Abstract
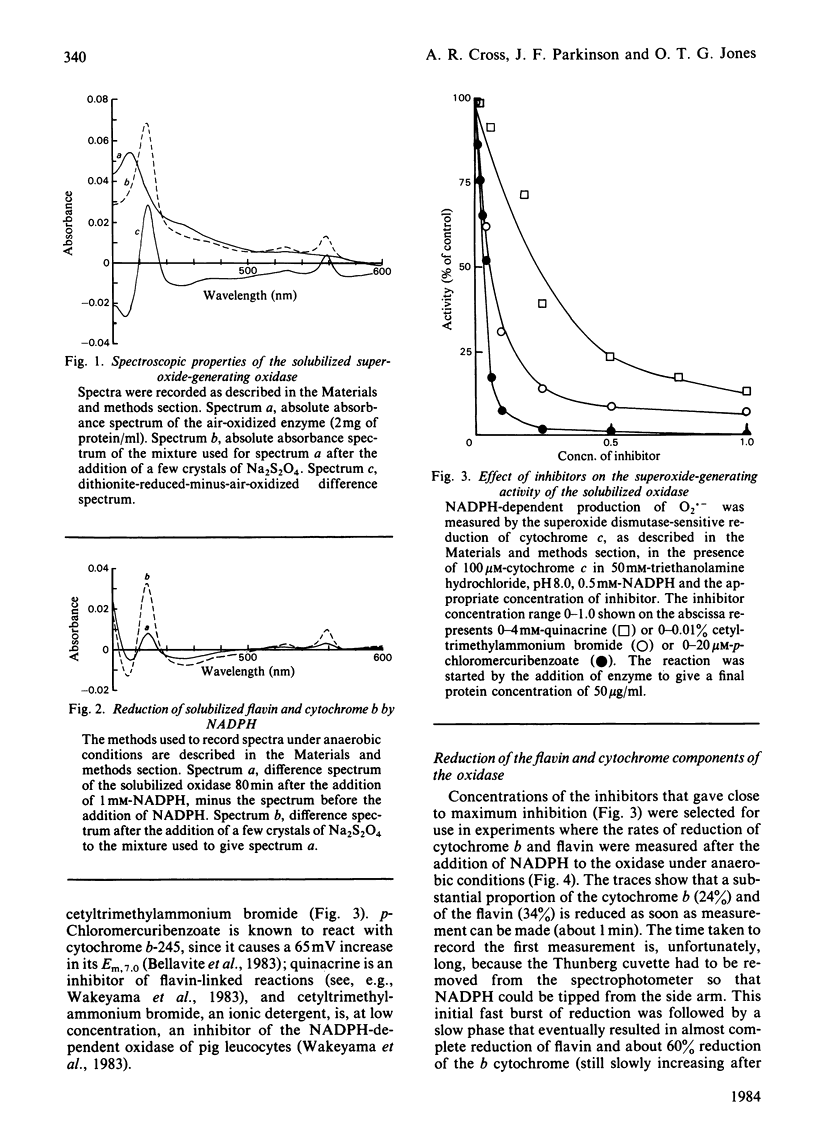
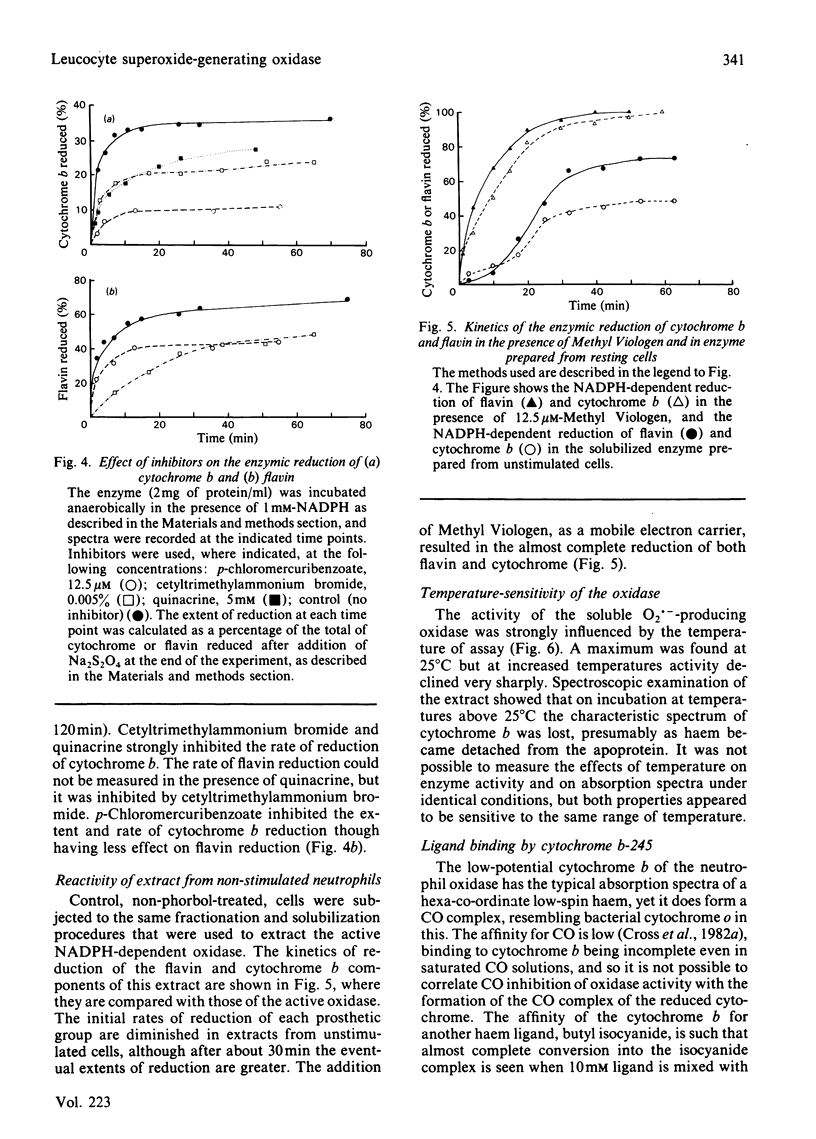
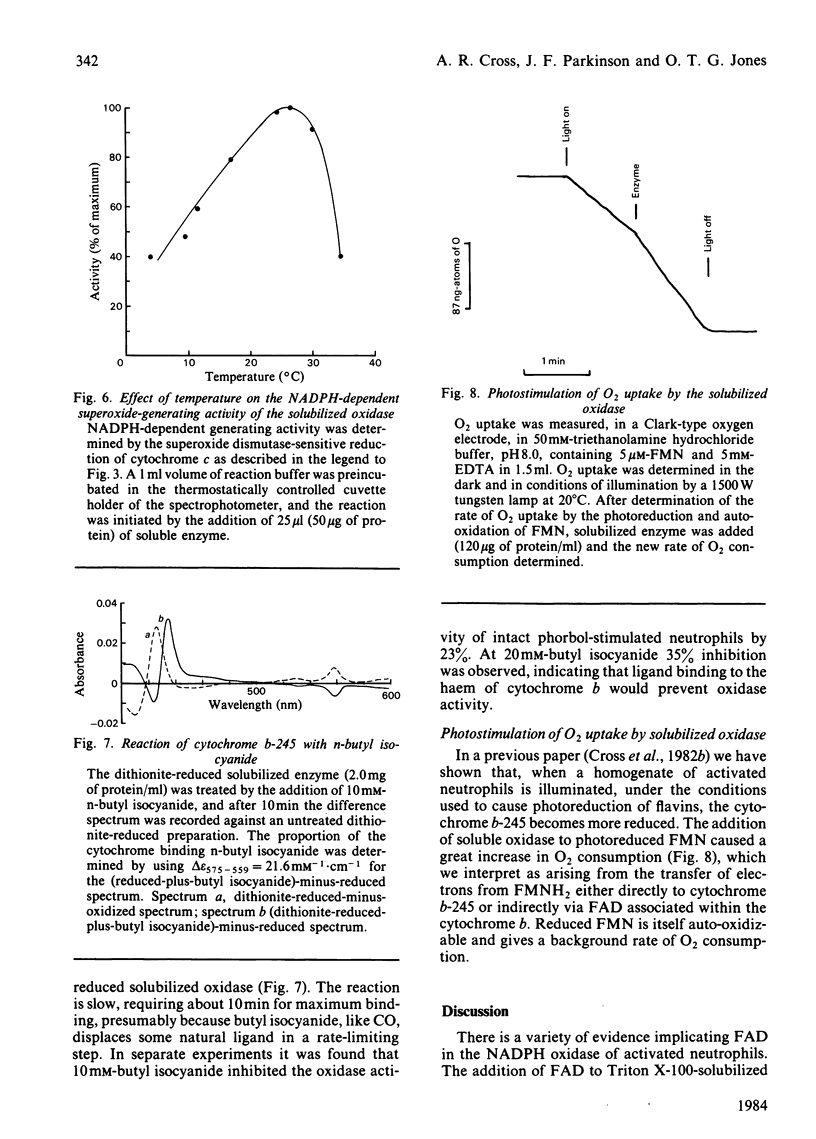
An NADPH-dependent O2.- -generating oxidase was solubilized from phorbol 12-myristate 13-acetate-activated pig neutrophils by using a mixture of detergents. Recovery of oxidase was approx. 40%. The extract contained cytochrome b-245 (331 pmol/mg of protein) and FAD (421 pmol/mg of protein); approx. 30% of each was reduced within 60s when NADPH was added to anaerobic incubations. Three different additives, quinacrine, p-chloromercuribenzoate and cetyltrimethylammonium bromide, strongly inhibited O2.- generation; they also inhibited the reduction by NADPH of cytochrome b at the same low concentrations. In the presence of p-chloromercuribenzoate cytochrome b reduction was strongly inhibited and flavin reduction was less inhibited. A detergent extract prepared from non-stimulated neutrophils also contained flavin and cytochrome b, but its rate of O2.- production was less than 1% of that from activated cells; its initial rate of cytochrome b and flavin reduction was low, although the state of reduction at equilibrium was similar to that of extracts of activated cells. Even in the non-activated cell extract the reduction of flavin and cytochrome was made fast and complete when Methyl Viologen was added to the anaerobic incubations. The oxidase was temperature-sensitive, with a sharp maximum at 25 degrees C; temperatures above this caused loss of O2.- generation, and this coincided with loss of the characteristic cytochrome b spectrum, indicate of denaturation of the cytochrome. The cytochrome b formed a complex with butyl isocyanide (close to 100% binding at 10mM); butyl isocyanide also inhibited the oxidase activity of stimulated whole neutrophils (22.5% inhibition at 10mM). Photoreduced FMN stimulated O2 uptake by the oxidase. The results support a scheme of electron transport within the oxidase complex involving NADPH, FAD, cytochrome b-245 and O2 in that sequence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bellavite P., Cross A. R., Serra M. C., Davoli A., Jones O. T., Rossi F. The cytochrome b and flavin content and properties of the O2- -forming NADPH oxidase solubilized from activated neutrophils. Biochim Biophys Acta. 1983 Jul 28;746(1-2):40–47. doi: 10.1016/0167-4838(83)90008-0. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Tauber A. I. Subcellular localization of the human neutrophil NADPH oxidase. b-Cytochrome and associated flavoprotein. J Biol Chem. 1984 Jan 10;259(1):47–52. [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Higson F. K., Jones O. T., Harper A. M., Segal A. W. The enzymic reduction and kinetics of oxidation of cytochrome b-245 of neutrophils. Biochem J. 1982 May 15;204(2):479–485. doi: 10.1042/bj2040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Garcia R., Segal A. W. The association of FAD with the cytochrome b-245 of human neutrophils. Biochem J. 1982 Dec 15;208(3):759–763. doi: 10.1042/bj2080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Harper A. M., Segal A. W. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem J. 1981 Feb 15;194(2):599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L. Redox potentiometry: determination of midpoint potentials of oxidation-reduction components of biological electron-transfer systems. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- Faeder E. J., Siegel L. M. A rapid micromethod for determination of FMN and FAD in mixtures. Anal Biochem. 1973 May;53(1):332–336. doi: 10.1016/0003-2697(73)90442-9. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., Lefker B. A. Catalytic properties of the resolved flavoprotein and cytochrome B components of the NADPH dependent O2- . generating oxidase from human neutrophils. Biochem Biophys Res Commun. 1984 Jan 30;118(2):430–436. doi: 10.1016/0006-291x(84)91321-4. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., Schervish E. W., Santinga J. T. Functional relationship of the cytochrome b to the superoxide-generating oxidase of human neutrophils. J Biol Chem. 1982 Apr 25;257(8):4114–4119. [PubMed] [Google Scholar]
- Gabig T. G. The NADPH-dependent O-.2-generating oxidase from human neutrophils. J Biol Chem. 1983 May 25;258(10):6352–6356. [PubMed] [Google Scholar]
- Hamers M. N., de Boer M., Meerhof L. J., Weening R. S., Roos D. Complementation in monocyte hybrids revealing genetic heterogeneity in chronic granulomatous disease. Nature. 1984 Feb 9;307(5951):553–555. doi: 10.1038/307553a0. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D. R., Walsh C., O'Callaghan A. M., Goetzl E. J., Tauber A. I. Characteristics of the cofactor requirements for the superoxide-generating NADPH oxidase of human polymorphonuclear leukocytes. Biochemistry. 1981 Mar 17;20(6):1468–1476. doi: 10.1021/bi00509a010. [DOI] [PubMed] [Google Scholar]
- Muir Wood P. The redox potential of the system oxygen--superoxide. FEBS Lett. 1974 Aug 15;44(1):22–24. doi: 10.1016/0014-5793(74)80297-8. [DOI] [PubMed] [Google Scholar]
- ROSSI F., ZATTI M. CHANGES IN THE METABOLIC PATTERN OF POLYMORPHO-NUCLEAR LEUCOCYTES DURING PHAGOCYTOSIS. Br J Exp Pathol. 1964 Oct;45:548–559. [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Cross A. R., Garcia R. C., Borregaard N., Valerius N. H., Soothill J. F., Jones O. T. Absence of cytochrome b-245 in chronic granulomatous disease. A multicenter European evaluation of its incidence and relevance. N Engl J Med. 1983 Feb 3;308(5):245–251. doi: 10.1056/NEJM198302033080503. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. Absence of cytochrome b reduction in stimulated neutrophils from both female and male patients with chronic granulomatous disease. FEBS Lett. 1980 Jan 28;110(1):111–114. doi: 10.1016/0014-5793(80)80035-4. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. Reduction and subsequent oxidation of a cytochrome b of human neutrophils after stimulation with phorbol myristate acetate. Biochem Biophys Res Commun. 1979 May 14;88(1):130–134. doi: 10.1016/0006-291x(79)91706-6. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. The subcellular distribution and some properties of the cytochrome b component of the microbicidal oxidase system of human neutrophils. Biochem J. 1979 Jul 15;182(1):181–188. doi: 10.1042/bj1820181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeyama H., Takeshige K., Minakami S. NADPH-dependent reduction of 2,6-dichlorophenol-indophenol by the phagocytic vesicles of pig polymorphonuclear leucocytes. Biochem J. 1983 Feb 15;210(2):577–581. doi: 10.1042/bj2100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeyama H., Takeshige K., Takayanagi R., Minakami S. Superoxide-forming NADPH oxidase preparation of pig polymorphonuclear leucocyte. Biochem J. 1982 Sep 1;205(3):593–601. doi: 10.1042/bj2050593. [DOI] [PMC free article] [PubMed] [Google Scholar]


