphorylation in the coupled state with complex I and II substrates; ETS, maximum mitochondria respiratory activity; Activity of Complex IV, uncoupled mitochondria respiratory activity linked to complex IV upon inhibition of complex I by rotenone (n = 7–10). h Cytosolic concentrations of ATP, lactate and activity of PDH and CS by ELISA (n = 7). i, j Net flux of oxygen absorption in colon epithelium by NMT technology (n = 5). k, l Hypoxia stain images are shown and Pimonidazole staining was quantified by scoring blinded sections of proximal colon (n = 5). Data are displayed as Mean ± SEM; Independent-Sample t test were performed; Data non normally distributed were analyzed by Mann-Whitney U test. (*p < 0.05, **p < 0.01, ***p < 0.001).
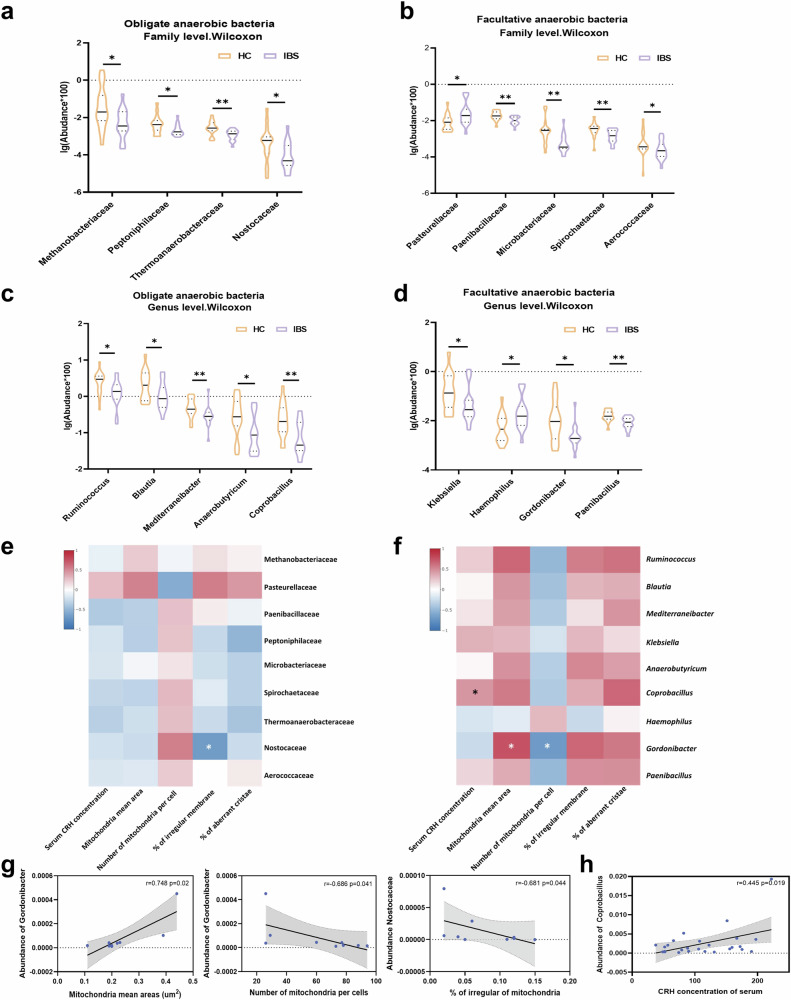
Fig. 2. Gut dysbiosis and correlation analysis in clinical trial.
a–d The Wilcoxon rank-sum test demonstrates significant differences in abundance at the family (a, b) and genus (c, d) levels based on microbiota profiling of feces from HC and patients with IBS (n = 16). The violin plot displays the median (center line inside the violin), interquartile range (IQR, dotted line inside the violin), minimums and maximums within 1.5 times the IQR (bound of the violin); Independent-Sample t test was performed. (*p < 0.05, **p < 0.01). e, f Heatmap shows correlation analysis between abundance of microbiota and host stress indicators in clinical participants at family (e) and genus (f) levels. g Correlation analysis between the abundance of microbiota and mitochondria damage in clinical participants. h Correlation analysis between the abundance of microbiota and CRH levels in clinical participants. The black line shows the best linear fit. The bule of the dots represent the density of the connectivity values. The gray shadow shows the 95% confidence interval. Correlation analysis was performed based on clinical participants (n = 9 and 13). Symbols indicate significance: *p < 0.05.
For further explore the relationship between HPA axis activation, colonocytes’mitochondria ultrastructure damage, and gut dysbiosis in patients with IBS, we conducted a correlation analysis of these indicators (Fig. 2e, f). Our findings revealed that the abundance of facultative anaerobes, such as the abundance of Gordonibacter at the genus level, positively correlated with damaged mitochondria ultrastructure (expanding mitochondria mean areas) (Fig. 2g left and middle). Conversely, the obligate anaerobes, such as the abundance of Nostocaceae at the family level, exhibited a negative correlation with damaged mitochondria ultrastructure (increasing ratio of irregular mitochondria membrane) (Fig. 2g right). Furthermore, our analysis showed that the obligate anaerobes Coprobacillus at the family level positively related to CRH concentration (Fig. 2h)
In brief, we observed increased serum CRH concentration, damaged colonocytes’ mitochondria and gut dysbiosis in patients with IBS, as well found that gut dysbiosis related to increased CRH level and damaged colonocytes’ mitochondria.
Acute stress impairs colonocytes’ mitochondria and triggers dysbiosis
Based on the results of clinical research and other background references, we supposed that stress may damage colonocytes’ mitochondria via CRH to trigger mitochondria. Thus, for further investigate the relationship between activated HPA axis, damaged colonocytes’ mitochondria, and disrupted gut microbiota, we developed the acute water avoidance stress (WAS) mice which recapitulates the transfer of psychological stress from the brain to gut changes as well as avoids the influence of the altered gut microbiota on host24,25. Accurately, we used 6 weeks SPF male C57BL/6 J mice and randomly assigned them to either the WAS group or the Sham group. Mice in WAS group were placed on a narrow platform surrounding by water, while those in the Sham group were placed on an identical platform but without water surrounded it. Both groups underwent this intervention for one hour (Fig. 3a). Mice exposed to acute stress exhibited elevated corticosterone and CRH concentrations in their serum, alone with increased expression of CRHR1 in colonocytes (Fig. 3b, c). We observed barely intestinal inflammation and intestinal barrier dysfunction in both WAS and Sham group mice (Supplementary Fig. 6a, b). However, ultrastructural analysis revealed significant damaged in the colonocytes’ mitochondria in the WAS group mice, accurately the decreased number of mitochondria per cell and an increased ratio of aberrant mitochondria (Fig. 1d, e). Consistent with these mitochondria ultrastructure defects, WAS group mice exhibited increasing expression of mitochondria fission genes, including DRP1, (encoding dynamin-1-like protein), FIS1 (encoding mitochondria fission 1 protein), and decreasing expression of fusion genes such as OPA1 (encoding mitochondria dynamin like GTPase) and MFN2 (encoding outer mitochondria membrane GTPase for dynamics) (Supplementary Fig. 6c)26,27.
In terms of mitochondria function, we evaluated the colonocytes’ mitochondria respiration using Oxygeaph-2k Oroboros technology, alone with intracellular level of OXPHOS metabolites28. WAS group mice exhibited decreased OXPHOS activity, reduced levels of the electronic transfer system, and impaired complex IV activity. These indicators directly reflected compromised mitochondria respiration. Notably, complex IV, which is responsible for catalyzing the conversion of oxygen to water during the electronic transfer chain, provides the most direct measure of oxygen consumption ability (Fig. 1f, g). Correspondingly, mice exposed to stress demonstrated a significant reduction in pyruvate dehydrogenase (PDH) activity, which catalyzes the oxidative decarboxylation of pyruvate to form acetyl-CoA, as well as decreased citrate synthetase (CS) activity, which is involved in the irreversible condensation of acetyl-CoA with oxaloacetate to produce citrate. Additionally, there was a decrease in ATP levels without any change in lactate level (Fig. 1h). This suggests that short-term stress, such as the one-hour intervention used in this study, is insufficient to shift cell metabolism from OXPHOS to oxygenation. Consistent with the attenuated mitochondria respiration observed, WAS group mice exhibited decreasing expression of mitochondria function and DNA-transcription related genes. These include CYB, encoding cytochrome b; PHB, encoding prohibitin, a mitochondria chaperone for function; TUFM, encoding a protein involved in protein translation within mitochondria; and TFB2M, encoding transcription factor B2 protein, which is involved in transcription initiation from the mitochondria promoter (Supplementary Fig. 6d). Epithelial hypoxia is crucial for maintaining colonic homeostasis, and this state is sustained by the high oxygen consumption of colonocytes’ mitochondria11. To assess whether mitochondria dysfunction impairs epithelial hypoxia, we employed two distinct techniques: NMT (Fig. 3i) and hypoxia staining (Fig. 3k). Our findings revealed that the real-time net flux of oxygen absorption in the colon epithelium of WAS group mice was only half of that in the Sham group (Fig. 3j). Furthermore, when we visualized epithelial hypoxia using the exogenous hypoxic marker pimonidazole, we observed a significant diminish in epithelial hypoxia in the WAS group mice compared to controls (Fig. 3l). Notably, NMT is typically used to investigate the influx and efflux rates of selected ions and molecules in the microenvironment surrounding plant living cells or intact organisms29. However, we innovatively employed NMT to assess epithelial oxygen absorption in mice. Specifically, we measure the oxygen molecule concentrations at both near and far distances within the colonic mucosal tissue over a period of time. Subsequently, we imported these measured oxygen concentration data into the Nernst equation to calculate the oxygen flux rate and direction to calculate mouse epithelial oxygen uptake.
To explore the influence of acute stress to gut microbiota, we conducted a comprehensive analysis of fecal samples from both Sham and WAS group mice using metagenomic sequencing (Supplementary Fig. 7, 8). Compared to the Sham group, WAS group mice were characterized by a significant reduction in the abundance of obligate anaerobes at both the family and genus levels (Supplementary Fig. 9), such as Helicobacteraceae and Akkermansiaceae (Fig. 4a, c). Conversely, there was an increase in the abundance of facultative anaerobes at both the family and genus levels, including Moraxellaceae and Pseudomonadaceae (Fig. 4b, d). Correspondingly, microbial reactions in the WAS group mice demonstrated reduced levels of anaerobic reactions and increased levels of aerobic reactions (Supplementary Fig. 10). Furthermore, WAS mice microbiota exhibited an increasing level of infectious disease, drug resistance and starch metabolism, while showing a decreasing level of amino acid metabolism and digestive capacity (Supplementary Fig. 10). Correlation analysis in WAS and Sham group mice showed a relationship between colonocytes’ mitochondria characteristics and gut microbiota (Fig. 4e, f). In terms of mitochondria ultrastructure, damaged mitochondria ultrastructure promoted an increase in the abundance of facultative anaerobes increased, such as Moraxellaceae, Pseudomonadaceae at the family level and Acinetobacter, Pseudomonas and Sarcina at the genus level. Meanwhile, damaged mitochondria ultrastructure decreased the abundance of obligate anaerobes, such as Helicobacteraceae, Deferribacteraceae and Akkermansiaceae at the family level, and Helicobacter, Akkermansia and Catenibacterium at the genus level (Fig. 4e, f). In terms of mitochondria respiration, a decreased level of mitochondria oxidative phosphorylation dramatically reduced the abundance of obligate anaerobes, such as Akkermansiaceae at the family level and Akkermansia at the genus level, while elevating the abundance of facultative anaerobes like Candidatus_Borkfalkiaceae at the family level and Sarcina at the genus level (Fig. 4g, h). Correspondingly, reduced net colon epithelium oxygen absorption also altered microbiota composition, similar to the microbiota changes tendency under damaged mitochondria oxygen consumption ability, with a decreased abundance of obligate anaerobes such as Akkermansiaceae at the family level and Akkermansia at the genus level, and an elevated abundance of facultative anaerobes such as Moraxellaceae at the family level, Acinetobacte at the genus level (Fig. 4i, j).
In short, acute stress exposure in mice activates the HPA axis, leading to the release of CRH. Elevated levels of CRH subsequently enhance the expression of CRHR1 in colonocytes. This cascade of events ultimately leads to mitochondria dysfunction, colon epithelial hypoxia diminishing, and microbiota dysbiosis.
Enhanced CRH-CRHR1 signaling damages mitochondria in vitro
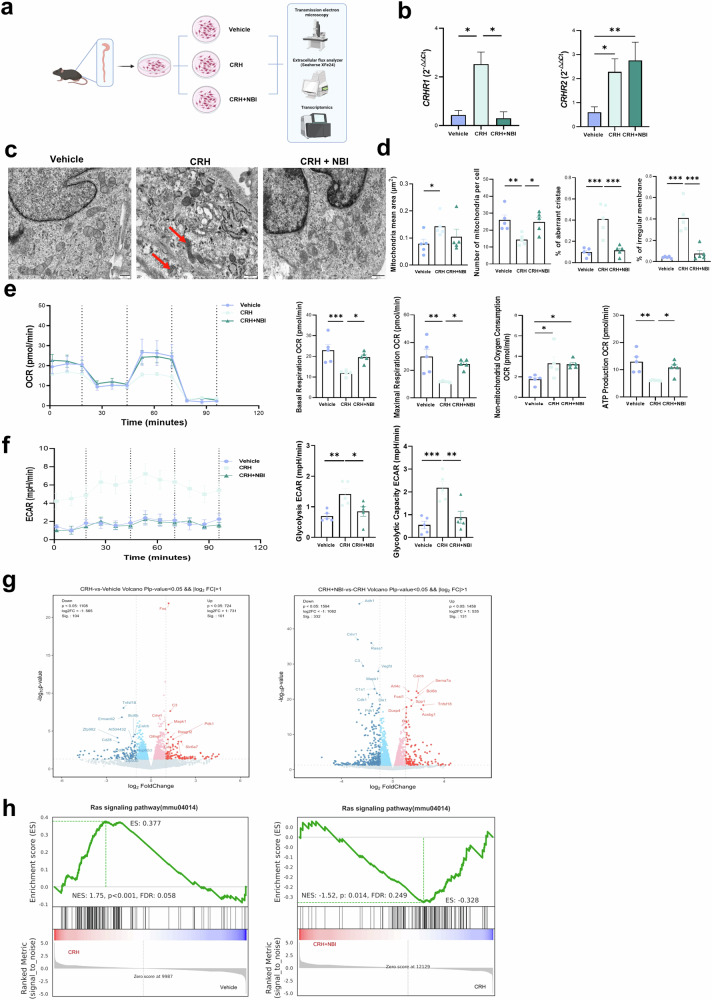
Given our studies that psychological stress increases CRH concentration and up-regulates CRHR1 expression, leading to damage of colonocytes’ mitochondria in mice, we aimed to explore whether the CRH-CRHR1 signaling pathway mediates stress-induced colonocyte’s mitochondria damage in vitro. We isolated primary colon epithelial cells from C57BL/6 J mice and exposed them to CRH (100 nM), CRH + NBI30775 (NBI in abbreviation, a potent small-molecule CRHR1 antagonist with high affinity for CRHR1 and 1000-fold weaker activate at CRHR28) or vehicle (DMSO) for 30 min respectively (Fig. 5a).
Fig. 5. CRH alters mitochondria function and ultrastructure of primary colon epithelial cells via CRHR1.
a Graphic of primary colon epithelial cells from male C57BL/6 J mice experiment created with BioRender.com. b CRHR1 and CRHR2 genes expression level were determined by RT-PCR from colonocyte preparation. c TEM images of colon epithelial cells mitochondria in Vehicle, CRH and CRH + NBI group cells, red arrow aims to damaged mitochondrion (scale bar = 500 nm). d Mitochondria quantitative and morphological changes: Mitochondria mean area; Number of mitochondria per cells; Percentage of aberrant cristae mitochondria; Percentage of irregular. e Mitochondria respiration function of different cells group, measured through oxygen consumption rate (OCR) using seahorse. Each indicates of mitochondria respiration function in different cells group: Basal respiration; Maximal respiration; non-mitochondria oxygen consumption; ATP production. f Mitochondria respiration function of different cells group, measured through extracellular acidification rate (ECAR) using seahorse. g Volcano plot displayed the upregulation or downregulation of gene expression in the colonocytes in the CRH and CRH + NBI group. Differential expression analysis was performed using DESeq2, selecting differentially expressed genes with p < 0.05. h GSEA demonstrated the downregulation of “Ras signaling pathway” in the CRH and CRH + NBI group. The Wilcoxon test followed by FDR correction was used for GSEA enrichment analysis. All cell experiments were performed in n = 5 independent replicates. Data are displayed as Mean ± SEM; one-way ANOVA and Bonferroni’s post hoc comparison test were performed. (*p < 0.05, **p < 0.01, ***p < 0.001).
After exposure to CRH, the cells exhibited a significant increase in the expression of both CRHR1 and CRHR2. However, the presence of NBI effectively inhibited the expression of CRHR1 (Fig. 5b). TEM analysis revealed that cells treated with CRH displayed characteristics such as decreased mitochondria numbers and an increased ratio of aberrant mitochondria, including swollen mitochondria, irregular membranes and aberrant cristae (Fig. 5c, d). Analysis of seahorse cell energy metabolism tecnology30 demonstrated that CRH drastically reduced mitochondria basal respiration, maximal respiration and ATP production, while increased non-mitochondria oxygen consumption (Fig. 5e). In contrast, CRH significantly increased the level of glycolysis and glycolysis capacity to provide energy for colonocytes (Fig. 5f). Correspondingly, exposure of cells to CRH altered the expression of genes related to mitochondria dynamics, including increased expression of fission genes such as DRP1 and FIS1, and decreased expression of fusion genes like OPA1 and MFN2 (Supplementary Fig. 11a); as well decreased expression of genes related to mitochondria function and DNA-transcription, including CYB, PHB, TUFM and TFB1M (Supplementary Fig. 11b). However, the presence of NBI significantly repaired the mitochondria damage caused by CRH in both respiration and ultrastructural, and also regulated the expression level of mitochondria-related genes that were altered by CRH. To gain a deeper underlying of the underlying mechanisms associated with CRHR1 regulation and its impact on mitochondria, we conducted a transcriptome analysis on primary colon epithelial cells derived from C57BL/6J mice. Our findings revealed that 101 genes were upregulated and 104 genes were downregulated in the CRH group compared to the vehicle group (Fig. 5g left). Additionally, we observed that 131 genes were upregulated and 332 genes were downregulated in the CRH + NBI group compared with the CRH group (Fig. 5g right panel). Notably, the genes such as Camp, Mapk1, Cdk1 and Pdk1 were upregulated in CRH group but downregulated in the CRH + NBI group. Further Gene Set Enrichment Analysis (GSEA) demonstrated significant regulation of pathways enriched in both the GO and KEGG databases, including the upregulation of in the “Ras signaling pathway” in the CRH group and its downregulation in the CRH + NBI group (Fig. 5g). Specifically, CRHR1 combined with CRH to activate the cAMP-Ras-MAPK signaling pathway. Subsequently, MAPK phosphorylated Drp1 via Cdk1, promoting mitochondrial fission and activate PDH kinase (Pdk1), which inhibited PDH activity and reduced mitochondria respiration.
Briefly, our findings clarify that the enhanced CRH-CRHR1 signaling pathway can damage the colonocytes’ mitochondria function and ultrastructure in vitro.
Stress transfer to gut via CRH-CRHR1-mitochondria pathway
Although we have verified that an enhanced CRH-CRHR1 signaling pathway can damage the colonocytes’ mitochondria in vitro, we further aimed to explore whether this pathway mediates the transfer of stress from the brain to the gut, potentially triggering gut dysbiosis, and whether inhibiting CRHR1 or activating mitochondria could serve as therapeutic targets to ameliorate this dysbiosis in vivo. 5-amino salicylic (5-ASA, the active ingredient in mesalazine), a PPAR-γ agonist that activates mitochondria bioenergetics specifically in the colonocytes31. For the purpose of simulating the release of central CRH from the PVN, we used stereotactic injection to inject CRH or a vehicle into the PVN of mice. Immediately following this, we administered NBI or 5-ASA to the CRH-treated mice via intraperitoneal injection (Fig. 6a). Mice exposed to CRH exhibited significantly elevated concentrations of corticosterone and CRH in their serum, alone with increased expression levels of CRHR1 and CRHR2 in colonocytes. Notably, NBI treatment significantly reduced the CRH-induced elevation of colonocytes’ CRHR1, while 5-ASA treatment did not affect these indicators, thus excluding any potential influence of NBI and 5-ASA on the HPA axis (Fig. 6b, c). Mice exposed to CRH did not exhibit intestinal inflammation and intestinal barrier dysfunction (Supplementary Fig. 12a), but did show significant damage to mitochondria ultrastructure, including a decrease in the numbers of mitochondria per cell and the presence of swollen mitochondria (Fig. 6d, e). Treatment with NBI restored the mitochondria defects, while 5-ASA had no effect to these defects (Fig. 6d, e). Correspondingly, exposure to CRH led to increased expression of mitochondria fission genes DRP1 and FIS1, while decreased expression of fusion genes OPA1 and MFN2 in mice. NBI treatment reversed these CRH-induced alterations in mitochondria dynamics genes, whereas 5-ASA had no effect on these gene changes (Supplementary Fig. 12c). In the aspect of mitochondria function, mice exposed to CRH exhibited significant impairments of mitochondria respiration, marked by decreased levels of OXPHOS and electronic transport system, as well reduced activity of complex IV. Both NBI and 5-ASA treatments effectively reversed these changes, indicating a restoration of mitochondria respiration function (Fig. 6g). Furthermore, CRH exposure led to a significant reduction in the activity of PDH and CS, accompanied by a decrease in the concentration of cytoplasm ATP in colonocytes of mice. Notably, CRH treatment did not lead to an elevation in lactate concentration in colonocytes, once more suggesting that this stimulation was not sufficient to shift the colonocytes’ metabolism way (Fig. 6f). Both NBI and 5-ASA treatment effectively improved mitochondria respiration, leading to an increase in PDH and CS activity, and ATP levels in the colonic epithelium (Fig. 6f). At the molecular level, exposure to CRH resulted in decreased expression of mitochondria function and DNA-transcription-related genes, including CYB, PHB, TUFM, and TFB2M, while both NBI and 5-ASA treatment were able to increase the expression levels of these genes (Supplementary Fig. 12d). Consistent with the mitochondria damage, the epithelium hypoxia of CRH-treated mice was impaired significantly. Both NBI and 5-ASA treatment restored epithelial hypoxia effectively (Fig. 6h, i). Collectively, these results demonstrate that treatment with NBI and 5-ASA effectively blunted or abrogated the CRH-triggered mitochondria damage and epithelial hypoxia diminish.
Fig. 6. CRH-CRHR1 signaling pathway damaged mitochondria and impaired epithelial hypoxia in mice.
a Graphic of stereotactic injection with CRH and intraperitoneal injection with NBI or 5-ASA in mice. b Corticosterone and CRH concentration of serum were determined by ELISA (n = 7). c CRHR1 and CRHR2 genes expression level were determined by RT-PCR isolated from colonocyte preparation (n = 7). d TEM images of colon epithelial cells mitochondria in Vehicle, CRH, CRH + NBI, CRH + 5-ASA group, red arrow aims to damaged mitochondrion (scale bar = 500 nm). e Mitochondria quantitative and morphological changes: Mitochondria mean area; Number of mitochondria per cells; Percentage of aberrant cristae mitochondria; Percentage of irregular (n = 5). f Cytosolic concentrations of ATP, lactate and activity of PDH and CS by ELISA (n = 7). g OXPHOS, maximum oxidative phosphorylation in the coupled state with complex I and II substrates, pyruvate and ADP; ETS, maximum mitochondria respiratory activity; Activity of Complex IV, uncoupled mitochondria respiratory activity linked to complex IV upon inhibition of complex I by rotenone (n = 7). h, i, Representative hypoxia stain images are shown and Pimonidazole staining was quantified by scoring blinded sections of proximal colon (n = 5) Data are displayed as Mean ± SEM; one-way ANOVA and Bonferroni’s post hoc comparison test were performed. (*p < 0.05, **p < 0.01, ***p < 0.001).
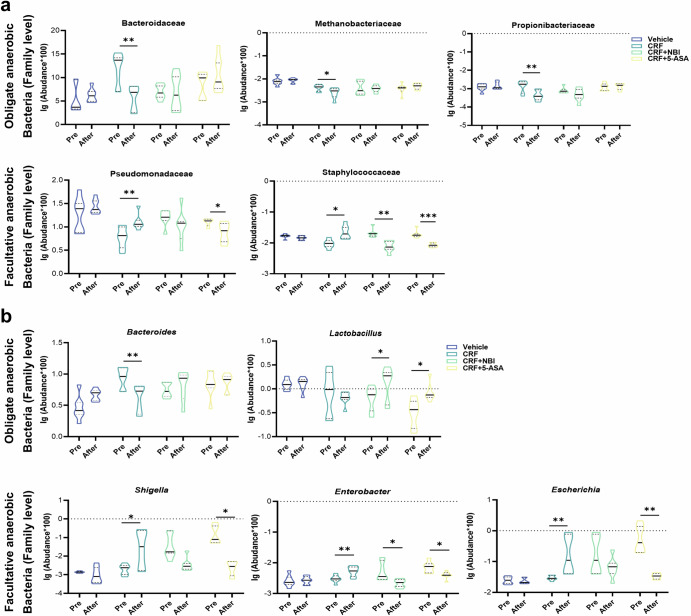
Metagenomic analysis revealed the microbiota profiles of mice in the Vehicle, CRH, CRH + NBI, and CRH + 5-ASA groups (Supplementary Fig. 13). By comparing the metagenomic sequencing results before and after intervention in mice, we observed that mice subjected to CRH intervention exhibited a significant increase in facultative anaerobes, such as Pseudomonadaceae and Enterobacter, while showing a decrease in obligate anaerobes, such as Bacteroidaceae and Propionibacteriaceae compared to pre-intervention levels. Notably, antagonizing CRHR1 expression or activating mitochondria was found to prevent CRH-induced gut dysbiosis. Moreover, there interventions led to a more significantly increase in obligate anaerobes like Lactobacillus and a decrease in facultative anaerobes such as Enterobacter compared to pre-intervention levels (Fig. 7).
Fig. 7. Comparison of gut microbiota in mice in Vehicle, CRH, CRH + NBI, and CRH + 5-ASA group before and after intervention.
a, b The Wilcoxon rank-sum test demonstrates significant differences in abundance at the family (a) and genus (b) levels based on microbiota profiling of feces from Vehicle, CRH, CRH + NBI, and CRH + 5-ASA group before and after intervention (n = 7). The violin plot displays the median (center line inside the violin), interquartile range (IQR, dotted line inside the violin), minimums and maximums within 1.5 times the IQR (bound of the violin); Independent-Sample t test were performed; Data non normally distributed were analyzed by Mann-Whitney U test. (*p < 0.05, **p < 0.01, ***p < 0.001).
Taken together, these findings verified the CRH-CRHR1-mitochondria pathway as a mechanism that transfers stress from the brain to the gut and triggers gut dysbiosis. Antagonizing the CRHR1 signaling pathway or activating mitochondria can alleviate the risks of stress-induced gut dysbiosis.
Discussion
Psychological stress exerts significant yet poorly understood impact mechanism on gut dysbiosis and brain-gut axis disordered diseases such as IBS32–34. Although pioneering studies have demonstrated that the brain-tuned neuropsychological signals to be perceived by the gut, but the specific mechanism involved remain elusive. Here we reveal a pathway where colonocytes’ mitochondria relay stress signals from the brain, leading to injure epithelium hypoxia and remodel gut microbiota. Specifically, this brain-to-gut circuit entails colonocytes’ CRHR1 as a niche signal that hampers mitochondria respiration to broke epithelium hypoxia and shift the dominant strains from obligate to facultative anaerobes in microbiota.
According to the clinical results, patients with IBS exhibit increased concentration of serum CRH, damaged colonocyte mitochondria ultrastructure, and gut dysbiosis compared with HC. Interestingly, we found the pattern of gut microbiome changes in patients with IBS is that microbiota shift from obligate anaerobes such as Methanobacteriaceae and Ruminococcus to facultative anaerobes such as Pasteurellaceae and Haemophilus, as well accompanying with the microbiota function shifts from anaerobic to aerobic reaction. Furthermore, correlation analysis revealed that increased facultative anaerobes and decreased obligate anaerobes related to increased CRH level and damaged colonocytes’ mitochondria ultrastructure. Although previous studies have also reported decreased levels of obligate anaerobes such as Methanobacteriaceae and Ruminococcus in patients with IBS, the underlying mechanism remains unclear35,36. Notably, colonocytes’ mitochondria is important for maintaining the colonic surface in a state of physiological hypoxia, which limits the amount of oxygen emanating from the mucosal surface, thereby checking aerobic growth of facultative anaerobes. Conversely, reduced mitochondria bioenergetics lower epithelial oxygen consumption, thereby diffusion of oxygen into the intestinal lumen37–39. Here, our findings provided a novel hypothesis, suggesting that facultative anaerobes increasing and obligate anaerobes decreasing might due to escaped oxygen from CRH-induced colonocytes’ mitochondria damage during IBS.
Since patients with IBS experienced the complex chronic stress have altered gut microbiota, whereas gut microbiota and its’ metabolism would conversely impact the gut health. To avoid this confounding factor, we designed WAS mice model to simulate acute psychological stress as well avoid the interaction between host and microbiota. As results, we observed a cascade of changes propagating from the brain to the gut in mice. Specifically, stress activated HPA axis, leading to the release of CRH and subsequent upregulated the expression of CRHR1 in colonocytes. This, in turn, damaged the mitochondria’s ultrastructure and respiration function, resulting in impaired epithelial hypoxia and escaped oxygen from intestinal surface to lumen. Here, our correlation analysis between mitochondria and microbiota in mice revealed that damaged mitochondria and impaired epithelium hypoxia exhibited negatively associated with obligate anaerobe such as Akkermansiaceae, while positively associated with facultative anaerobe such as Pseudomonadaceae and Moraxellaceae. This suggests that oxygen leaking from the epithelium into the lumen is a critical factor in disrupting microbiota balance. It is noteworthy that Akkermansiaceae, a bacterium known to interact with host intestinal cells, plays a vital role in regulating gut barrier function, antimicrobial peptide production, immune regulation, mucus layer thickness and inflammation. A decrease in the abundance of Akkermansiaceae has been linked to various diseases, including obesity, diabetes, liver steatosis, inflammation, and an impaired response to cancer immunotherapies40. On the other hand, facultative anaerobes like Pseudomonadaceae and Moraxellaceae are recognized as potential pathogens. They can invade the mucosa through virulence factors and induce inflammation41,42. Notably, while prior research has examined the influence of intestinal cell mitochondria on intestinal homeostasis, much of the focus has been on the interaction between mitochondria and the microbiota31,43. However, our findings provide a novel perspective, revealing that colonocyte mitochondria play a crucial role in transducing stress signals to the colon epithelium and initiating gut dysbiosis, which expands the research boundaries of the brain-gut axis.
Although clinical and WAS mice experiments have revealed the HPA axis activates, colonocytes’ mitochondria damages and gut dysbiosis are both triggered by stress, how CRH signal involved remain less well understood. To further explore the CRH mechanisms underlying how stress triggers these changes, we designed both in vitro and in vivo experiments. Using colonocytes treated with CRH and a stereotactic injection CRH-induced mouse model, we found that stress triggers gut dysbiosis via the CRH-CRHR1-mitochondria pathway. Specifically, mice treated with CRH exhibited a dramatical increase in the abundance of facultative anaerobes, such as Pseudomonadaceae and Enterobacter, and a decrease in obligate anaerobes, such as Bacteroidaceae and Propionibacteriaceae, compared to pre-intervention mice. Notably, the growth of Pseudomonadaceae or Enterobacter can induce intestinal inflammation and intestinal barrier dysfunction through their virulence factor, such as exotoxins and hemolysins44,45, while the decline of Bacteroidaceae and Propionibacteriaceae restricts the production of beneficial metabolites, such as acetic acid and propionic acid46,47. However, mice treated with CRH combined with NBI or -5ASA inhibited the growth of facultative anaerobes while promoting the abundance of obligated anaerobes. It is worth noting that although either antagonizing CRHR1 or activating mitochondria can both improve CRH-induced mitochondria respiration reduction and epithelium hypoxia diminish, only antagonizing CRHR1 can restore mitochondria dynamics defects. For these reasons, antagonizing CRHR1 expression may be a more effective strategy than activating mitochondria to alleviate oxidative stress triggered by escaped mt-DNA or mt-ROS from damaged mitochondria48. Although previous researches have reported that CRHR1 signaling plays a role in dorsal root ganglia (DRG) to mediate visceral hypersensitive and stimulate colonic motor function, our findings reveal that the CRHR1 signaling pathway also transmits stress to colonocytes, resulting in mitochondria damage. This refreshes the traditional understanding of the effect of CRHR149.
In general, although our series of clinical and basic studies have provided experimental support for the theme that colonocytes’ mitochondria relay the stress from the brain to the gut and triggers gut dysbiosis via oxygen escape, there are still some limitations should be addressed. Firstly, although we elucidated in-depth mechanism by which CRHR1 impairs mitochondria in the cAMP-Ras-MAPK signaling pathway, revealing other potential effects, such as immune signaling in the colonocyte microenvironment is necessary in the future. Secondly, further investigation is required to understand how changes in other components of the brain-gut axis, such as the enteric nervous system under stress, affect gut microbiota. Finally, given the limited number of clinical participants, a multiple-center study exploring IBS is needed to validate the link between colonocytes’ mitochondria and gut microbiota.
Based on our current understanding, our study has, for the first time, elucidated a novel mechanism in which the CRH-CRHR1-mitochondria pathway functions as a conduit for stress transmission from the brain to the gut, ultimately leading to gut microbiota dysbiosis. Furthermore, we conducted transcriptomic analysis and further revealed the in-depth mechanism by which CRHR1 damages colonocytes’ mitochondria via cAMP-Ras-MAPK signaling. This finding not only enhances our comprehension of the Brain-Gut-Microbiota interactions but also identifies potential therapeutic targets for the treatment of stress-induced gastrointestinal diseases.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant. NO. 82470578, No. 82170557 to L.D and 82201744 to X.L), the National Key Research and Development Program of China (grant No. 2021YFA1301304, 2019YFA0905600 to L.D)
Author contributions
Y.Z. conceived, designed, and supervised the project. Y.Z. and X.L. performed all the experiments and analysis, and wrote the paper. S.L., H.G., J.Z. and K.W. provided clinical trial’s data. Z.Z., H.Z., and C.Z. participated in the mice studies. F.P. instructed the images of histopathology. Y.Z. and X.L. wrote and revised the manuscript with inputs from L.D. All authors validated and approved the final manuscript.
Data availability
Metagenome sequencing datasets have been deposited in the National Center for Biotechnology Information under accession no. PRJNA1073694.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yiming Zhang, Xiaoang Li.
Supplementary information
The online version contains supplementary material available at 10.1038/s41522-024-00571-z.
References
- 1.Morais, L. H., Schreiber, H. L. T. & Mazmanian, S. K. The gut microbiota-brain axis in behaviour and brain disorders. Nature reviews. Microbiology19, 241–255, 10.1038/s41579-020-00460-0 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Camilleri, M. Diagnosis and Treatment of Irritable Bowel Syndrome: A Review. JAMA325, 865–877, 10.1001/jama.2020.22532 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Stewart Campbell, A. et al. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: an open-label phase 1b/2a trial. Nat. Med28, 528–534, 10.1038/s41591-022-01683-9 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Dalile, B., Van Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol.16, 461–478, 10.1038/s41575-019-0157-3 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Zhang, J. D. et al. Berberine alleviates visceral hypersensitivity in rats by altering gut microbiome and suppressing spinal microglial activation. Acta Pharmacologica Sin.42, 1821–1833, 10.1038/s41401-020-00601-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhuiyan, P., Wang, Y. W., Sha, H. H., Dong, H. Q. & Qian, Y. N. Neuroimmune connections between corticotropin-releasing hormone and mast cells: novel strategies for the treatment of neurodegenerative diseases. Neural Regenerat. Res.16, 2184–2197, 10.4103/1673-5374.310608 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, Y. et al. Lidocaine ameliorates intestinal barrier dysfunction in irritable bowel syndrome by modulating corticotropin-releasing hormone receptor 2. Neurogastroenterol. Motil.35, e14677, 10.1111/nmo.14677 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Battaglia, C. R., Cursano, S., Calzia, E., Catanese, A. & Boeckers, T. M. Corticotropin-releasing hormone (CRH) alters mitochondrial morphology and function by activating the NF-kB-DRP1 axis in hippocampal neurons. Cell Death Dis.11, 1004, 10.1038/s41419-020-03204-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borcherding, N. & Brestoff, J. R. The power and potential of mitochondria transfer. Nature623, 283–291, 10.1038/s41586-023-06537-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rath, E., Moschetta, A. & Haller, D. Mitochondrial function - gatekeeper of intestinal epithelial cell homeostasis. Nat. Rev. Gastroenterol. Hepatol.15, 497–516, 10.1038/s41575-018-0021-x (2018). [DOI] [PubMed] [Google Scholar]
- 11.Litvak, Y., Byndloss, M. X. & Bäumler, A. J. Colonocyte metabolism shapes the gut microbiota. Sci. (N. Y., N. Y.)362, eaat9076, 10.1126/science.aat9076 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solis, A. G., Klapholz, M., Zhao, J. & Levy, M. The bidirectional nature of microbiome-epithelial cell interactions. Curr. Opin. Microbiol.56, 45–51, 10.1016/j.mib.2020.06.007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, J. Y., Tsolis, R. M. & Bäumler, A. J. The microbiome and gut homeostasis. Sci. (N. Y., N. Y.)377, eabp9960, 10.1126/science.abp9960 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Jackson, D. N. & Theiss, A. L. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut microbes11, 285–304, 10.1080/19490976.2019.1592421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith, A. L. et al. Age-associated mitochondrial DNA mutations cause metabolic remodelling that contributes to accelerated intestinal tumorigenesis. Nat. cancer1, 976–989, 10.1038/s43018-020-00112-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho, G. T. & Theiss, A. L. Mitochondria and Inflammatory Bowel Diseases: Toward a Stratified Therapeutic Intervention. Annu. Rev. Physiol.84, 435–459, 10.1146/annurev-physiol-060821-083306 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong, X. et al. Activation of Drp1 promotes fatty acids-induced metabolic reprograming to potentiate Wnt signaling in colon cancer. Cell death Differ.29, 1913–1927, 10.1038/s41418-022-00974-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordin, E., Hellström, P. M., Brunius, C. & Landberg, R. Modest Conformity Between Self-Reporting of Bristol Stool Form and Fecal Consistency Measured by Stool Water Content in Irritable Bowel Syndrome and a FODMAP and Gluten Trial. Am. J. Gastroenterol.117, 1668–1674, 10.14309/ajg.0000000000001942 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Lohiniemi, S., Mäki, M., Kaukinen, K., Laippala, P. & Collin, P. Gastrointestinal symptoms rating scale in coeliac disease patients on wheat starch-based gluten-free diets. Scand. J. Gastroenterol.35, 947–949, 10.1080/003655200750023002 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Martoni, C. J., Srivastava, S. & Leyer, G. J. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 Improve Abdominal Pain Severity and Symptomology in Irritable Bowel Syndrome: Randomized Controlled Trial. Nutrients12, 363, 10.3390/nu12020363 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drossman, D. A. et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am. J. Gastroenterol.95, 999–1007, 10.1111/j.1572-0241.2000.01941.x (2000). [DOI] [PubMed] [Google Scholar]
- 22.Meng, J., Du, J., Diao, X. & Zou, Y. Effects of an evidence-based nursing intervention on prevention of anxiety and depression in the postpartum period. Stress health : J. Int. Soc. Investig. Stress38, 435–442, 10.1002/smi.3104 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Wang, Q. S. et al. Puerarin from Pueraria lobata alleviates the symptoms of irritable bowel syndrome-diarrhea. Food Funct.12, 2211–2224, 10.1039/d0fo02848g (2021). [DOI] [PubMed] [Google Scholar]
- 24.Zheng, G. et al. Glucocorticoid receptor-mediated Nr1d1 chromatin circadian misalignment in stress-induced irritable bowel syndrome. iScience26, 107137, 10.1016/j.isci.2023.107137 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu, C. et al. Low FODMAP Diet Relieves Visceral Hypersensitivity and Is Associated with Changes in Colonic Microcirculation in Water Avoidance Mice Model. Nutrients15, 1155, 10.3390/nu15051155 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan, D. C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol.15, 235–259, 10.1146/annurev-pathmechdis-012419-032711 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Sánchez-Rodríguez, R. et al. OPA1 drives macrophage metabolism and functional commitment via p65 signaling. Cell death Differ.30, 742–752, 10.1038/s41418-022-01076-y (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gómez-Valadés, A. G. et al. Mitochondrial cristae-remodeling protein OPA1 in POMC neurons couples Ca(2+) homeostasis with adipose tissue lipolysis. Cell Metab.33, 1820–1835.e1829, 10.1016/j.cmet.2021.07.008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, B., Zhang, J. & Ye, N. Noninvasive micro-test technology: monitoring ion and molecular flow in plants. Trends plant Sci.28, 123–124, 10.1016/j.tplants.2022.10.008 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Song, R., Dasgupta, C., Mulder, C. & Zhang, L. MicroRNA-210 Controls Mitochondrial Metabolism and Protects Heart Function in Myocardial Infarction. Circulation145, 1140–1153, 10.1161/circulationaha.121.056929 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, J. Y. et al. High-Fat Diet and Antibiotics Cooperatively Impair Mitochondrial Bioenergetics to Trigger Dysbiosis that Exacerbates Pre-inflammatory Bowel Disease. Cell host microbe28, 273–284.e276, 10.1016/j.chom.2020.06.001 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honarpisheh, P., Bryan, R. M. & McCullough, L. D. Aging Microbiota-Gut-Brain Axis in Stroke Risk and Outcome. Circul. Res.130, 1112–1144, 10.1161/circresaha.122.319983 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer, E. A., Nance, K. & Chen, S. The Gut-Brain Axis. Annu. Rev. Med.73, 439–453, 10.1146/annurev-med-042320-014032 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Margolis, K. G., Cryan, J. F. & Mayer, E. A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology160, 1486–1501, 10.1053/j.gastro.2020.10.066 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen, H. et al. Alternation of the gut microbiota in irritable bowel syndrome: an integrated analysis based on multicenter amplicon sequencing data. J. Transl. Med.21, 117, 10.1186/s12967-023-03953-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villanueva-Millan, M. J. et al. Methanogens and Hydrogen Sulfide Producing Bacteria Guide Distinct Gut Microbe Profiles and Irritable Bowel Syndrome Subtypes. Am. J. Gastroenterol.117, 2055–2066, 10.14309/ajg.0000000000001997 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu, Z. et al. Mitochondrial transcription factor A in RORγt(+) lymphocytes regulate small intestine homeostasis and metabolism. Nat. Commun.12, 4462, 10.1038/s41467-021-24755-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peña-Cearra, A. et al. Mitochondrial dysfunction-associated microbiota establishes a transmissible refractory response to anti-TNF therapy during ulcerative colitis. Gut microbes15, 2266626, 10.1080/19490976.2023.2266626 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konjar, Š. et al. Mitochondria maintain controlled activation state of epithelial-resident T lymphocytes. Sci. Immunol.3, eaan2543, 10.1126/sciimmunol.aan2543 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cani, P. D., Depommier, C., Derrien, M., Everard, A. & de Vos, W. M. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol.19, 625–637, 10.1038/s41575-022-00631-9 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Manner, C. et al. A genetic switch controls Pseudomonas aeruginosa surface colonization. Nat. Microbiol.8, 1520–1533, 10.1038/s41564-023-01403-0 (2023). [DOI] [PubMed] [Google Scholar]
- 42.Rubiola, S. et al. Shotgun metagenomic sequencing of bulk tank milk filters reveals the role of Moraxellaceae and Enterobacteriaceae as carriers of antimicrobial resistance genes. Food Res. Int. (Ott., Ont.)158, 111579, 10.1016/j.foodres.2022.111579 (2022). [DOI] [PubMed] [Google Scholar]
- 43.Yoo, W. et al. High-fat diet-induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Sci. (N. Y., N. Y.)373, 813–818, 10.1126/science.aba3683 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonis, A. et al. Discovery of highly neutralizing human antibodies targeting Pseudomonas aeruginosa. Cell186, 5098–5113.e5019, 10.1016/j.cell.2023.10.002 (2023). [DOI] [PubMed] [Google Scholar]
- 45.Sengupta, P., Muthamilselvi Sivabalan, S. K., Singh, N. K., Raman, K. & Venkateswaran, K. Genomic, functional, and metabolic enhancements in multidrug-resistant Enterobacter bugandensis facilitating its persistence and succession in the International Space Station. Microbiome12, 62, 10.1186/s40168-024-01777-1 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, X. et al. Bacteroides methylmalonyl-CoA mutase produces propionate that promotes intestinal goblet cell differentiation and homeostasis. Cell host microbe32, 63–78.e67, 10.1016/j.chom.2023.11.005 (2024). [DOI] [PubMed] [Google Scholar]
- 47.Dikeocha, I. J., Al-Kabsi, A. M., Ahmeda, A. F., Mathai, M. & Alshawsh, M. A. Investigation into the Potential Role of Propionibacterium freudenreichii in Prevention of Colorectal Cancer and Its Effects on the Diversity of Gut Microbiota in Rats. Int J. Mol. Sci.24, 8080, 10.3390/ijms24098080 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, Y., Zhang, J. & Duan, L. The role of microbiota-mitochondria crosstalk in pathogenesis and therapy of intestinal diseases. Pharm. Res186, 106530, 10.1016/j.phrs.2022.106530 (2022). [DOI] [PubMed] [Google Scholar]
- 49.Nozu, T. & Okumura, T. Corticotropin-releasing factor receptor type 1 and type 2 interaction in irritable bowel syndrome. J. Gastroenterol.50, 819–830, 10.1007/s00535-015-1086-8 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Metagenome sequencing datasets have been deposited in the National Center for Biotechnology Information under accession no. PRJNA1073694.