Abstract
The sole treatment for snakebite envenomation (SBE), the anti-snake venom (ASV), suffers from considerable drawbacks, including side effects and limited species specificity. Additionally, despite its existence for more than a century, uniform availability of good quality ASV does not yet exist. The present review describes the journey of a SBE victim and highlights the global crisis of SBE management. A detailed analysis of the current ASV market has also been presented along with the worldwide snake distribution. The current production of country specific licensed ASV throughout the globe along with their manufacturers has been examined at the snake species level. Furthermore, a detailed analysis of on-ground situation of SBE management in antivenom manufacturing countries has been done using the most recent literature. Additionally, the export and import of different ASVs have been discussed in terms of procurement policies of individual countries, their shortcomings, along with the possible solution at the species level. It is interesting to note that in most countries, the existence of ASV is really either neglected or overstated, implying that it is there but unsuitable for use, or that it is not present but can be obtained from other countries. This highlights the urgent need of significant reassessment and international collaborations not just for development and production, but also for procurement, distribution, availability, and awareness. A PROMISE (Practical ROutes for Managing Indigenous Snakebite Envenoming) approach has also been introduced, offering simple, economical, and easy to adopt steps to efficiently alleviate the worldwide SBE burden.
Keywords: Anti-snake venom, Anti-snake venom manufacturing, Export and import, Global policy, PROMISE approach, Snakebite envenoming management
Introduction
Anti-snake venom (ASV) is the only approved treatment for snakebite envenoming (SBE), the biggest public health crisis, that arises when a snake injects poisonous venom following a bite [1–3]. Despite the availability of ASVs for more than 100 years, more than 150,000 people are likely to die from snakebites globally, especially the poor in rural areas in developing countries [2, 4, 5]. The World Health Organization (WHO), in its 2019 and 2021 roadmaps, has aimed to halve the annual global SBE burden by 2030 [6, 7]. One of the four pillars of this roadmap is availability of safe, effective, and economical ASVs to people, and its improved control and regulation [8]. However, there have been reports about the flooding of market with low quality ASVs following the cessation of production by a major global ASV manufacturer [9]. The complexity of the venom components, geographical limitations, low funding, and improper ASV regulation are other issues in SBE management [4, 10, 11]. The latter is particularly important since even countries with capabilities of ASV production fail to properly manage SBE without proper ASV distribution policies. Thus, to reduce SBE burden and to ensure that no victim is left without treatment, it is necessary to understand the ASV market for better disbursement, regulation, and regional control. This will help in bilateral improvements in health policies with direct benefit to the poor victims and marked reduction in SBE induced fatalities and disability adjusted life years.
The present review summarizes the major causes of global crisis in SBE management and current ASV manufacturing practices of all the recognized ASV producers in a single map at the species level. To the best of our knowledge, this is the highest resolution snake biodiversity and antivenom manufacturing map ever published. For countries that do not produce, but import ASVs, respective import routes and procurement flaws have been highlighted, and alternative vendor countries have been suggested in terms of species specific ASVs availability. Finally, a PROMISE (Practical ROutes for Managing Indigenous Snakebite Envenoming) approach has also been proposed comprising of straightforward, cost-effective, and easily implementable measures for immediate reduction in the global SBE burden.
This article is expected to aid policymakers for improved fund allocation, regulation of ASV production, procurement, and distribution, with learning from case studies of several countries around the world. This information should also be useful for decision making for countries who wish to procure ASV from alternative countries that produce more specific ASVs. Additionally, it will aid in educating the physicians about the ASVs available in one’s own and neighboring countries and improved SBE patient management. Finally, the PROMISE approach should serve as easy to implement ideas for any country interested in reducing SBE burden without significant economical investments.
Method
Initial search was done on Scopus with the term ‘snakebite’ or ‘antivenom’ along with individual ‘country name’ in ‘article title, abstract, and keyword’ section. Region specific studies from 2020 onwards that documented the on-ground situation of antivenom availability in the country were included. Studies focused on alternative antivenoms, patient case studies, venom characterizations, non-human victims, other animals, and antivenom manufacturing were excluded. In case no relevant article could be found on Scopus, a general internet search was done using the same keywords to find the relevant literature. Where appropriate, the reference list of included articles was also screened to identify the most appropriate article.
The Global SBE Management Crisis
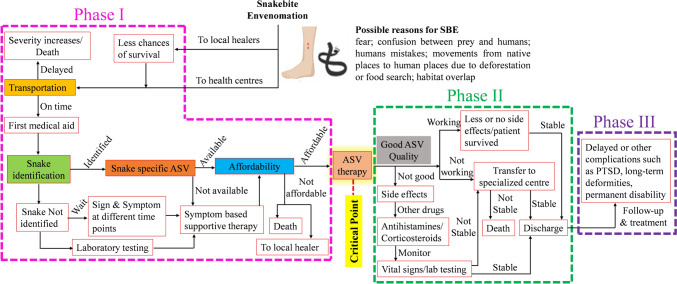
A victim’s journey from SBE to receive ASV therapy is filled with multiple hurdles (Fig. 1). The first is transportation to a health centre because SBE is largely the disease of the poor people from rural or remote areas in developing or low-income countries. These places often lack good health care centres and have poor transportation facilities which also force patients to resort to traditional healers over modern medicine [12–14]. Nevertheless, once a patient manages to reach a health care center, the next hurdle is SBE identification, because not all snakes present with a distinct clinically identifiable set of symptoms, the snake description reported by the patient or the attendant is often inadequate, and many health care practitioners (HCPs) have only limited knowledge about SBE [2, 15]. Other hurdles include availability of the species-specific and good quality ASV in appropriate quantity. Affordability is another major hurdle, not just that the ASVs are expensive, but the costs of additional therapies and hospital further limits affordability for the poor [16]. For a patient who crosses all these hurdles, the administration of the ASV serves as a critical life-saving point and marks the end of, what we call, the phase I of the patient’s journey to SBE therapy.
Fig. 1.
The journey of a SBE victim. The coloured boxes indicate the major hurdles in ASV therapy. The outcome of the antivenom therapy can be lifesaving or fatal depending upon the quality, specificity, efficacy, dosage, time of administration, and side effects of the antivenom administered. Note that affordability includes ASV cost as well as other hospital expenses
The administration of only a safe, affordable, specific, and good quality ASV can mitigate the effects of SBE [17]. This is the beginning of phase II of the patient’s post therapy struggles because the administration of the ASV does not guarantee permanent cure. Low quality ASV can lead to mild to severe side effects in patients [18]. Further, ASV may not always be effective in preventing SBE mediated damage. This is often the case when the patient arrives hours after the bite or the ASV administration is delayed because of phase I hurdles. Studies recommend early ASV administration within 2–6 h post bite [19–21]. This is because delayed ASV therapy can prevent further damages, but it cannot reverse the damage already done [22]. Additionally, delay in bite to needle time not only increases systemic envenomation, but also increases the number of ASV vials required for treatment, along with length of hospital stay, as well as the risk of morbidity and mortality [19].
Unfortunately, even after administration of a good quality and effective ASV, complete cure from SBE effects is not guaranteed and late adverse reactions of ASV and disability adjusted life years are common [2, 18]. This is phase III of the patient struggle which may continue for the entire lifespan. Nevertheless, the earliest possible administration of safe and specific ASV is the only available treatment paradigm to save a SBE victim’s life and this is still not accessible in many countries. A deeper analysis about the targeted species, ASV production, and procurement strategies is thus needed to fully understand the global status of ASVs.
The Global Status of Anti-Snake Venoms
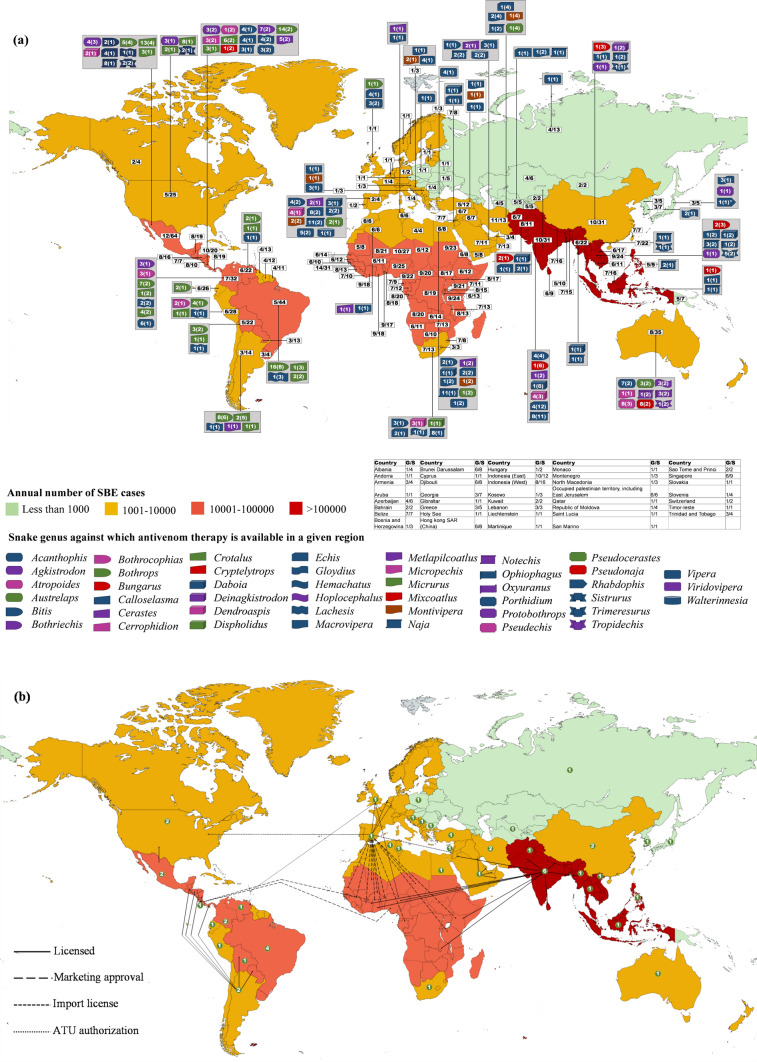
Although SBE continues to be a cause of worldwide concern, it has affected some parts of the world worse than others (Fig. 2). The global distribution of SBE burden, number of snake genera and species found in each country, ASV production in various countries, targeted genera, and the number of anti-snake venoms manufactured against each genus in different regions, as listed in the WHO database [23], are summarized in Fig. 2a. The corresponding export and import network of anti-snake venoms along with the number of ASV manufacturers in each country are presented in Fig. 2b.
Fig. 2.
Geographical distribution of snakes, snakebites, anti-snake venom production, the targeted genera, and species. a Global snakebite cases, snake distribution, ASV production, its distribution, and the targeted genera. Numbers in white boxes represent the number of snake genera followed by number of species found in respective countries. Each symbol represents a genus targeted by the antivenom(s) produced in each country. The number of targeted species of a genus is written inside the corresponding symbol. Numbers in parentheses represent the total number of antivenoms produced by the manufacturing country against the given genus. * represents investigational product. G/S correspond, respectively, to the number of genera and species present in a country. Note: Ecuador shut down ASV manufacturing in 2012, and the last batch of Croatia ASV expired in 2019. Both countries are now dependent on imports [24] [25]. b Antivenom manufacturers and import/export. Numbers inside the circles represent the number of antivenom manufacturers in the corresponding country. Note: Kenya recalled Indian antivenoms in 2023 due to inefficacy and is aiming self-manufacturing [26] [27]. Based on published data and WHO [23, 28]
The number of ASV manufacturers have increased from 46 to 51 since 2018 [23, 29]. However, the variability in the global ASV distribution and the limited availability of ASV against some genera is still quite evident. Furthermore, the ASV against many genera are produced by a single manufacturer in a given country. In fact, 29 out of 37 countries have a single ASV manufacturer. A detailed description of the ASV status around the world is discussed below.
Asia
This region has the highest burden of SBE in the world and contributes to 70% SBE mortality globally [30]. There are about 300 snake species including 70 venomous snakes. However, most countries manufacture ASV against only a few species. As a result, the use of non-specific ASVs for treating bites from non-targeted species is frequent across almost all countries. For example, in mainland China, envenomations with green pit viper are recommended to be treated with ASVs against snakes from the same subfamily Crotalinae with unsatisfactory therapeutic efficacy [31]. Even in India, which is a leading producer and exporter of ASVs in the world, ASVs against the big four species is used to treat all SBE cases. However, this polyvalent ASV is ineffective in treating bites from other medically important snake species present in the region, such as H.hypnale, Echis sochureki, Naja kaouthia, and C. malabaricus, voicing the need for availability of appropriate ASVs [32–35]. Also, the Indian polyvalent ASV exhibits limited efficacy against identical species in different parts of the country due to differences in the venom composition [32, 36–38].
Of the many manufacturing countries, India and Iran are the only ASV exporters in the region. While the Iranian ASV is permitted in a few Middle East countries, India exports its ASV to Africa, Middle East, and South Asia. It can be said that the South Asian region is majorly dependent on the Indian ASVs. Nevertheless, since South Asia bears 70% of the global SBE burden, special attention must be given to the Indian ASV manufacturers [30]. No information about the ASVs available in Bangladesh and Afghanistan could be found in the WHO database despite the significantly high SBE burden in these regions.
Africa
In Africa, a region with a high annual SBE incidence of up to 100 thousand, most afflicted countries do not produce ASV. The continent has only four ASV manufacturers listed in the WHO database, one each in Algeria, Tunisia, Egypt, and South Africa. Antivenoms from neither of these 4 manufacturers is approved in other African countries. The sub-Saharan Africa region, which bears the second largest burden of SBE after Asia, thus has only one ASV producer in this region i.e. South Africa [9]. Here also, there is discrepancy in ASV availability between rural and urban areas [39]. The African countries depend on imports from other continents, which risks the supply chain and raises the cost of already expensive ASVs which is estimated to be US$100–153 in the region [9]. Further, the market is unstable and the production of many ASVs has been stopped in the past. Chad, Ghana, Central Africa Republic, and Nigeria have already faced increased mortality due to the poor quality of some ASVs when a leading foreign ASV manufacturer stopped supply [9]. Furthermore, efficacy data of the imported ASVs is majorly lacking [40]. In addition, the venoms used for ASV production are sourced from limited suppliers only. Given the vastness of this region and the biogeographical venom variation of the multitude of species found here [41], extensive cross-reactivity studies of existing ASVs can be an interim solution to the SBE problem.
North America
There are more than 30 species of pit vipers in the region and almost 9,000 SBE cases are reported annually [42]. Within the family, rattlesnakes account for the majority of the casualties because of their potent venom and widespread distribution. The first ASV was approved as early as 1954. Costa Rica is the major ASV producer in the area followed by Mexico and USA, in terms of number of targeted snake species. Costa Rica is also a major exporter of ASVs and has product marketing approvals in neighbouring as well as African countries.
South America
Manufacturing of ASVs is an old tradition since 1901 when the first ASV was developed in Brazil [43]. However, only limited amounts of ASV are produced due to the restricted capacities of the only four ASV producing laboratories [44]. Antivenom is also produced in Argentina, Peru, Colombia, Venezuela, and Bolivia. However, despite the presence of multiple ASV manufacturers, many countries are dependent on import and ASV supply is usually insufficient [45]. Also, not all the ASVs are undergo quality control and preclinical efficacy assessments [43]. The importance of the former can be understood from Eucador, where ASV manufacturing was decommissioned owing to flaws in production process, and the manufacturing plant was closed in 2012 [24]. Ecuador is now dependent on ASV import from Costa Rica.
Europe
It has a burden of approximately 8000 SBE annually and the medically relevant venomous snakes belong to the Viperinae family [46, 47]. There is a long history of ASV production in Europe and almost 13 laboratories produced it in early 1980s. However, in Croatia, the last ASV batch expired in 2019 and Croatia, despite possessing ASV manufacturing capabilities, is currently dependent on imports [25]. Nevertheless, trust in immunotherapy, has recently increased with availability of purified and well tolerated ASVs. There are several ASVs for vipers in Europe [47, 48]. Recently, a new polyvalent ASV has been shown to be effective against European vipers [49]. Only two manufacturers from United Kingdom and Spain export ASVs. The Spanish ASV is a cornerstone product for many African countries. This is a Pan-African ASV which is claimed to target 22 snake species from 4 genera. However, this product has not been recommended by the WHO. The WHO has terminated its risk–benefit assessment without making any recommendations [23]. Also, none of the European ASVs is licensed by the European Medical Agency [48].
Australia
Snakebite envenomings are rare in Australia, but some cases may be life-threatening [50]. Brown snake, tiger snakes, black snakes, death adder, taipan, and sea snakes are the major clinically important snakes in the region [51]. ASV is produced in the region since 1930 and ASVs were developed against all major snake groups as early as 1962 [52]. However, there is only a single manufacturer, and it does not export its ASVs. Commercial snake venom detection tests are available, however, due to the polyvalent nature of Australian ASVs and problems with the kit's results, the emphasis has turned to identifying envenoming in patients rather than snake identification [53]. The region also has diverse species of sea snakes but a single monovalent ASV, raised against a Malaysian snake, is used to treat all sea snake envenomations [21].
Despite the aforementioned issues, different countries of the world have made attempts to lower the SBE burden in a variety of ways, but a number of barriers still exist. Table 1 summarizes the most recent information on these.
Table 1.
Effective steps and barriers to SBE management
| Region | Countrya | Manufacturer(s) | Effective steps | Barriers in SBE management | References |
|---|---|---|---|---|---|
| Asia | China |
National Institute of Preventive Medicine Shanghai Serum Bio-technology Co Ltd |
Haphazard ASV supply Non-specific ASVs ASV shortage |
[31] | |
| India |
Biological E Limited Premium Serums and Vaccines Pvt. Ltd VINS Bioproducts Ltd King Institute of Preventative Medicine and Research Haffkine Biopharmaceutical Corporation Ltd Bharat Serums and Vaccines Limited |
Emergency ambulance service with lifesaving equipment and drugs, including ASV |
Under reporting of SBE and mortality Lack of safe and effective antivenoms Poor healthcare facilities Inclination towards traditional healers ASV manufactured only against big four Untrained medical staff |
[91–94] | |
| Indonesia | PT Bio Farma (Persero) |
Antivenom cross-neutralization data required for marketing approval SBE treatment costs covered on co-payment basis |
A single ASV is available Costly and limited ASV Antivenom ineffective against many Indonesian species Absence of antivenom in nearby health care facilities and lack of transportation Absence of cold-chain storage Cultural barriers |
[75, 95] | |
| Iran |
Razi Vaccine & Serum Research Institute Padra Serum Alborz |
Toxicology trained physicians National unified protocol for SBE management |
Under-reporting of SBE cases Preference of traditional treatment No formal clinical trial of one antivenom Antivenom starting dose not established by formal clinical trial Wound incision and fasciotomy still practiced |
[74] | |
| Israel | Kamada limited |
Short distance to hospital in some areas Uncertain snake identification in many cases |
Lack of uniform treatment protocol for SBE No antivenom available against one of the venomous endemic species-Atractaspis engaddensis |
[96, 97] | |
| Japan | KM Biologics Co. Ltd | Snake institute to help physicians | Unapproved antivenom against R. tigrinus | [98] | |
| Myanmar | Myanmar pharmaceutical factory |
Myanmar Snakebite Project Antivenom usage reported to ministry |
lack of pharmaceutical logistic system affects antivenom distribution | [75] | |
| Pakistan | National Institute of Health |
Inclination towards traditional healers Long transportation times Manufactures liquid antivenom but has poor refrigeration facilities Low domestic supply No guidance protocol for antivenom production Inadequately trained health care workers |
[99] | ||
| Philippines | Biologicals Manufacturing Division (Research Institute for Tropical Medicine) |
Subsidised antivenom production SBE treatment cost included in health insurance |
Seek traditional healers ASV shortage Ineffective ASV supply chain |
[75] | |
| Republic of Korea | KoreaVaccine Co Ltd | National reference standard for antivenom |
Costly antivenoms Validated guideline for antivenoadministration unavailable No ASV against R. tigrinus |
[100–102] | |
| Saudi Arabia | National Antivenom & Vaccine Production Center (NAVPC) |
Good medical facility Antivenom available even in remote areas Established national records |
[103] | ||
| Thailand | Queen Saovabha Memorial Institute |
Well-established supply chain real-time antivenom inventory |
No national training on SBE management since 2016 | [75] | |
| Vietnam | Institute of Vaccines and Biological Substances (IVAC) |
Inclination towards traditional practices Poor documentation of SBE records Lack of SBE statistics Limited studies on clinical presentation of SBE No national protocol for SBE management Antivenom shortage and high cost Long distance to hospitals Lack of trained HCPs Lack of SBE education and public awareness Monovalent antivenoms prevalent which require accurate snake identification Use of antivenoms of unknown efficacy and potency Only one study on adverse reaction to antivenom so far |
[68] | ||
| Africa | South Africa | South African VaccinProducers (SAVP) |
Free toxicology advice to HCPs and public at all hours Trained physicians Free snake identification charts |
Discrepancy in antivenom availability in urban and rural areas SBE reporting not mandatory Need of cold chain transport Short expiry of ASVs |
[39, 104] |
| Tunisia | Institut Pasteur de Tunis |
No validated scale of SBE severity Limited studies on SBE Lack of health facilities in rural areas Delay between bite and hospital arrival Propensity for at home first aid such as tourniquets |
[105] | ||
| North America | Costa Rica | Instituto Clodomiro Picado |
Free antivenom SBE notification mandatory Traditional medicine rarely used Quality control of antivenoms Good cold chain National protocol for SBE diagnosis and treatment Regular training sessions on SBE management |
A part of population is devoid of SBE related governmental aids, such as agricultural workers Access to health facility delayed in some regions |
[106] |
| Mexico |
Birmex (Instituto Nacional de Higiene) Laboratorios Silanes, S. A. de C. V |
Inclination towards traditional healers Limited data on SBE epidemiology |
[107] | ||
| United States |
BTG International Inc Wyeth (owned by Pfizer) |
Cost transparency Concerns about insurance cover Antivenom not available at all facilities Costly antivenom Controversial maintenance therapy |
[108, 109] | ||
| South America | Bolivia | Ministerio de Salud y Deportes, Instituto Nacional de Laboratorios De Salud |
Snake misidentification Untrained HCPs |
[110] | |
| Brazil |
Fundacao Ezequiel Dias (FUNED) Centro de Producao e Pesquisas de Immunobiol Instituto Butantan Instituto Vital Brazil S.A |
Compulsory case notification Free antivenom |
Inclination towards traditional practices No antivenom in rural areas Lack of trust in local health care Low confidence among clinicians in SBE management Overdosing and under-dosing of antivenoms Antivenom expiration owing to lack of inventory Antivenom storage issues due to lack of stable electricity Limited ASV production capacity |
[64, 111] | |
| Colombia |
Instituto Nacional de Salud (CO) Laboratorios Biologicos PROBIOL Ltda |
Mandatory reporting |
Regular antivenom shortage Lack of cold chain transport No policy for antivenom distribution |
[63] | |
| Ecuador | Instituto Nacional de Higiene y Medicina Tropical "Leopoldo Izquieta Pérez" | Fixed maximum price for antivenom |
Antivenom production stopped in 2012 Dependent on import of ASV from Costa Rica |
[24] | |
| Europe | Croatia | Imunološki Zavod (Institute of Immunology) | Low reported mortality |
Last antivenom batch expired in 2019 Dependent on imports now |
[25] |
| Serbia | Institute of Virology, Vaccine and Sera TORLAK |
Antivenom available Snakes protected by law |
HCPs unawareness of species in their areas Snake misidentification common |
[85] | |
| Spain | INOSAN BIOPHARMA S. A. (Spain) | Very few cases and fatalities | [112] | ||
| The United Kingdom | Micropharm Ltd | 24 h rapid clinical advice available through poison centres | Exotic snakebites a challenge | [113] | |
| Australia | Australia | Seqirus Pty Ltd |
Costly ASV Need of cold chain transport Limited shelf-life Single antivenom to treat envenoming by all sea snakes – due to rarity of sea snake envenoming-more time for administering antivenom than terrestrial SBE cases Limited clinical evidence to support use of this antivenom for sea snake envenoming |
[21] |
The table lists the anti-snake venom (ASV) manufacturing countries and companies. Additionally, it presents a non-exhaustive list of effective steps taken by ASV manufacturing countries and the existing gaps in snakebite management. The information has been sourced from the WHO snake ASV database [23] and the most recent literature (2020-present) to provide insights into the present situations in these countries. The list is by no means exhaustive and additional steps or gaps might be present in the respective countries
aFor Algeria, Argentina, Bulgaria, Egypt, Peru, Poland, Russia, Turkey, Uzbekistan, and Venezuela, no relevant literature, published 2020 onwards, could be found
The Problem with Imported Anti-Snake Venoms
It is important to remember that, even when ASVs from one nation are used to treat SBE in another, the ASV may not be successful because of regional differences, leading to less effective ASVs on the market, as seen in the case of Sri Lanka [54–56]. Also, there are cases where the imported ASVs are not raised against any endemic species of a country. For example, in Jordan, one of the countries in middle east with maximum number of snakebites, the single imported ASV is raised against African snakes that do not occur in Jordan and is clinically ineffective [57]. Analysis of the efficacy of imported ASVs against envenomation by endemic species is important as their efficacy is not always guaranteed. Recently, Kenya recalled the Indian ASV upon evidence of its inefficacy in treating Kenyan snake envenomation cases [26]. It must be noted that the recalled ASV is listed in the WHO snake ASV database and is claimed to target many species found in Kenya [23]. Kenya may now partner with Costa Rica for technology transfer and aim local ASV production in next three years, as per the reports [27]. However, bringing Kenyan ASV to market will not be an easy task. Sri Lanka had earlier collaborated with Costa Rica and US for a similar goal [22]. However, it has been almost a decade since the collaboration, but the clinical trial data has not been published. In their another collaboration with a leading antivenom manufacturer in India, a product has shown better pre-clinical efficacy than currently used Indian ASV, however, its additional pre-clinical and clinical studies are still required [58]. The country is still dependent on import from India, whose ASV has been reported to bear limited efficacy against Sri Lankan snake species [54, 58].
For almost all countries, the imported ASVs do not target all regional species. Some of these missed species are even of the highest medical importance with their respective ASVs available for sale in other countries. The list of possible vendor countries where ASV is available for each of the missed species of each importing country (as per WHO database) is provided in Table 2. Organizations like WHO that deal in the purchase and distribution of ASVs in underdeveloped nations like sub-Saharan Africa should also find Table 2 useful [8]. For example, the Spanish ASVs may be useful in targeting species of Jordan and the Brazilian ASV for targeting snakes in Paraguay. Greater international cooperation between countries is thus needed to import other/additional ASVs. Still, for many other species no ASV is available in any part of the world. Production of newer ASVs, inclusion of venoms of these species in immunogen mixtures used for ASV production, or cross-reactivity studies of existing ASVs should be undertaken for dealing envenomation cases with these species.
Table 2.
The global export–import network of anti-snake venom (ASV)
| Region | Importing country | Exporting country | Antivenom name | Claimed species targeted in importing country | Missed species [possible vendor countries] | Missed species—no antivenom available |
|---|---|---|---|---|---|---|
| Asia | Israel | Spain | Inoserp™ MENA | Cerastes cerastes, Cerastes gasperettii, Daboia palaestinae, Echis coloratus, Pseudocerastes fieldi, Walterinnesia aegyptia | None | Atractaspis engaddensis, Montivipera bornmuelleri |
| Israel | Israel | Vipera palaestinae Antiserum (Equine source) | Daboia palaestinae | |||
| Israel | Israel | Echis coloratus Antiserum (Equine source) | Echis coloratus | |||
| Asia | Jordan | India | Snake Venom Antitoxin (Menaven) | None | Cerastes gasperettii [Spain, Saudi Arabia], Daboia palaestinae [Egypt, Israel, Spain], Echis coloratus [Egypt, Israel, Saudi Arabia, Spain, The United Kingdom], Macrovipera lebetina [Algeria, Croatia, Egypt, Iran (Islamic Reublic of), Spain, Serbia, Tunisia, Turkey, Uzbekistan], Pseudocerastes fieldi [Egypt, Spain], Walterinnesia aegyptia [Saudi Arabia, Spain] | Atractaspis engaddensis |
| Snake Venom Antiserum (Echis) | ||||||
| Asia | Kuwait | Saudi Arabia | Polyvalent Snake Antivenom—Equine | Cerastes gasperettii, Walterinnesia morgani | None | None |
| Asia | Myanmar | India | Snake Venom Antiserum I.P. (Asia) | None | Bungarus candidus [Thailand], Bungarus fasciatus [Indonasia, Thailand], Naja siamensis [Thailand], Naja sumatrana [Thailand], Ophiophagus hannah [Thailand], Protobothrops mucrosquamatus [China], Trimeresurus albolabris [Thailand, Vietnam], Trimeresurus erythrurus [Thailand], Trimeresurus purpureomaculatus [Thailand] | Bungarus bungaroides, Bungarus niger, Bungarus flaviceps, Bungarus magnimaculatus, Bungarus wanghaotingi, Naja mandalayensis, Protobothrops jerdonii, Protobothrops kaulbacki, Trimeresurus gumprechti, Trimeresurus guoi, Trimeresurus yunnanensis |
| Myanmar | Myanmar | Anti-Viper (Russell's viper) | Daboia siamensis | |||
| Myanmar | Myanmar | Siamese cobra antivenin | Naja kaouthia | |||
| Asia | Nepal | India | Snake Venom Antiserum I.P. (Asia) | Bungarus caeruleus,Daboia russelii, Naja naja | Bungarus fasciatus [Indonasia, Thailand], Naja kaouthia [Myanmar, Thailand, Vienam], Ophiophagus hannah [Thailand] | Bungarus bungaroides, Bungarus lividus, Bungarus niger, Bungarus walli, Gloydius himalayanus, Protobothrops jerdonii, Protobothrops himalayanus, Trimeresurus salazar, Trimeresurus septentrionalis, Trimeresurus tibetanus |
| Asia | Oman | Saudi Arabia | Polyvalent Snake Antivenom—Equine | Bitis arietans, Cerastes gasperettii, Echis coloratus, Echis omanensis, Naja arabica | Echis carinatus [India, Iran (Islamic Republic of), Pakistan, Spain, Uzbekistan], Echis khosatzkii [Spain], Pseudocerastes persicus [Iran (Islamic Republic of), Spain], Vipera ammodytes [Bulgaria, Croatia, Egypt, Serbia, Spain, Turkey], Vipera berus [Bulgaria, Croatia, Poland, Russian Federation, Serbia, Spain, The United Kingdom], Vipera ursinii [Bulgaria, Croatia] | Atractaspis andersonii, Vipera nikolskii |
| Asia | Pakistan | India | Snake Venom Antiserum I.P. (Asia) | Bungarus caeruleus, Daboia russelii, Echis carinatus, Naja naja | Macrovipera lebetina [Algeria, Croatia, Egypt, Iran (Islamic Reublic of), Spain, Serbia, Tunisia, Turkey, Uzbekistan], Pseudocerastes persicus [Iran (Islamic Republic of), Spain] | Bungarus persicus, Eristicophis macmahonii, Gloydius himalayanus |
| Pakistan | Pakistan | Polyvalent Antisnake Venom Serum | Bungarus caeruleus, Bungarus sindanus, Daboia russelii, Echis carinatus, Naja naja, Naja oxiana | |||
| Asia | Qatar | Saudi Arabia | Polyvalent Snake Antivenom—Equine | Cerastes gasperettii | None | None |
| Asia | Sri Lanka | India | Snake Venom Antiserum I.P. (Asia) | Bungarus caeruleus, Daboia russelii, Echis carinatus, Naja naja | None | Bungarus ceylonicus, Hypnale hypnale, Hypnale nepa, Hypnale zara, Trimeresurus trigonocephalus |
| Asia | UAE | Saudi Arabia | Polyvalent Snake Antivenom—Equine | Cerastes gasperettii, Echis omanensis | Echis carinatus [India, Iran (Islamic Republic of), Pakistan, Spain, Uzbekistan], Pseudocerastes persicus [Iran (Islamic Republic of), Spain] | None |
| Africa | Mali | Spain | Inoserp™ Pan-Africa | Bitis arietans, Dendroaspis polylepis, Echis leucogaster, Echis ocellatus, Echis pyramidum, Naja haje, Naja katiensis, Naja nigricollis, Naja pallida, Naja savannula, Naja senegalensis, Naja subfulva | Cerastes cerastes [Algeria, Egypt, India, Saudi Arabia, Spain, Tunisia], Dispholidus typus [South Africa] | Atractaspis fallax, Atractaspis micropholis, Atractaspis watsoni, Echis hughesi, Echis jogeri,Naja ashei, Thelotornis mossambicanus |
| Africa | Morocco | Spain | Inoserp™ MENA | Bitis arietans, Cerastes cerastes, Daboia mauritanica, Echis leucogaster, Naja haje | None | Vipera monticola |
| Africa | Nigeria | Costa Rica | EchiTAb-plus-ICP Liquid | Bitis arietans, Bitis gabonica, Bitis nasicornis, Echis leucogaster, Echis ocellatus, Echis romani | Dispholidus typus [South Africa], Naja katiensis [Spain], Naja senegalensis [Spain] | Atheris broadleyi, Atheris chlorechis, Atheris squamigera, Atractaspis irregularis, Atractaspis micropholis, Atractaspis watsoni, Pseudohaje goldii, Pseudohaje nigra, Thelotornis kirtlandii |
| Nigeria | India | Snake Venom Antiserum (Africa) | Bitis arietans, Bitis gabonica, Dendroaspis jamesoni, Dendroaspis viridis, Echis leucogaster, Echis ocellatus, Naja haje, Naja melanoleuca, Naja nigricollis, Naja savannula, Naja subfulva | |||
| Nigeria | The United Kingdom | EchiTAbG | Echis ocellatus, Echis romani | |||
| Africa | Republic of Congo | Mexico | Antivipmyn® Africaa | Bitis arietans, Bitis gabonica, Dendroaspis polylepis, Naja haje, Naja melanoleuca, Naja nigricollis, Naja subfulva | Bitis nasicornis [Costa Rica, India, Spain], Dendroaspis jamesoni [India, Spain, South Africa], Dispholidus typus [South Africa] | Atheris broadleyi, Atheris squamigera, Atractaspis bibronii, Atractaspis irregularis, Naja anchietae, Naja annulata, Naja christyi, Pseudohaje goldii, Thelotornis kirtlandii, Thelotornis capensis |
| Africa | Sierra Leone | Spain | Inoserp™ Pan-Africa | Bitis arietans, Bitis nasicornis, Bitis rhinoceros, Dendroaspis polylepis, Dendroaspis viridis, Naja guineensis, Naja nigricollis, Naja savannula | Dispholidus typus [South Africa] | Atheris chlorechis, Echis jogeri, Pseudohaje nigra, Thelotornis kirtlandii |
| Africa | Senegal | Spain | Inoserp™ Pan-Africa | Bitis arietans, Dendroaspis polylepis, Dendroaspis viridis, Echis leucogaster, Echis ocellatus, Naja katiensis, Naja nigricollis, Naja savannula, Naja senegalensis | Dispholidus typus [South Africa] | Atractaspis microlepidota, Atractaspis micropholis, Atractaspis watsoni, Echis jogeri |
| Africa | Tanzania | Spain | Inoserp™ Pan-Africa | Bitis arietans, Bitis gabonica, Bitis nasicornis, Dendroaspis angusticeps, Dendroaspis jamesoni, Dendroaspis polylepis, Naja haje, Naja nigricollis, Naja pallida, Naja subfulva | Dispholidus typus [South Africa], Naja mossambica [Costa Rica, Egypt, South Africa] | Atheris squamigera, Atractaspis bibronii, Atractaspis fallax, Atractaspis irregularis, Naja annulata, Naja ashei, Pseudohaje goldii, Proatheris superciliaris, Thelotornis capensis, Thelotornis kirtlandii, Thelotornis mossambicanus, Thelotornis usambaricus |
| Africa | Togo | Spain | Inoserp™ Pan-Africa | Bitis arietans, Bitis nasicornis, Bitis rhinoceros, Dendroaspis jamesoni, Dendroaspis viridis, Echis ocellatus, Naja guineensis, Naja katiensis, Naja nigricollis, Naja savannula, Naja senegalensis | Dispholidus typus [South Africa] | Atheris chlorechis, Atractaspis irregularis, Atractaspis watsoni, Pseudohaje nigra, Thelotornis kirtlandii |
| Africa | Gabon | Spain | Inoserp™ Pan-Africa | Bitis arietans, Bitis gabonica, Bitis nasicornis, Dendroaspis jamesoni, Naja melanoleuca, Naja nigricollis | None | Atheris broadleyi, Atheris squamigera, Atractaspis irregularis, Naja annulata, Pseudohaje goldii, Thelotornis kirtlandii |
| Africa | Ghana | India | Snake Venom Antiserum (Africa) | Bitis arietans, Dendroaspis jamesoni, Dendroaspis viridis, Echis ocellatus, Naja guineensis, Naja nigricollis, Naja savannula | Dispholidus typus [South Africa] | Atheris chlorechis, Atractaspis irregularis, Atractaspis watsoni, Pseudohaje goldii, Pseudohaje nigra, Thelotornis kirtlandii |
| Ghana | Mexico | Antivipmyn® Africaa | Bitis arietans, Dendroaspis viridis, Echis ocellatus, Naja guineensis, Naja nigricollis, Naja savannula | |||
| Ghana | Spain | Inoserp™ Pan-Africa | Bitis arietans, Bitis nasicornis, Bitis rhinoceros, Dendroaspis jamesoni, Dendroaspis viridis, Echis ocellatus, Naja guineensis, Naja katiensis, Naja nigricollis, Naja savannula, Naja senegalensis | |||
| Africa | Guinea | Spain | Inoserp™ Pan-Africa | Bitis arietans, Bitis gabonica, Bitis nasicornis, Bitis rhinoceros, Dendroaspis jamesoni, Dendroaspis polylepis, Dendroaspis viridis, Echis ocellatus, Naja guineensis, Naja katiensis, Naja melanoleuca, Naja nigricollis, Naja savannula, Naja senegalensis | Acanthophis laevis [Australia], Acanthophis rugosus [Australia], Dispholidus typus [South Africa], Micropechis ikaheka [Australia], Oxyuranus scutellatus [Australia], Pseudechis papuanus [Australia], Pseudechis rossignolii [Australia], Pseudechis rossignolii [Australia] | Atheris chlorechis, Atheris squamigera, Atractaspis irregularis, Atractaspis micropholis, Echis jogeri, Naja annulata, Pseudohaje goldii, Pseudohaje nigra, Thelotornis kirtlandii |
| Africa | Ivory Coast | Spain | Inoserp™ Pan-Africa | Bitis arietans, Bitis nasicornis, Bitis rhinoceros, Dendroaspis polylepis, Dendroaspis viridis, Echis ocellatus, Naja guineensis, Naja katiensis, Naja nigricollis, Naja savannula, Naja senegalensis | Dispholidus typus [South Africa] | Atheris chlorechis, Atractaspis irregularis, Atractaspis micropholis, Pseudohaje goldii, Pseudohaje nigra, Thelotornis kirtlandii |
| Africa | Zambia | India | Snake Venom Antiserum (Africa) | Bitis arietans, Bitis gabonica, Dendroaspis polylepis, Naja nigricollis, Naja subfulva | Dispholidus typus [South Africa], Naja annulifera [South Africa], Naja mossambica [Costa Rica, Egypt, South Africa] | Atractaspis bibronii, Naja annulata, Naja anchietae, Thelotornis capensis, Thelotornis kirtlandii, Thelotornis mossambicanus |
| Africa | Benin | Mexico | Antivipmyn® Africaa | Bitis arietans, Bitis gabonica, Dendroaspis polylepis, Dendroaspis viridis, Echis leucogaster, Echis ocellatus, Naja melanoleuca, Naja nigricollis, Naja savannula | Dispholidus typus [South Africa] | Atractaspis micropholis, Atractaspis watsoni, Pseudohaje nigra, Thelotornis kirtlandii |
| Spain | Inoserp™ Pan-Africa | Bitis arietans, Bitis gabonica, Bitis nasicornis, Dendroaspis jamesoni, Dendroaspis polylepis, Dendroaspis viridis, Echis leucogaster, Echis ocellatus, Naja katiensis, Naja melanoleuca, Naja nigricollis, Naja savannula, Naja senegalensis | ||||
| Africa | Burkina Faso | Costa Rica | EchiTAb-plus-ICP Liquid | Bitis arietans, Echis leucogaster, Echis ocellatus, Naja nigricollis | Dispholidus typus [South Africa] | Atractaspis micropholis, Atractaspis watsoni |
| Burkina Faso | Spain | Inoserp™ Pan-Africa | Bitis arietans, Dendroaspis polylepis, Echis leucogaster, Echis ocellatus, Naja katiensis, Naja nigricollis, Naja savannula, Naja senegalensis | |||
| Africa | Cote d'Ivoire | India | Snake Venom Antiserum (Africa) | Bitis arietans, Dendroaspis polylepis, Dendroaspis viridis, Echis ocellatus, Naja guineensis, Naja nigricollis, Naja savannula | Bitis nasicornis [Costa Rica, India, Spain], Bitis rhinoceros [Costa Rica, India, Spain], Dispholidus typus [South Africa], Naja katiensis [Spain], Naja senegalensis [Spain] | Atheris chlorechis, Atractaspis irregularis, Atractaspis micropholis, Pseudohaje goldii, Pseudohaje nigra, Thelotornis kirtlandii |
| Africa | Kenya | India | Snake Venom Antiserum (Africa) | Bitis arietans, Bitis gabonica, Dendroaspis jamesoni, Dendroaspis polylepis, Naja haje, Naja nigricollis, Naja subfulva | Dispholidus typus [South Africa] | Thelotornis mossambicanus, Atheris desaixi, Atheris squamigera, Atractaspis bibronii, Atractaspis fallax, Atractaspis irregularis, Naja ashei, Pseudohaje goldii, Thelotornis usambaricus |
| Kenya | Spain | Inoserp™ Pan-Africa | Bitis arietans, Bitis gabonica, Bitis nasicornis, Dendroaspis angusticeps, Dendroaspis jamesoni, Dendroaspis polylepis, Echis pyramidum, Naja haje, Naja nigricollis, Naja pallida, Naja subfulva | |||
| Africa | Chad | Spain | Inoserp™ MENA | Bitis arietans, Cerastes cerastes, Echis leucogaster, Naja haje, Naja nigricollis, Naja nubiae | Dispholidus typus [South Africa], Echis romani [Costa Rica, India, Mexico, The United Kingdom], Naja savannula [Egypt, India, Mexico, Spain, South Africa], Naja subfulva [Egypt, India, Mexico, Spain, South Africa] | Atractaspis micropholis, Atractaspis watsoni, |
| North America | Guatemala | Costa Rica | PoliVal-ICP Lyophilized | Agkistrodon bilineatus, Bothriechis bicolor, Bothriechis schlegelii, Bothriechis thalassinus, Bothrops asper, Cerrophidion godmani, Crotalus simus, Metlapilcoatlus mexicanus, Metlapilcoatlus occiduus, Metlapilcoatlus olmec, Porthidium nasutum, Porthidium ophryomegas | Bothriechis aurifer [Colombia], Crotalus tzabcan [Mexico], Micrurus nigrocinctus [Costa Rica, Mexico] | Agkistrodon russeolus, Micrurus browni, Micrurus diastema, Micrurus elegans, |
| Guatemala | Argentina | Suero Antiofidico Centroamericano BIOL | Bothrops asper, Crotalus simus | |||
| North America | Honduras | Argentina | Suero Antiofidico Centroamericano BIOL CLB | Bothrops asper, Crotalus simus | None | Agkistrodon howardgloydi, Bothriechis guifarroi, Cerrophidion wilsoni, Micrurus alleni, Micrurus diastema |
| Honduras | Costa Rica | PoliVal-ICP Liquid | Bothriechis marchi, Bothriechis schlegelii, Bothriechis thalassinus, Bothrops asper, Crotalus simus, Metlapilcoatlus indomitus, Metlapilcoatlus mexicanus, Metlapilcoatlus occiduus, Porthidium nasutum, Porthidium ophryomegas | |||
| Honduras | Costa Rica | CoRal-ICP Liquid | Micrurus nigrocinctus | |||
| Honduras | Costa Rica | PoliVal-ICP Lyophilized | Bothriechis marchi, Bothriechis schlegelii, Bothriechis thalassinus, Bothrops asper, Crotalus simus, Metlapilcoatlus indomitus, Metlapilcoatlus mexicanus, Metlapilcoatlus occiduus, Micrurus nigrocinctus, Porthidium nasutum, Porthidium ophryomegas | |||
| North America | Martinique | The United Kingdom | BothroFAV | Bothrops lanceolatus | None | None |
| North America | Nicaragua | Argentina | Suero Antiofidico Centroamericano BIOL CLB | Bothrops asper, Crotalus simus | None | Agkistrodon howardgloydi, Cerrophidion wilsoni, Micrurus alleni |
| Nicaragua | Costa Rica | PoliVal-ICP Liquid | Bothriechis schlegelii, Bothrops asper, Crotalus simus, Metlapilcoatlus mexicanus, Porthidium nasutum, Porthidium ophryomegas | |||
| Nicaragua | Costa Rica | PoliVal-ICP Lyophilized | Bothriechis schlegelii, Bothrops asper, Crotalus simus, Metlapilcoatlus mexicanus, Porthidium nasutum, Porthidium ophryomegas | |||
| Nicaragua | Costa Rica | CoRal-ICP Liquid | Micrurus nigrocinctus | |||
| North America | Panama | Argentina | Suero Antiofidico Centroamericano BIOL CLB | Bothrops asper | Bothriechis nigroviridis [Colombia], Micrurus mipartitus [Colombia] | Micrurus alleni, Bothrops punctatus, Cerrophidion sasai, Micrurus clarki, Micrurus dissoleucus, Micrurus dumerilii |
| Panama | Costa Rica | PoliVal-ICP Liquid | Atropoides picadoi, Bothriechis lateralis, Bothriechis schlegelii, Bothriechis supraciliaris, Bothrops asper, Lachesis acrochorda, Lachesis stenophrys, Metlapilcoatlus mexicanus, Porthidium lansbergii, Porthidium nasutum | |||
| Panama | Costa Rica | PoliVal-ICP Lyophilized | Atropoides picadoi, Bothriechis lateralis, Bothriechis schlegelii, Bothriechis supraciliaris, Bothrops asper, Lachesis acrochorda, Lachesis stenophrys, Metlapilcoatlus mexicanus, Porthidium lansbergii, Porthidium nasutum | |||
| Panama | Costa Rica | CoRal-ICP Liquid | Micrurus nigrocinctus | |||
| North America | USA | Mexico | Anavip® | Agkistrodon contortrix, Agkistrodon piscivorus, Crotalus adamanteus, Crotalus atrox, Crotalus horridus, Crotalus molossus, Crotalus oreganus, Crotalus ruber, Crotalus scutulatus, Crotalus viridis, Sistrurus miliarius | None | Crotalus cerberus, Crotalus lepidus, Crotalus ornatus, Crotalus pricei, Crotalus pyrrhus, Crotalus stephensi, Crotalus tigris, Crotalus willardi, Micruroides euryxanthus, Sistrurus tergeminus |
| Spain | Inoserp™ MENA | Cerastes cerastes | ||||
| Inoserp™ Pan-Africa | None | |||||
| USA | USA | CroFab (Crotalidae Polyvalent Immune Fab (Ovine)) | Agkistrodon contortrix, Agkistrodon piscivorus, Agkistrodon taylori, Crotalus adamanteus, Crotalus atrox, Crotalus horridus, Crotalus molossus, Crotalus oreganus, Crotalus ruber, Crotalus scutulatus, Crotalus viridis, Sistrurus catenatus, Sistrurus miliarius | |||
| USA | USA | North American Coral Snake Antivenin (Equine) | Micrurus fulvius, Micrurus tener | |||
| South America | Bolivia | Argentina | Suero Antiofidico Polivalente BIOL | Bothrops diporus, Crotalus durissus | Bothrocophias hyoprora [Peru], Bothrocophias microphthalmus [Peru], Bothrops bilineatus [Brazil, Colombia], Bothrops brazili [Brazil, Costa Rica, Peru], Bothrops mattogrossensis [Brazil, Colombia, Mexico], Bothrops moojeni [Argentina, Brazil, Costa Rica], Bothrops taeniatus [Brazil], Micrurus lemniscatus [Brazil, Colombia], Micrurus spixii [Colombia], Micrurus surinamensis [Colombia] | Bothrocophias andianus, Bothrops sanctaecrucis, Micrurus annellatus, Micrurus hemprichii, Micrurus narduccii, Micrurus obscurus, Micrurus pyrrhocryptus |
| Bolivia | Bolivia | Suero Antiofidico Polivalente Botropico/Crotalico | Bothrops atrox, Bothrops jonathani,Bothrops neuwiedi, Crotalus durissus | |||
| Bolivia | Bolivia | Suero Antiofidico Polivalente Botropico/Laquesico | Bothrops atrox, Bothrops neuwiedi, Lachesis muta | |||
| South America | Paraguay | Argentina | Suero Antiofidico Polivalente BIOL | Bothrops alternatus, Bothrops diporus, Crotalus durissus | Bothrops jararaca [Argentina, Brazil, Colombia, Costa Rica], Bothrops jararacussu [Argentina, Brazil, Costa Rica], Bothrops mattogrossensis [Brazil, Colombia, Mexico], Bothrops moojeni [Argentina, Brazil, Costa Rica], Bothrops pubescens [Brazil], Micrurus corallinus [Argentina, Brazil], Micrurus lemniscatus [Brazil, Colombia] | Micrurus altirostris, Micrurus frontalis, Micrurus pyrrhocryptus |
| Europe | Belgium | Spain | Inoserp™ MENA | None | Vipera berus [Bulgaria, Croatia, Poland, Russian Federation, Serbia, Spain, The United Kingdom] | None |
| Inoserp™ Pan-Africa | None | |||||
| Europe | Cyprus | Spain | Inoserp™ Pan-Africa | None | Macrovipera lebetina [Algeria, Croatia, Egypt, Iran (Islamic Republic of), Spain, Serbia, Tunisia, Turkey, Uzbekistan] | None |
| Europe | France | Spain | Inoserp™ Pan-Africa | None | Vipera ursinii [Bulgaria, Croatia] | Vipera seoanei |
| The United Kingdom | ViperaFAV | Vipera aspis, Vipera berus | ||||
| Europe | Germany | Spain | Inoserp™ Pan-Africa | None | Vipera aspis [Bulgaria, Croatia, Serbia, Spain, The United Kingdom], Vipera berus [Bulgaria, Croatia, Poland, Russian Federation, Serbia, Spain, The United Kingdom] | None |
| Europe | Netherlands | Spain | Inoserp™ MENA | None | Vipera berus [Bulgaria, Croatia, Poland, Russian Federation, Serbia, Spain, The United Kingdom] | None |
| Inoserp™ Pan-Africa | None | |||||
| Europe | United Kingdom | Spain | Inoserp™ MENA | None | None | None |
| Inoserp™ Pan-Africa | None | |||||
| The United Kingdom | EchiTAbG | None | ||||
| ViperaFAV | Vipera berus | |||||
| ViperaTAb® | Vipera berus |
The claimed species lists the species present in the respective country and claimed to be targeted by a given ASV as per the WHO database [23]. The missed species list the snake species present in a country which are not targeted by any antivenom available in the respective country. The countries which manufacture antivenom(s) against these missed species are mentioned in parentheses. The missed species for which no antivenom is manufactured in any part of the world are listed under ‘missed species-no antivenom available’
aRegistration expired
Lessons from the World-The PROMISE Approach
Now that we have established that ASV administration is the crucial part of the SBE treatment process, it is obligatory to investigate the current ASV market. Nevertheless, other obstacles also play a key role while treating SBE (Fig. 1 and Table 1). Although infrastructure improvements and research into developing next generation ASVs [59] are definitely needed to ensure availability of ASVs in distant areas and overcoming limitations of current ASVs, as also discussed in other roadmaps [60], these are not expected to be encouraged given the neglected nature of SBE. Thus, to improve the other aspects of SBE management, we recommend the PROMISE (Practical ROutes for Managing Indigenous Snakebite Envenoming) approach which comprises of economical and simple yet effective steps to be undertaken by all countries interested in reducing SBE in their regions. The approach is discussed below in detail and summarized in Fig. 3.
Fig. 3.
The PROMISE approach for SBE management. The PROMISE (Practical ROutes for Managing Indigenous Snakebite Envenoming) approach is an amalgamation of economical and easy remedies whose implementation can significantly reduce the SBE burden
Alliance with Local Healers
The integration of traditional healers into the SBE management pathway should be considered. In this regard, a registry of legitimate healers must be made and advertised among the masses. While the registry would ensure the exclusion of non-qualified self-claimed healers who can worsen patient’s condition, the release of a list of authorized healers from the regulatory agencies would help in gaining the trust of those local people who prefer traditional therapists over hospital care. The HCPs can teach these registered local healers about the initial SBE management and local healers may help with diagnosis, first aid, and timely referral to health care centres. Besides saving lives, their referrals would also help in estimating SBE statistics more accurately and reduce under-reporting. Success with such an integrated approach has been reported in African countries for Human African Trypanosomiasis (sleeping sickness), HIV, and psychosis in terms of improved diagnosis or clinical outcomes for patients [14, 61].
Improved Communication and Overcoming Transport Barrier
Improvements in phone and internet services, and transportation can enhance the health seeking nature among the masses, as in Ethiopia [62]. The transportation barrier can also be partially lifted by facilitating ASV distribution and establishment of poison information centers. In absence of a centralized ASV distribution program based on local SBE epidemiology, concentration of ASVs in populated areas over others and increased product cost may occur, as in Colombia [63]. Also, victims in rural and remote areas may remain deprived of ASV despite in-house production and free distribution, such as in Brazil [64]. Further, strict vigilance is needed for adequate distribution of ASVs across all levels of healthcare systems. In Kenya, ASV is unavailable in dispensaries despite governmental guidelines [65]. Additionally, the teleconsultation services of poison information centers can also prevent patient transport, help in knowledge propagation among clinicians, and potentially lower expenses and expedite diagnosis and treatment, such as in Taiwan [66]. The guidelines for establishing such centres have recently been updated by the WHO [67]. Also, their establishment should also be followed by its publicity among the HCPs. In South Africa, many physicians do not call such centres simply because they do not know that such centres exist [39].
Gaining Trust
Patients in Healthcare System
In Brazil, even if some rural areas have access to ASVs, people prefer to travel long distances in a search for more trustworthy treatment [64]. This is one important issue which is usually ignored by most policy makers. A potential solution could involve the circulation of annual reports from healthcare centers regarding SBE cases treated and their corresponding outcomes, both within the healthcare community and to the public.
Doctors in ASV Therapy
The trust of doctors on ASV therapy is also important. In a survey conducted in Vietnam, doctors advocated the superiority of traditional healers over ASV for SBE management [68]. Also, in Kenya, health care workers have reported non-administration of available ASV to victims because of fear of adverse reactions [69]. Sharing case reports of successful ASV therapy on a regular basis can be beneficial in this regard.
Doctors in Themselves
Treatment of SBE is not included in the medical course syllabus even in countries with high SBE burden such as India [15, 70]. Further, due to the least number of victims or the available treatments, usually the preparedness of the health workers towards SBE is minimum. In multiple surveys as well, HCPs from different countries have admitted their lack of knowledge in SBE management [15]. Management of SBE and ASV associated adverse reactions must thus be included in the curriculum of undergraduate medical students. Also, the clinicians should be regularly updated about SBE case classification and management through training programs. Simultaneously, educating the HCPs about dry bites, that are snakebites without venom injection, is also paramount [71]. A survey in a Saudi Arabia University revealed that while medical students received training for SBE management, around 80% were unaware of dry bites [72].
One country One Protocol
A national unified protocol for SBE management should be prepared to reduce ASV usage, treatment cost, and also the duration of hospital stay, as in case of Iran [73, 74]. The protocol reduced the ASV usage by 4 vials, treatment cost by $196, and hospital stay by 1 day per patient. The existing protocols of neighboring countries and the treatment guidelines for SBE by the WHO might serve as a reference for preparing such national protocols. For countries like India, which have a wide variety of snake species and different qualities and cross-reactivities of antivenoms, it is advisable to implement more rigorous quality control measures and modify the procedure to accommodate the specific snake diversity of each region. Alternatively, it may be beneficial to develop protocols that are specific to individual states or regions.
Accurate Snake Identification
An inventory of regional snakes, available ASVs and their targeted species, and an SBE management chart should be available in every treating facility, especially in the rural areas. This information must be available in local languages to ensure understanding of common masses. This will aid in correct snake identification and boost the confidence of both patients and HCPs in SBE management. For example, the use of a snake atlas, as in Ethiopia, can be a simple yet powerful tool in this regard [62]. The non-venomous species must also be documented in the snake atlas to minimize the confusion and the associated unnecessary treatment or side effects. If possible, a real-time inventory should be made such as in Thailand, to map required ASV availability in nearby areas [75]. Additionally, HCPs must be made aware of the online databases, such as the WHO snakebite platform, WCH clinical toxicology, and iNaturalist, which can be used not only for self-education but also for accurate snake identification with the patient attendants to facilitate their recall with the snake pictures available in the database [23, 76, 77]. Mobile application based databases are also available. One such application is’SnakeHub’ which is a free mobile app that provides description of 114 snake species found in Kerala, India in English as well as in local language [78]. Efforts must also be made for developing mobile applications for image based correct snake identification such as those using artificial intelligence and SnakeSnap app [79, 80]. When creating such apps, one can contact local rescuers for assistance. Adequate awareness about such apps must be spread among both the HCPs as well as common masses for improving snake identification.
Another way of aiding clinicians in ASV selection, improving therapeutic efficacies of SBE treatment and reducing mortality is the development of venom detection kits [81]. However, efforts must be made to ensure that the kits are economical and cause a minimum increment in the cost of ASV therapy. Further, the sensitivity and specificity of such kits must be thoroughly tested. Currently, despite availability of venom detection kits, the Australian clinicians are focusing more on diagnosing envenoming rather than the kit results [53].
Good ASV Quality
Only Good Manufacturing Practices (GMPs) and stringent quality control can ensure the production of a safe and effective ASV [17]. However, sudden imposition of stringent regulations or reforms, after years of neglect, should be avoided. This important lesson can be learnt from Ecuador, a country with ASV manufacturing history since 1981, which shut down ASV manufacturing following sudden imposition of GMPs and identification of other production flaws [24]. Winning the manufacturer’s confidence, for example, by providing time and incentives for upgrading production practices, should be a priority to ensure a sustainable approach for effective implementation of governmental policies.
Public–Private Partnerships
The onus to prove ASV specificity lies on the manufacturer, but it can be accelerated with public–private partnerships. The governments or the regulatory authorities should also partner with the manufacturers in a search for expanding the ASV cross-reactivity to other endemic species found in the country. Collaborations with academia may also be promoted in this regard, since full expertise might not be available under a single roof. Thus, while efficacy data would ensure that the imported ASV would be clinically suitable, the cross-reactivity studies would expand the customer’s base. Such studies offer a promising approach to decrease the burden of SBE while newer ASVs are being developed. In many studies, using a mouse model, a single ASV was shown to exhibit cross-reactivity against several heterologous medically significant snakes from sub-Saharan Africa [82, 83]. However, certain conditions must be met before using these cross-neutralization test-passed ASVs against heterologous venoms. For example, the disparities in metabolic activity between mice and humans call for additional clinical testing [84]. Additionally, such studies should include long-term assessment for toxicities of different venom components. For example, neutralizing the paralytic effects of N. sumatrana venom in mice for 24 h does not guarantee that the hematoxic or other effects will be neutralized, and the animals will survive [84].
Further, the manufacturers must be encouraged to stay updated about the recent advancements in ASV production and incorporate appropriate changes in their production processes to generate broadly specific, affordable, safe, and effective (BASE) ASVs [17].
Correct Calculations of ASV Demand
The strategy for calculating ASV demand needs also be finalized in such a way that access to ASV is guaranteed. As reported in a Colombian study, the different methods used to calculate ASV usage can yield widely varying results [63]. While one method, based on the theoretical average number of ASV vials used for treatment, suggested a yearly need of 25,380 vials, another method based on accident severity suggested a need of 50,021 vials in the country. Yet another method, that used multiple variables, such as underreporting and number of accidents in the previous decade, suggested the need of 54,440 vials. In addition to doing reasonable calculations, it is imperative to have contingency plans to handle unexpected surges in ASV demand, particularly during emergency situations like floods that may result in an increase in snakebite cases.
Record Maintenance
Mandatory reporting of snakebites will not only help in better understanding of the regional SBE epidemiology, but also aid in estimating the actual ASV demand, better snake identification, and decision making for ASV administration. This can be achieved with manuals that include details about SBE case presentation, diagnosis, treatment, and clinical course description along with the name and amount of ASV administered. The regional records can also be combined into a centralized data bank accessible to public, physicians, researchers, and policymakers for effective SBE management. Extra care must also be taken when entering the species name. Incorrect snake name entries in medical publications can be a cause of concern [85]. It is also advisable to integrate pictures of the biting species wherever feasible.
Task Force
A task force comprising of frontline healthcare professionals should be established to conduct surveillance and collect data on occurrences of SBE, mortality and socio-economic burden. Such studies, even if done to cover only a fraction of population, as underway in India, can be valuable in managing the distribution of ASV and for better governmental policies such as fund allocation for SBE management [86]. It must be noted that the choice of protocol can affect the estimates of SBE burden. For example, utilizing disability weights determined from the community, rather than those derived from the global burden of disease (GBD), can lead to more accurate estimations of SBE burden [87]. Furthermore, protocols must be created in compliance with international guidelines such as the STROBE guidelines for cross-sectional studies [88].
Media and Education
The media should exclusively distribute accurate information in a responsible manner. For example, in Serbia, reports regarding the occurrences of even the non-venomous snakes and rare bites, have been reported to be dramatized by media [85]. A role reversal of the media, as also mentioned by the author, can help in disseminating accurate knowledge among the masses about SBE and its treatment. Another simple strategy to educate the masses can be the introduction of SBE management in the school curriculum. Alternatively, annual workshops can be conducted by schoolteachers for the same. Another approach can be ‘A Snake A Month’ (ASAM) session, where school children are taught about one regional snake every month.
Prohibit Snake Trade and Cross Breeding
The trend of rearing venomous snakes by individuals results in increased number of SBE [25, 89]. Even in countries, such as Brazil, where snake trading is prohibited, illegal ownership of venomous snakes has been reported [90]. In Switzerland, where private housing of snakes is legal, physicians are often untrained in dealing patients envenomed by exotic pets [89]. Similarly, the cross-breeding of snakes to create hybrids of related snake species should be strictly banned, since the hybrids have unknown venom composition and ASV efficacy in bitten victims cannot be guaranteed [89].
Conclusions
The present study provides a comprehensive review about the gaps in SBE therapy, current status of ASV manufacturing, global distribution, and the economical remedies to provide a holistic approach for SBE management. For an SBE victim, the acquisition of ASV demands a painful journey with multiple hurdles with unsure outcomes. The critical point in this journey is the administration of a safe and effective ASV, which is often not available due to the uneven distribution of SBE burden and ASV manufacturing across the world with the latter being limited to a few countries. Even in countries with ASV production abilities, ASVs are produced against a few species only and only a few manufacturers export their ASVs to other countries. Furthermore, the quality of the imported ASVs is not guaranteed and some countries even import ASVs against species that are not even found in their country, despite the availability of specific anti-snake venoms in other countries. A self-reassessment for the currently imported ASVs and enhanced support for ASV supply is thus needed at the international level. Also, although significant advancements have been made in the strategies for ASV production, it is still underproduced and is in short supply in different countries. Many countries, especially in Africa, do not have approval for imported ASVs despite the high SBE burden. Consequently, there is a huge imbalance between ASV demand and supply and the need for ASV is far more than estimated. We thus call for a reassessment of the global ASV demand, procurement and distribution policies, and enhanced responsibility of both manufacturers and importers for ASV quality. Also, urgent attention is needed towards ASV manufacturing and/or cross-reactivity studies for species for which no ASV is available. Additionally, a PROMISE approach, comprising several simple and economical steps have been proposed in this article that can be taken to overcome the hurdles to SBE management without major costs. The findings of this study could also serve as a ready reference for the clinicians and policy makers who want to educate themselves about the ASVs available in their country, and neighboring areas.
Acknowledgements
No funding was received to assist with the preparation of this manuscript.
Author Contributions
RK and ASR conceived the paper. RK performed literature search, data analysis, and drafted the manuscript. ASR reviewed and edited the paper. Both authors edited and approved the final version of the manuscript.
Data Availability
This published article contains all the generated and analyzed data, thus no additional data source is required.
Declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nat Rev Dis Prim. 2017;3:17063. 10.1038/NRDP.2017.63. [DOI] [PubMed] [Google Scholar]
- 2.Ralph R, Faiz MA, Sharma SK, Ribeiro I, Chappuis F. Managing snakebite. BMJ. 2022;376: e057926. 10.1136/bmj-2020-057926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annan K. Snakebite: The biggest public health crisis you’ve never heard of 2018. https://www.kofiannanfoundation.org/combatting-hunger/public-health-snakebite/ (Accessed Nov 3, 2023).
- 4.Warrell DA, Williams DJ. Clinical aspects of snakebite envenoming and its treatment in low-resource settings. Lancet. 2023;401:1382–98. 10.1016/S0140-6736(23)00002-8. [DOI] [PubMed] [Google Scholar]
- 5.Pucca MB, Cerni FA, Janke R, Bermúdez-Méndez E, Ledsgaard L, Barbosa JE, et al. History of envenoming therapy and current perspectives. Front Immunol. 2019;10:1598. 10.3389/fimmu.2019.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snakebite envenoming: a strategy for prevention and control. Geneva: World Health Organization; 2019. [DOI] [PubMed]
- 7.Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030. Geneva World Heal. Organ., 2020, p. 162–5.
- 8.Williams DJ, Faiz MA, Abela-Ridder B, Ainsworth S, Bulfone TC, Nickerson AD, et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl Trop Dis. 2019;13: e0007059. 10.1371/journal.pntd.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habib AG, Musa BM, Iliyasu G, Hamza M, Kuznik A, Chippaux JP. Challenges and prospects of snake antivenom supply in Sub-Saharan Africa. PLoS Negl Trop Dis. 2020;14: e0008374. 10.1371/journal.pntd.0008374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potet J, Beran D, Ray N, Alcoba G, Habib AG, Iliyasu G, et al. Access to antivenoms in the developing world: a multidisciplinary analysis. Toxicon X. 2021;12: 100086. 10.1016/j.toxcx.2021.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casewell NR, Jackson TNW, Laustsen AH, Sunagar K. Causes and consequences of snake venom variation. Trends Pharmacol Sci. 2020;41:570–81. 10.1016/j.tips.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longbottom J, Shearer FM, Devine M, Alcoba G, Chappuis F, Weiss DJ, et al. Vulnerability to snakebite envenoming: a global mapping of hotspots. Lancet. 2018;392:673–84. 10.1016/S0140-6736(18)31224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuat M, Alcoba G, Eyong J, Wanda F, Comte E, Nkwescheu A, et al. Dealing with snakebite in rural Cameroon: A qualitative investigation among victims and traditional healers. Toxicon X. 2021;9–10: 100072. 10.1016/j.toxcx.2021.100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinhorst J, Tianyi FL, Habib AG, Oluoch GO, Lalloo DG, Stienstra Y. Uniting behind a common goal: Collaboration between traditional healers and allopathic health care workers to improve rural snakebite care. Toxicon X. 2022;16: 100140. 10.1016/j.toxcx.2022.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michael GC, Bala AA, Mohammed M. Snakebite knowledge assessment and training of healthcare professionals in Asia, Africa, and the Middle East: A review. Toxicon X. 2022;16: 100142. 10.1016/j.toxcx.2022.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babo Martins S, Bolon I, Alcoba G, Ochoa C, Torgerson P, Sharma SK, et al. Assessment of the effect of snakebite on health and socioeconomic factors using a One Health perspective in the Terai region of Nepal: a cross-sectional study. Lancet Glob Heal. 2022;10:e409–15. 10.1016/S2214-109X(21)00549-0. [DOI] [PubMed] [Google Scholar]
- 17.Rathore AS, Kumar R, Tiwari OS. Recent advancements in snake antivenom production. Int J Biol Macromol. 2023;240: 124478. 10.1016/j.ijbiomac.2023.124478. [DOI] [PubMed] [Google Scholar]
- 18.De Silva HA, Ryan NM, De Silva HJ. Adverse reactions to snake antivenom, and their prevention and treatment. Br J Clin Pharmacol. 2016;81:446–52. 10.1111/bcp.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayaraman T, Dhanasinghu R, Kuppusamy S, Gaur A, Sakthivadivel V. Bite-to-needle time – an extrapolative indicator of repercussion in patients with snake bite. Indian J Crit Care Med. 2022;26:1173–6. 10.5005/jp-journals-10071-24344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochoa C, Rai M, Babo Martins S, Alcoba G, Bolon I, Ruiz de Castañeda R, et al. Vulnerability to snakebite envenoming and access to healthcare in the Terai region of Nepal: a geospatial analysis. Lancet Reg Heal-Southeast Asia. 2023;9:100103. 10.1016/j.lansea.2022.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston CI, Tasoulis T, Isbister GK. Australian sea snake envenoming causes myotoxicity and non-specific systemic symptoms - Australian Snakebite Project (ASP-24). Front Pharmacol. 2022;13:1–9. 10.3389/fphar.2022.816795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willyard C. A fanged crisis. Nature. 2023;621:S40–7. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Expert derived snake distributions n.d. https://snbdatainfo.who.int/?data_id=dataSource_10-187d5a34599-layer-4%3A31 (Accessed Dec 17, 2023).
- 24.Ortiz-Prado E, Yeager J, Andrade F, Schiavi-Guzman C, Abedrabbo-Figueroa P, Terán E, et al. Snake antivenom production in ecuador: poor implementation, and an unplanned cessation leads to a call for a renaissance. Toxicon. 2021;202:90–7. 10.1016/j.toxicon.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Pejak DT, Adam VN, Srzić I. Venomous snakebites in croatia, clinical presentation diagnosis and treatment. Acta Clin Croat. 2022;61:59–66. 10.20471/acc.2022.61.s1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obar M. KEMSA Recalls Indian Medicine Manufactured From Snake Venom 2023. https://www.kenyans.co.ke/news/83712-kemsa-recalls-indian-medicine-manufactured-snake-venom (accessed October 23, 2023).
- 27.Malesi T. Ineffective’ India-made antivenoms recalled in Kenya, country faces snakebite crisis 2023.
- 28.Kasturiratne A, Wickremasinghe AR, De Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:1591–604. 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutiérrez JM. Global availability of antivenoms: the relevance of public manufacturing laboratories. Toxins (Basel). 2019. 10.3390/toxins11010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ralph R, Sharma SK, Faiz MA, Ribeiro I, Rijal S, Chappuis F, et al. The timing is right to end snakebite deaths in South Asia. BMJ. 2019;364: k5317. 10.1136/bmj.k5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng ZY, Huang PY, Du JY, Liu YX, Guo SG, Zeng LS, et al. Effect of Agkistrodon halys antivenom in patients bit by green pit viper and the prognostic role of the disease–a retrospective cohort study. Clin Toxicol. 2022;60:808–17. 10.1080/15563650.2022.2041200. [DOI] [PubMed] [Google Scholar]
- 32.Senji Laxme RR, Khochare S, de Souza HF, Ahuja B, Suranse V, Martin G, et al. Beyond the ‘big four’: venom profiling of the medically important yet neglected Indian snakes reveals disturbing antivenom deficiencies. PLoS Negl Trop Dis. 2019;13: e0007899. 10.1371/journal.pntd.0007899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitaker R, Martin G. Diversity and Distribution of Medically Important Snakes of India. In: Gopalakrishnakone P, Faiz A, Fernando R, Gnanathasan CA, Habib AG, Yang C-C, editors. Clinical Toxinology in Asia Pacific and Africa. Dordrecht, Springer: Netherlands; 2015. p. 115–36. 10.1007/978-94-007-6386-9_16. [Google Scholar]
- 34.Gopal G, Selvaraj H, Venkataramanan SK, Venkataraman S, Saravanan K, Bibina C, et al. Systematic review and meta-analysis on the efficacy of Indian polyvalent antivenom against the Indian snakes of clinical significance. Arch Toxicol. 2024;98:375–93. 10.1007/s00204-023-03643-9. [DOI] [PubMed] [Google Scholar]
- 35.Khochare S, Senji Laxme RR, Jaikumar P, Kaur N, Attarde S, Martin G, et al. Fangs in the Ghats: preclinical insights into the medical importance of pit vipers from the Western Ghats. Int J Mol Sci. 2023. 10.3390/ijms24119516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senji Laxme RR, Attarde S, Khochare S, Suranse V, Martin G, Casewell NR, et al. Biogeographical venom variation in the indian spectacled cobra (Naja naja) underscores the pressing need for pan-india efficacious snakebite therapy. PLoS Negl Trop Dis. 2021;15: e0009150. 10.1371/journal.pntd.0009150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rashmi U, Khochare S, Attarde S, Laxme RRS, Suranse V, Martin G, et al. Remarkable intrapopulation venom variability in the monocellate cobra (Naja kaouthia) unveils neglected aspects of India’s snakebite problem. J Proteom. 2021;242: 104256. 10.1016/j.jprot.2021.104256. [DOI] [PubMed] [Google Scholar]
- 38.Senji Laxme RR, Khochare S, Attarde S, Suranse V, Iyer A, Casewell NR, et al. Biogeographic venom variation in Russell’s viper (Daboia russelii) and the preclinical inefficacy of antivenom therapy in snakebite hotspots. PLoS Negl Trop Dis. 2021;15: e0009247. 10.1371/journal.pntd.0009247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neumann NR, du Plessis A, van Hoving DJ, Hoyte CO, Lermer A, Wittels S, et al. Antivenom supply and demand: an analysis of antivenom availability and utilization in South Africa. African J Emerg Med. 2023;13:245–9. 10.1016/j.afjem.2023.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ainsworth S, Menzies SK, Casewell NR, Harrison RA. An analysis of preclinical efficacy testing of antivenoms for sub-Saharan Africa: Inadequate independent scrutiny and poor-quality reporting are barriers to improving snakebite treatment and management. PLoS Negl Trop Dis. 2020;14: e0008579. 10.1371/journal.pntd.0008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Offor BC, Muller B, Piater LA. A review of the proteomic profiling of african viperidae and elapidae snake venoms and their antivenom neutralisation. Toxins (Basel). 2022;14:723. 10.3390/toxins14110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cocchio C, Johnson J, Clifton S. Review of North American pit viper antivenoms. Am J Heal Pharm. 2020;77:175–87. 10.1093/ajhp/zxz278. [DOI] [PubMed] [Google Scholar]
- 43.Gutiérrez JM, Fan HW. Improving the control of snakebite envenomation in Latin America and the Caribbean: a discussion on pending issues. Trans R Soc Trop Med Hyg. 2018;112:523–6. 10.1093/trstmh/try104. [DOI] [PubMed] [Google Scholar]
- 44.Schneider MC, Min K, Hamrick PN, Montebello LR, Ranieri TM, Mardini L, et al. Overview of snakebite in Brazil: possible drivers and a tool for risk mapping. PLoS Negl Trop Dis. 2021;15: e0009044. 10.1371/journal.pntd.0009044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mora-Obandoid D, Plaid D, Lomonteid B, Guerrero-Vargasid JA, Ayerbe S, Calveteid JJ. Antivenomics and in vivo preclinical efficacy of six latin american antivenoms towards Southwestern Colombian bothrops asper lineage venoms. PLoS Negl Trop Dis. 2021;15: e0009073. 10.1371/journal.pntd.0009073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chippaux JP. Epidemiology of snakebites in Europe: a systematic review of the literature. Toxicon. 2012;59:86–99. 10.1016/j.toxicon.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Di Nicola MR, Pontara A, Kass GEN, Kramer NI, Avella I, Pampena R, et al. Vipers of Major clinical relevance in Europe: taxonomy, venom composition, toxicology and clinical management of human bites. Toxicology. 2021;453: 152724. 10.1016/j.tox.2021.152724. [DOI] [PubMed] [Google Scholar]
- 48.Lamb T, de Haro L, Lonati D, Brvar M, Eddleston M. Antivenom for European Vipera species envenoming. Clin Toxicol. 2017;55:557–68. 10.1080/15563650.2017.1300261. [DOI] [PubMed] [Google Scholar]
- 49.García-Arredondo A, Martínez M, Calderón A, Saldívar A, Soria R. Preclinical assessment of a new polyvalent antivenom (Inoserp europe) against several species of the subfamily viperinae. Toxins (Basel). 2019;11:149. 10.3390/toxins11030149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welton RE, Liew D, Braitberg G. Incidence of fatal snake bite in Australia: a coronial based retrospective study (2000–2016). Toxicon. 2017;131:11–5. 10.1016/j.toxicon.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Whyte I, Buckley N. Antivenom update. Aust Prescr. 2012;35:152–5. 10.18773/austprescr.2012.069. [Google Scholar]
- 52.Featherstone PJ, Ball CM. The development of snake antivenoms in Australia. Anaesth Intensive Care. 2022;50:342–4. 10.1177/0310057X221108562. [DOI] [PubMed] [Google Scholar]
- 53.Isbister GK. Antivenom availability, delays and use in Australia. Toxicon X. 2023;17: 100145. 10.1016/j.toxcx.2022.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maduwage K, Silva A, O’Leary MA, Hodgson WC, Isbister GK. Efficacy of Indian polyvalent snake antivenoms against Sri Lankan snake venoms: lethality studies or clinically focussed in vitro studies. Sci Rep. 2016;6:26778. 10.1038/srep26778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sintiprungrat K, Watcharatanyatip K, Senevirathne WDST, Chaisuriya P, Chokchaichamnankit D, Srisomsap C, et al. A comparative study of venomics of Naja naja from India and Sri Lanka, clinical manifestations and antivenomics of an Indian polyspecific antivenom. J Proteomics. 2016;132:131–43. 10.1016/j.jprot.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Heckmann X, Lambert V, Mion G, Ehrhardt A, Marty C, Perotti F, et al. Failure of a Mexican antivenom on recovery from snakebite-related coagulopathy in French Guiana. Clin Toxicol. 2021;59:193–9. 10.1080/15563650.2020.1786108. [DOI] [PubMed] [Google Scholar]
- 57.Abu Baker MA, Al-Saraireh M, Amr Z, Amr SS, Warrell DA. Snakebites in Jordan: a clinical and epidemiological study. Toxicon. 2022;208:18–30. 10.1016/j.toxicon.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Patra A, Kalita B, Khadilkar MV, Salvi NC, Shelke PV, Mukherjee AK. Assessment of quality and pre-clinical efficacy of a newly developed polyvalent antivenom against the medically important snakes of Sri Lanka. Sci Rep. 2021;11:18238. 10.1038/s41598-021-97501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uko SO, Malami I, Ibrahim KG, Lawal N, Bello MB, Abubakar MB, et al. Revolutionizing snakebite care with novel antivenoms: Breakthroughs and barriers. Heliyon. 2024;10: e25531. 10.1016/j.heliyon.2024.e25531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukherjee AK, Mackessy SP. Prevention and improvement of clinical management of snakebite in Southern Asian countries: a proposed road map. Toxicon. 2021;200:140–52. 10.1016/j.toxicon.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Boum Y, Kwedi-Nolna S, Haberer JE, Leke RRG. Traditional healers to improve access to quality health care in Africa. Lancet Glob Heal. 2021;9:e1487–8. 10.1016/S2214-109X(21)00438-1. [DOI] [PubMed] [Google Scholar]
- 62.Steegemans I, Sisay K, Nshimiyimana E, Gebrewold G, Piening T, Menberu Tessema E, et al. Treatment outcomes among snakebite patients in north-west Ethiopia—a retrospective analysis. PLoS Negl Trop Dis. 2022;16: e0010148. 10.1371/journal.pntd.0010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Estrada-Gómez S, Vargas-Muñoz LJ, Higuita-Gutiérrez LF. Epidemiology of snake bites linked with the antivenoms production in Colombia 2008–2020: produced vials do not meet the needs. Drug Healthc Patient Saf. 2022;14:171–84. 10.2147/DHPS.S367757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rocha GDS, Farias AS, Alcântara JA, Machado VA, Murta F, Val F, et al. Validation of a culturally relevant snakebite envenomation clinical practice guideline in Brazil. Toxins (Basel). 2022;14:1–14. 10.3390/toxins14060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ooms GI, van Oirschot J, Okemo D, Waldmann B, Erulu E, Mantel-Teeuwisse AK, et al. Availability, affordability and stock-outs of commodities for the treatment of snakebite in Kenya. PLoS Negl Trop Dis. 2021;15: e0009702. 10.1371/journal.pntd.0009702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang JD, Tsan YT, Mao YC, Wang LM. Venomous snakebites and antivenom treatment according to a protocol for pediatric patients in Taiwan. J Venom Anim Toxins Incl Trop Dis. 2009;15:667–79. 10.1590/S1678-91992009000400006. [Google Scholar]
- 67.WHO. Guidelines for Establishing a Poison Centre. 2020
- 68.Patra A, Mukherjee AK. Assessment of snakebite burdens, clinical features of envenomation, and strategies to improve snakebite management in Vietnam. Acta Trop. 2021;216: 105833. 10.1016/j.actatropica.2021.105833. [DOI] [PubMed] [Google Scholar]
- 69.Barnes K, Ngari C, Parkurito S, Wood L, Otundo D, Harrison R, et al. Delays, fears and training needs: perspectives of health workers on clinical management of snakebite revealed by a qualitative study in Kitui County. Kenya Toxicon X. 2021;11: 100078. 10.1016/j.toxcx.2021.100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gajbhiye RK, Munshi H, Bawaskar HS. National programme for prevention & control of snakebite in India. Indian J Med Res. 2023. 10.4103/ijmr.ijmr_2424_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pucca MB, Knudsen C, Oliveira IS, Rimbault C, Cerni FA, Wen FH, et al. Current knowledge on snake dry bites. Toxins (Basel). 2020;12:4–7. 10.3390/toxins12110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alqahtani SS, Banji D, Banji OJF, Syed MH, Syed NK, Meraya AM, et al. Knowledge and attitude of first-aid treatments for snakebites, and the perception of snakes among the students of health sciences at Jazan university. Saudi Arabia Healthc. 2022;10:2226. 10.3390/healthcare10112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monzavi SM, Salarian AA, Khoshdel AR, Dadpour B, Afshari R. Effectiveness of a clinical protocol implemented to standardize snakebite management in Iran: initial evaluation. Wilderness Environ Med. 2015;26:115–23. 10.1016/j.wem.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 74.Dehghani R, Monzavi SM, Mehrpour O, Shirazi FM, Hassanian-Moghaddam H, Keyler DE, et al. Medically important snakes and snakebite envenoming in Iran. Toxicon. 2023;230: 107149. 10.1016/j.toxicon.2023.107149. [DOI] [PubMed] [Google Scholar]
- 75.Patikorn C, Ismail AK, Abidin SAZ, Blanco FB, Blessmann J, Choumlivong K, et al. Situation of snakebite, antivenom market and access to antivenoms in ASEAN countries. BMJ Glob Heal. 2022. 10.1136/bmjgh-2021-007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clinical Toxinology Resources n.d. http://www.toxinology.com/.
- 77.iNaturalist n.d. https://www.inaturalist.org/ (accessed November 5, 2023).
- 78.SnakeHub n.d. https://www.indriyambiologics.com/snake-hub (accessed May 17, 2024).
- 79.Patel A, Cheung L, Khatod N, Matijosaitiene I, Arteaga A, Gilkey JW. Revealing the unknown: real-time recognition of Galápagos snake species using deep learning. Animals. 2020. 10.3390/ani10050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.SnakeSnap n.d. https://apps.apple.com/us/app/snakesnap/id1466825376.
- 81.Puzari U, Mukherjee AK. Recent developments in diagnostic tools and bioanalytical methods for analysis of snake venom: a critical review. Anal Chim Acta. 2020;1137:208–24. 10.1016/j.aca.2020.07.054. [DOI] [PubMed] [Google Scholar]
- 82.Khochare S, Jaglan A, Rashmi U, Dam P, Sunagar K. Harnessing the cross-neutralisation potential of existing antivenoms for mitigating the outcomes of snakebite in Sub-Saharan Africa. Int J Mol Sci. 2024;25:4213. 10.3390/ijms25084213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee LP, Tan KY, Tan CH. Toxicity and cross-neutralization of snake venoms from two lesser-known arboreal pit vipers in Southeast Asia: Trimeresurus wiroti and Trimeresurus puniceus. Toxicon. 2020;185:91–6. 10.1016/j.toxicon.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 84.Cham G, Lim F, Earnest A, Gopalakrishnakone P. Cross-reactivity against Naja sumatrana (Black Spitting Cobra) Envenoming from the Haffkine Antivenom in a Mouse Model. ISRN Toxicol. 2013;2013: 247645. 10.1155/2013/247645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nikolić S. Venomous snakebites in Serbia through 125 years : what we do (not) know in comparison with neighboring countries. a literature review. Acta Med Median. 2020;49:95–103. 10.5633/amm.2020.0413. [Google Scholar]
- 86.Menon JC, Bharti OK, Dhaliwal RS, John D, Menon GR, Grover A, et al. ICMR task force project-survey of the incidence, mortality, morbidity and socioeconomic burden of snakebite in India: a study protocol. PLoS ONE. 2022;17: e0270735. 10.1371/journal.pone.0270735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Menon JC, John D, Menon GR, Joseph JK, Suseela PR, Pillay V. Estimating epidemiological and economic burden and community derived disability weights for snake bite in Kerala: a study protocol. F1000Research. 2021;10:167. 10.12688/f1000research.50970.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gharaibeh A, Koppikar S, Bonilla-Escobar J, F. Strengthening the reporting of observational studies in epidemiology (STROBE) in the International Journal of Medical Students. Int J Med Stud. 2014;2:36–7. 10.5195/ijms.2014.76. [Google Scholar]
- 89.Fuchs J, Gessner T, Kupferschmidt H, Weiler S. Exotic venomous snakebites in Switzerland reported to the National Poisons Information Centre over 22 years. Swiss Med Wkly. 2022;152: w30117. 10.4414/SMW.2022.w30117. [DOI] [PubMed] [Google Scholar]
- 90.La Laina DZ, Nekaris KAI, Nijman V, Morcatty TQ. Illegal online pet trade in venomous snakes and the occurrence of snakebites in Brazil. Toxicon. 2021;193:48–54. 10.1016/j.toxicon.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 91.Suraweera W, Warrell D, Whitaker R, Menon G, Rodrigues R, Fu SH, et al. Trends in snakebite deaths in India from 2000 to 2019 in a nationally representative mortality study. Elife. 2020;9: e54076. 10.7554/eLife.54076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vanuopadath M, Rajan K, Alangode A, Nair SS, Nair BG. The need for next-generation antivenom for snakebite envenomation in India. Toxins (Basel). 2023. 10.3390/toxins15080510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bawaskar HS, Bawaskar PH, Bawaskar PH. Primary health care for snakebite in India is inadequate. Lancet. 2020;395:112. 10.1016/S0140-6736(19)31909-9. [DOI] [PubMed] [Google Scholar]
- 94.Gopal G, Muralidar S, Prakash D, Kamalakkannan A, Indhuprakash ST, Thirumalai D, et al. The concept of big four: road map from snakebite epidemiology to antivenom efficacy. Int J Biol Macromol. 2023;242: 124771. 10.1016/j.ijbiomac.2023.124771. [DOI] [PubMed] [Google Scholar]
- 95.Liwang F, Nuraeni F, Karyanti MR. Snake bite management in a toddler: a case report in Sumbawa Besar. Paediatr Indones. 2021;61:171–6. 10.14238/pi61.4.2021.171-4. [Google Scholar]
- 96.Avni-Maskit M, Pomp R, Chayen G, Jacob R. Latency of symptom progression in mild daboia palaestinae envenomation. Wilderness Environ Med. 2022;33:204–9. 10.1016/j.wem.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 97.Gross I, Maree A, Rekhtman D, Mujahed W, Hashavya S, Assaf J. Clinical characteristics and management of snake bite injuries in the Jerusalem area. J Clin Med. 2023;12:10–4. 10.3390/jcm12124132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hifumi T, Sakai A, Yamamoto A, Morokuma K, Otani N, Takahashi M, et al. Rhabdophis tigrinus (Yamakagashi) bites in Japan over the last 50 years: a retrospective survey. Front Public Heal. 2022;9:1–6. 10.3389/fpubh.2021.775458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Memon R, Erickson TB, Goldfine CE. Challenges in care of snake envenomation in rural Pakistan: a narrative review. Toxicol Commun. 2023;7:2223049. 10.1080/24734306.2023.2223049. [Google Scholar]
- 100.Han K, Song H, Choi CW, Park S, Kang YS, Jung K, et al. Standardization of the first Korean national reference standard for snake (Gloydius brevicaudus) antivenom. Toxicol Res. 2020;36:407–13. 10.1007/s43188-020-00047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moon JM, Koo YJ, Chun BJ, Park KH, Cho YS, Kim JC, et al. The effect of myocardial injury on the clinical course of snake envenomation in South Korea. Clin Toxicol. 2021;59:286–95. 10.1080/15563650.2020.1802473. [DOI] [PubMed] [Google Scholar]
- 102.Shin Y, Jang Y, Borzée A. Snakebite envenomings in the Republic of Korea from the 1970s to the 2020s: a review. Toxicon. 2021;196:8–18. 10.1016/j.toxicon.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 103.Al-Sadoon MK, Fahad Albeshr M, Ahamad Paray B, Rahman A-M. Envenomation and the bite rate by venomous snakes in the kingdom of Saudi Arabia over the period (2015–2018). Saudi J Biol Sci. 2021;28:582–6. 10.1016/j.sjbs.2020.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hardcastle T, Engelbrecht A, Lalloo V, Bell C, Toubkin M, Motara F, et al. Approach to the diagnosis and management of snakebite envenomation in South Africa in humans: Special patient groups and surgical aspects. South Afr Med J. 2023;113:16–21. 10.7196/SAMJ.2023.v113i6.1038. [DOI] [PubMed] [Google Scholar]
- 105.Chakroun-Walha O, Issaoui F, Nasri A, Bradai H, Farroukh A, Karray R, et al. Early severity predictors of snakebite envenomation in the southern region of Tunisia: a multivariate analysis. J Acute Dis. 2021;10:71. 10.4103/2221-6189.312155. [Google Scholar]
- 106.Gutiérrez JM, Maduwage K, Iliyasu G, Habib A. Snakebite envenoming in different national contexts: Costa Rica, Sri Lanka, and Nigeria. Toxicon X. 2021. 10.1016/j.toxcx.2021.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neri-Castro E, Bénard-Valle M, Gil G, Borja M, de León JL, Alagón A. Venomous snakes in Mexico: a review of the study of venoms, antivenom and epidemiology. Rev Latinoam Herpetol. 2020;03:5–22. [Google Scholar]
- 108.Tupetz A, Barcenas LK, Phillips AJ, Vissoci JRN, Gerardo CJ. BITES study: A qualitative analysis among emergency medicine physicians on snake envenomation management practices. PLoS ONE. 2022;17:1–16. 10.1371/journal.pone.0262215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chotai PN, Watlington J, Lewis S. Pediatric snakebites : comparing patients in two geographic locations in the United States. J Surg Res. 2021;265:297–302. 10.1016/j.jss.2021.03.045. [DOI] [PubMed] [Google Scholar]
- 110.Villca-Corani H, Nieto-Ariza B, León R, Rocabado JA, Chippaux JP, Urra FA. First reports of envenoming by South American water snakes Helicops angulatus and Hydrops triangularis from Bolivian Amazon: a one-year prospective study of non-front-fanged colubroid snakebites. Toxicon. 2021;202:53–9. 10.1016/j.toxicon.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 111.Schneider MC, Vuckovic M, Montebello L, Sarpy C, Huang Q, Galan DI, et al. Snakebites in rural areas of Brazil by race: indigenous the most exposed group. Int J Environ Res Public Health. 2021;18:9365. 10.3390/ijerph18179365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blasco Mariño R, Soteras Martínez I, Hernandez Roca AI, Zafren K. Isolated ptosis following a Vipera aspis bite. Wilderness Environ Med. 2022;33:245–7. 10.1016/j.wem.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 113.Jagpal PS, Williams HA, Eddleston M, Lalloo D, Warrell D, Sandilands EA, et al. Bites by exotic snakes reported to the UK National Poisons Information Service 2009–2020. Clin Toxicol. 2022;60:1044–50. 10.1080/15563650.2022.2077748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This published article contains all the generated and analyzed data, thus no additional data source is required.