Abstract
Cognitive symptoms persisting beyond the acute phase of COVID-19 infection are commonly described for up to 2 years after infection. The relationship between cognitive performance, in particular episodic memory processes observed chronically after infection, and cytokine levels in the acute phase of COVID-19 has not yet been identified in humans. To determine whether the levels of cytokines IL1β, IL-6 and TNFα secreted in the acute phase of SARS-CoV-2 infection are associated and predict verbal and visuospatial episodic memory performance in humans 6 to 9 months and 12 to 15 months post-infection. The associations and predictive value of the concentration of cytokines measured in acute phase (IL-1β, IL-6, TNFα) from plasma samples of N = 33 hospitalized COVID-19 patients (mean age 61 years, 39–78, 65% in intensive care) in relation to their verbal and visuospatial episodic memory performance measured at 6–9 months and 12–15 months post-infection were analyzed. To do this, we used Spearman correlations and generalised linear mixed models. IL-1β levels were associated with verbal episodic memory total recall scores 6–9 months post-infection. At 12–15 months post-infection IL-6 predicted verbal episodic memory score. This study demonstrated that the severity of inflammatory reaction at acute phase of SARS-CoV-2 infection predicts verbal episodic memory performance in the long-term post-infection.
Keywords: SARS-CoV-2, Cognition, Immunity, COVID-19, Post-COVID, Long COVID, Memory
Subject terms: Predictive markers, Human behaviour, Cytokines
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection leads to sequelae beyond the acute phase, manifested by a variety of physical, cognitive, and psychiatric symptoms (e.g. dyspnoea, memory loss, anxiety)1–3. These persistent symptoms are defined by the World Health Organisation as the post-COVID condition4. Studies mainly highlight the persistence of cognitive symptoms and fatigue5 up to 2 years post-infection6. In terms of prevalence, persistent cognitive symptoms one year after infection would represent between 14 and 34% of people hospitalized7. With regard to fatigue, 9 months after infection, around 19% of people (whether hospitalized or not) would still have clinically significant fatigue8. Curiously, the persistence of these symptoms are not entirely explained by the severity of the disease in the acute phase9. According to Morioka, et al.10, in a cohort study, more than a quarter of people with mild coronavirus disease (COVID-19) infection retained at least one physical or neurocognitive symptom between 6- and 24-months post-infection. However, there are disparities between the different research cohorts concerning the presence of post-COVID condition. During the 7 months following infection, multiple organs remain impacted by COVID-19, affecting between 30% and 70% of a heterogeneous population11.
There are multiple hypotheses regarding the pathophysiological pathways of post-COVID condition : some invoke the neurotropism of S proteins towards the ACE 2 receptor12 and systemic inflammatory consequences12 and others support the hypothesis of infiltration of the blood–brain barrier13. The first hypothesis is currently being studied by profiling leukocyte and cytokine markers that could explain the sequelae following infection by SARS-CoV-212,14–16. More specifically, overexpression of interleukin (IL)-1β and IL-6 during infection was associated with alterations in hippocampal neurogenesis from the post-acute phase (one week) in a mouse model17. Per the literature, in 2006, a cytokine model of memory underpinned by hippocampal substrates highlighted the involvement of tumor necrosis factor (TNF)α, IL-6 and IL-1β in memory and learning processes in animals18. Now, in the context of COVID-19 in humans, a previous study by Nuber-Champier et al.17 demonstrated relationships between the immunity displayed during the acute phase of the infection and the anosognosia of memory deficits 6–9 months post-infection. According to the authors19,20, TNFα predicted anosognosia of memory deficits and correlated with the functional connectivity of the hippocampal structures 6–9 months after infection19,20. Brain imaging studies in humans are currently showing that COVID-19 infection has anatomical (e.g., alteration of white and grey matter) and functional repercussions that could target the hippocampus, parahippocampal regions7,21,22 and a distributed cerebral network with behavioural consequences23–25.
In the light of these observations, one of the major concerns linked to the persistence of these symptoms and pathophysiological patterns lies in the risks associated with the development or acceleration of neurodegenerative pathologies26. Current work highlighting possible neurodegenerative trajectories associated with multiple types of viral infection (e.g. pneumonia, herpes)27 suggests that COVID-19 could also be a risk factor in the development of neurodegenerative pathologies28. Furthermore, the immune (IL-6 and IL-1β) and cognitive relationships in the context of neurodegenerative diseases seem to be increasingly robust29–31. It therefore seems of crucial interest to investigate in depth the immune and cognitive relationships following SARS-CoV-2 infection in order to better predict the associated very long-term risks to mental health. Furthermore, the investigation of immune-cognitive relationships appears to be crucial in understanding the persistence of cognitive symptoms after SARS-CoV-2 infection, which is still not well understood. To our knowledge, in the context of COVID-19, few studies have demonstrated associations between immunity during the acute phase and specific cognitive deficits observed beyond 6 months after infection in humans19,32,33.
Thus, given what is currently known about the relationships between cytokines (TNFα, IL-6 and IL-1β), brain function and associated cognitive processes18,31,34, and given the hypotheses of post-COVID cognitive decline whose origin appears to stem from systemic inflammation35, it is crucial to continue studying these interactions to better understand their effects. In other words, it seems necessary to study the relationships between memory capacity observed in humans at 6–9 months and 12–15 months post-infection alongside the levels of cytokines TNFα, IL-6 and IL-1β secreted during the acute phase. In this context, the main objective of this study was to explore the links between the cytokines TNFα, IL-6 and IL-1β secreted during the acute phase and verbal and visuospatial episodic memory performances in humans at 6–9 months and 12–15 months post-infection with SARS-CoV-2. With this objective in mind, and based on previous results linking immunity and cognition in the context of COVID-1919,32, we hypothesized that cytokine release syndrome at the time of acute COVID-19—including TNFα, IL-6 and IL-1β—would be associated with reduced episodic memory performance 6–9 months and 12–15 months post-infection. Furthermore, we expected that these biomarkers may predict verbal and visuospatial episodic memory performance 6–9 months and 12–15 months post-infection.
Results
Sociodemographic and clinical data
The sociodemographic and clinical data of the 33 patients included in this study are reported in Table 1. The sample comprised 65% patients hospitalized in intensive care and 35% hospitalized in conventional internal medicine.
Table 1.
Sociodemographic and clinical data from the sample of patients assessed 6–9 months and 12–15 months after infection with SARS-CoV-2.
| Patients included in the study | |
|---|---|
| N = 33 | |
| Mean age in years (± SD) | 61.30 (± 10.98) |
| Education level (1/2/3) | 1/13/19 |
| Sex (F/M) | 7/26 |
| Number of patients who required intermediate/intensive care in the acute phase | 11/22 |
| Mean days of hospitalization (± SD) | 30.16 (± 25.91) |
| Mean days between positive RT-PCR test and collection of immunological data (± SD) | 1.26 (± 2.85) |
| Diabetes (yes/no) | 6/27 |
| History of respiratory disorders (yes/no) | 7/26 |
| History of cardiovascular disorders (yes/no) | 8/25 |
| History of neurological disorders (yes/no) | 0/33 |
| History of psychiatric disorders (yes/no) | 0/33 |
| History of cancer (yes/no) | 0/33 |
| History of severe immunosuppression (yes/no) | 0/33 |
| History of developmental disorders (yes/no) | 0/33 |
Education level: 1 = compulsory schooling, 2 = post-compulsory schooling, and 3 = university degree or equivalent. Encephalopathy observed in the acute phase.
RT-PCR reverse transcription polymerase chain reaction, SD standard deviation, Sex F female, M mal.
Cytokines levels in the acute phase of COVID-19
The plasma cytokines – TNFα, IL-6, and IL-1β – concentrations assessment conducted via immunoassay at the time of admission to hospital for SARS-CoV2 infection, are presented in Table 2.
Table 2.
Concentration of cytokines extracted on admission to hospital for COVID-19 infection .
| Types of cytokines | Cytokine count on day 1 of hospitalization (N = 33) median [95% CI] |
|---|---|
| TNFα (pg/ml) | 3.86 [3.53; 6.61] |
| IL-6 (pg/ml) | 12.78 [14.32; 33.65] |
| IL-1β (pg/ml) | 0.69 [0.46; 1.39] |
IL interleukin, SD standard deviation, TNFα tumor necrosis factor-alpha.
Association between cytokines levels in the acute phase of COVID-19 and episodic memory performance 6–9 months and 12–15 months post-infection
Verbal episodic memory at 6–9 months
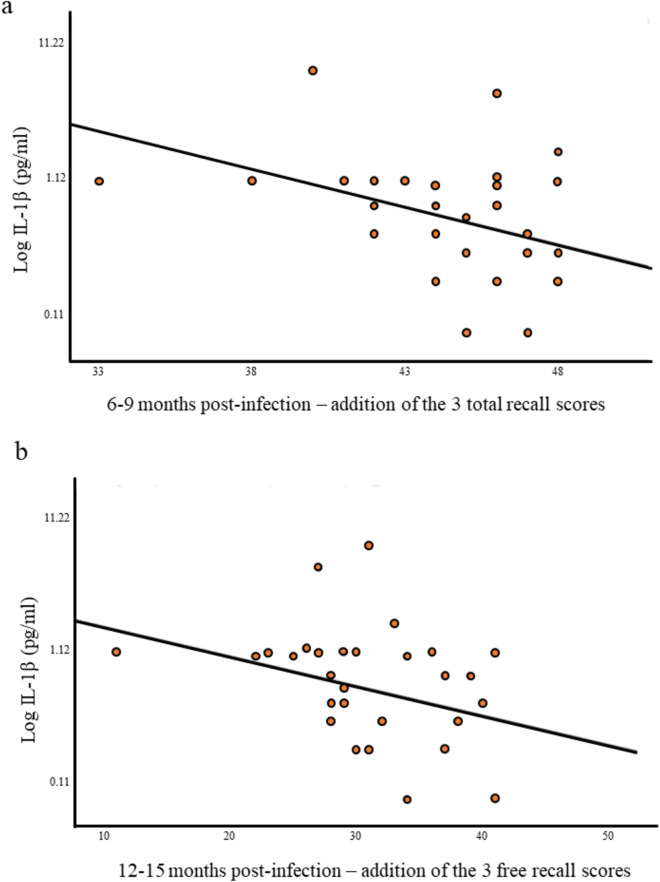
Correlations between RLRI raw scores obtained 6–9 months post-infection and cytokine levels measured in the acute phase of COVID-19 revealed all negative correlations between IL-1β plasma levels and all total scores and a free recall. IL-1β plasma levels correlated with RLRI free recall 2 (r = − 0.37; p = 0.034); total recall 1 scores (r = − 0.40; p = 0.019) total recall 2 scores (r = − 0.43; p = 0.013), the total of the three total scores (r = − 0.46; p = 0.006) (see Fig. 1), and total recall at 20-min scores (r = − 0.36; p = 0.037).
Fig. 1.
Semi-log association between IL-1β secreted during the acute phase of SARS-CoV-2 infection and episodic memory performance 6–9 months and 12–15 months post-infection. (a) Semi-log association between Log IL-1β and the total of the three RLRI total scores 6–9 months post infection. (b) Semi-log association between Log IL-1β and the addition of the three RLRI free recall scores 12–15 months post-infection. Log logarithmic, RLRI free/cued recall paradigm.
Visuospatial episodic memory at 6–9 months
None of the results were significant.
Verbal episodic memory at 12–15 months
RLRI free recall 2 scores correlated negatively with IL-1β plasma levels (r = − 0.41; p = 0.016) and the addition of the three free recall scores correlated negatively with IL-1β plasma levels measured on the acute phase of the infection (r = − 0.36; p = 0.039) (see Fig. 1).
Visuospatial episodic memory at 12–15 months
None of the results were significant.
Prediction of episodic memory performance 6–9 months post-infection by acute immunity
Verbal episodic memory
RLRI free recall 1 scores obtained 6–9 months post-infection were significantly predicted by IL-1β plasma levels measured in the acute phase of COVID-19 (F = 4.50; p = 0.043; 95% CI [− 4.80; − 0.08]). RLRI total recalls 2 scores were significantly predicted by IL-1β plasma levels (F = 7.02; p = 0.013; 95% CI [− 0.12; − 0.016]). In addition, the addition of the three total scores were predicted by IL-1β plasma levels measured in the acute phase of infection (F = 8.23; p = 0.008; 95% CI [− 6.58; − 1.10]). Finally, total recalls 1, 3 and at 20 min were significantly predicted by IL-1β plasma levels but did not survive FDR correction (total 1: F = 5.06; p = 0.032; 95% CI [− 0.31; − 0.015]); (total 3: F = 6.26; p = 0.018; 95% CI [− 0.11; − 0.012]) and (total 20 min :F = 4.76; p = 0.037; 95% CI [− 0.13; − 0.004]).
Visuospatial episodic memory
None of the results were significant.
Prediction of episodic memory performance 12–15 months post-infection by acute immunity
Verbal episodic memory
Free recall 1 score obtained 12–15 months after infection showed that IL-6 plasma levels was a significant predictive factor (F = 5.82; p = 0.022; 95% CI [− 2.84; − 0.23]).
Visuospatial episodic memory
None of the results were significant.
Discussion
The immune-cognitive relationship has been the subject of a great deal of research on animals and, more recently, on humans in infectious contexts36 or neurodegenerative diseases27,29. In particular, the cytokines IL-1, TNFα and IL-6 appear to be central inflammatory markers at the cerebral level and may have an impact on the performance of certain cognitive processes37.
This study, which explored the relationships between cytokines released during the acute phase of SARS-CoV-2 infection and cognitive functions in the post-infection context of COVID-19, has yielded interesting findings, especially in relation to memory processes. Indeed, our study supports our first hypothesis and suggests several significant associations, as shown by the correlation results. These analyzes at 6–9 months revealed negative associations between IL-1β and all the measures involving the verbal episodic memory storage process. Curiously, analyzes carried out 12–15 months post- infection revealed negative correlations associating retrieval processes in verbal episodic memory and the plasma concentration of IL-1β measured in the acute phase of SARS-CoV-2 infection. It would therefore appear, from these results, that marker IL-1β has a differential impact on verbal episodic memory sub-processes over time. Verbal episodic memory storage performance is influenced by the plasma concentration of IL-1β 6–9 months post-infection, while retrieval performance is influenced by the plasma concentration of IL-1β 12–15 months post-infection. This implies that immunity can modulate the performance of different processes of verbal episodic memory. However, no significant associations were found for visuospatial episodic memory. Given that only the verbal aspect of episodic memory is linked to the immunity secreted during the acute phase, it also seems that certain processes linked to a specific modality are more sensitive to inflammatory variations than others.
The present results also partially confirmed our second hypothesis, according to which acute levels of the cytokines IL-6, and IL-1β during the acute phase of COVID-19 could predict verbal episodic memory scores at 6–9 months and 12–15 months post-SARS-CoV-2 infection. We observed that, at 6–9 months post-infection, verbal episodic memory scores involving storage processes were predicted by IL-1β plasma levels. Analyzes of the prediction of memory scores at 12–15 months revealed different results to those observed at 6–9 months, with only one verbal episodic memory score (Free recall 1) reflecting more of a retrieval process, being predicted by IL-6 plasma levels. As regards the results relating to visuospatial episodic memory, none of the inflammatory markers proved to be predictive of these cognitive performances. Curiously, plasma TNFα levels were not linked to (or predictive of) these cognitive processes in this context. The links maintained between viral infection, inflammatory markers and episodic memory leave open the question of the trajectories of certain individuals with an potential risk of developing neurodegenerative pathology over time.
Viral infection can increase the risk of developing neurodegenerative pathologies over time27. According to Levine et al.27, hospitalization for influenza infection with pneumonia increases the risk of developing a neurodegenerative disease by a factor of two, 5 years later. There are many pathophysiological hypotheses, although the involvement of the immune system and neuroinflammation are currently interesting leads31. Several studies have since demonstrated links between the recruitment of IL-1β, IL-6 and TNFα type cytokines and the cognitive disorders observed in Alzheimer's disease30,31,38. It would appear that particularly high levels of IL-6 are associated with cognitive disorders observed in mild cognitive impairment and Alzheimer's disease and are also observed in greater quantities in Alzheimer's population than in a healthy population30,38. Recently, authors have highlighted an increased risk of developing a neurodegenerative pathology following infection with COVID-1926,39. According to Zarifkar et al.26, infection with COVID-19 is associated with a 3.5-fold greater risk of developing Alzheimer's disease compared with an uninfected person. The pathophysiological mechanisms connecting immune responses to cognitive disorders are not fully understood yet. However, the neurotrophic properties of IL-6 imply that a disruption in its regulation could play a role in the development of cognitive disorders31. Therefore, investigating the role of cytokine secretion by circulating immune cells and tissue-resident cells—such as glial cells—on cell-to-cell interaction and on the neuronal network may represent a promising avenue for future research by linking immune profile and long-term brain dysregulation. This could enhance our understanding of certain neurodegenerative mechanisms and the associated cognitive impairments40. This area of research becomes even more intriguing in the context of a systemic viral infection like SARS-CoV-2. The pathophysiological mechanisms unique to SARS-CoV-2 involve both specific and ubiquitous immune responses, which could potentially be mirrored in other viral contexts.
The present study has the advantage of having investigated cognitive performance precisely with specific tools for each cognitive function in a population in good health prior to infection with COVID-19. Thus, the impact on cognitive performance can less likely attributed to confounding factors linked to comorbidities. Moreover, cytokine levels measured soon after hospital admission avoid any influence from drugs administered during hospitalization. However, this study has several limitations, starting with the small sample size and the generalisability of the results, despite the power analysis we have carried out. A study involving a larger cohort seems necessary in order to be able to generalise the results statistically. A second limitation of this study is the lack of cytokine level measurements at various follow-up points post-SARS-CoV-2 infection, as well as the absence of immune cell profiling using flow cytometry phenotyping. A larger immune profiling description could be used to predict other cognitive disorders. In addition, the temporality between measurements of immunity in the acute phase and the cognitive measures (6–9 months post-infection) is likely to have an impact on the trajectories of individuals according to the different treatments received. Finally, the absence of a control group limits the interpretation of potential cognitive deficits in this population. However, despite the absence of a control group and of any interpretation as to the presence of potential cognitive deficits, we found evidence of variations in verbal episodic memory performance. This spectrum of performance may possibly span a continuum between normal and pathological performance. Furthermore, during the acute phase of the pandemic, it was ethically challenging to bring healthy individuals into the hospital. As a result, this cohort does not include a control group that was evaluated during the pandemic.
Conclusion
This study, conducted on a human population, highlights the predictive capacity of IL-1β plasma levels during the acute phase of SARS-CoV-2 infection on verbal episodic memory performance measured at 6–9 months. Plasma levels of IL-6 secreted during the acute phase predicted verbal episodic memory scores at 12–15 months post-infection. These findings reinforce the hypothesis of a connection between the acute immune system dysregulation and long-term cognitive functions.
Methods
General procedure and patient consent
This study extends the COVID-COG project. It utilizes existing data from the COVID-COG project, which includes samples from hospitalized and intensive care patients (all patients were hospitalized for SARS-CoV-2 infection), along with neuropsychological, sociodemographic, and clinical data (see section "COVID-COG cohort", "Participants included in the study" and "Sociodemographic and clinical data").
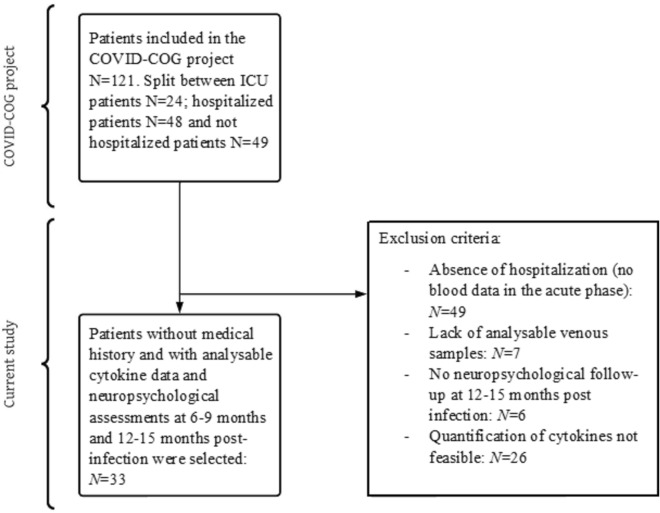
In the present study we specifically extracted and retrospectively analyzed acute cytokine data from hospitalization and intensive care patients with viable venous blood samples (see Fig. 2 and section "Retrospective analysis of cytokines" and "Cytokines levels in the acute phase of COVID-19"). We analyzed the relationship and predictability of episodic memory raw scores measured at 6–9 months and 12–15 months post-infection by acute cytokine levels. The study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the cantonal ethics committee of Geneva (CER-02186). Each patient included in the study was informed and freely consented in writing to participate in the study.
Fig. 2.
Study flowchart. In this study, we included 33 patients hospitalized in the acute phase of SARS-CoV-2 infection for whom a blood sample and cytokine analysis had been performed and who were assessed a neuropsychological examination at 6–9 months and 12–15 months post-infection. We excluded 88 patients from the cohort, who had not been hospitalized due to the absence of a blood sample, patients who had been hospitalized without a viable blood sample for cytokine analysis and patients who had not completed a follow-up neuropsychological examination 12–15 months post-infection. ICU intensive care unit.
COVID-COG cohort
The COVID-COG cohort is made up of 121 people eligible according to the following exclusion criteria: no neurological, psychiatric, cognitive and medical history that may affect cognition (e.g., cancer, HIV), neurodevelopmental pathologies, pregnancy, substance use, treatment affecting cognition and age over 80. A complete and validated neuropsychological test battery was administered to the participants 6–9 months and 12–15 months post-infection (see section "Measurement of episodic memory" and Voruz, et al.3) (free and informed consent of the patients was collected). The 121 people included in the COVID-COG project were divided into subgroups according to the severity of the infection in the acute phase. A first "mild" subgroup requiring no hospitalization (N = 49), a second "moderate" subgroup requiring hospitalization without mechanical ventilation (N = 48) and finally a "severe" subgroup requiring hospitalization in intensive care with mechanical ventilation (N = 24).
Participants included in the study
As described above, we extracted, from the COVID-COG cohort, the patients who benefited from a venous blood sample during the acute phase of the infection as well as a neuropsychological examination at the two measurement times (6–9 months and 12–15 months post-infection). Thus, 33 patients were included in this study, 22 from intensive care and 11 from intermediate care during the acute phase of the disease (see Fig. 2 and Table 1).
Measurement of episodic memory
Data from objective measures of episodic memory were extracted from the COVID-COG protocol. Based on the MNESIS model (Eustache et al.41), we measured two dimensions of episodic memory. We used two different episodic memory tasks to highlight verbal and visuospatial episodic memory processes. We used the following tools: free/cued recall paradigm (RLRI 16) (Grober and Buschke42) measuring verbal episodic memory (we collected free recall and total recall scores) and the Rey–Osterrieth Complex Figure test (Meyers and Meyers43) measuring visuospatial episodic memory (we collected the scores at 3-min recall and the scores at 20-min recall) (see supplementary materials S1 and S2). In clinical practice, these tests are used on a daily basis to assess different memory processes. Symptom validity was checked using the Behavior Rating Inventory of Executive Function (Abeare et al., 2021). Symptoms were validated for all patients in the cohort.
Retrospective analysis of cytokines
Cytokine measurements were conducted on the first day of hospitalization, prior to the administration of any therapies that could affect cytokine level variations. Blood samples were collected, on average, 1.26 ± 2.85 days following a positive PCR test (see Table 2). Cytokines (TNFα, IL-1β, IL-6) were measured (pg/ml) using commercially available multiplex bead immunoassays (Fluorokine MAP Multiplex Human Cytokine Panel, R&D Systems, Minneapolis, USA) and read using a Bioplex 200 array reader (Bio-Rad Laboratories, Hercules, CA, USA) and Luminex xMAP Technology (Luminex Corporation, Austin, TX, USA).
Statistical power
We set the type two β error at 0.80, the α threshold was set at 0.025 in view of our hypotheses. Finally, we estimated a correlation coefficient on the observed relationship between TNFα levels and post-COVID 19 cognitive symptoms obtained in Nuber-Champier, et al.19,44.
Thus, based on the calculation made by Sb et al.45, the necessary sample size is estimated at 21 participants per group.
Statistical analysis
Given the distribution of our cytokine data and the proximity of certain scores to zero, we performed a logarithmic (log) transformation of our data46. In addition, as the raw cognitive data were not normally distributed either, we used non-parametric tests, namely Spearman correlation tests and generalised linear mixed models (GLMM) gamma with log link or linear depending on data distribution46,47. We present the descriptive clinical and socio-demographic data of the sample in Table 1.
In relation to the hypothesis of an association between levels of cytokine secretion in the acute phase and memory performance at 6–9 months and 12–15 months post-infection, we performed Spearman correlations with a significance threshold set at 0.05. A total of four correlation matrices were produced: (i) relationship between cytokines levels in the acute phase of COVID-19 and verbal episodic memory scores measured at 6–9 months; (ii) relationship between cytokines levels in the acute phase of COVID-19 and visuospatial episodic memory scores measured at 6–9 months; (iii) relationship between cytokines levels in the acute phase of COVID-19 and verbal episodic memory scores measured at 12–15 months and (iv) relationship between cytokines levels in the acute phase of COVID-19 and visuospatial episodic memory scores measured at 12–15 months.
Concerning the hypothesis that memory performance measured at 6–9 months and 12–15 months could be predicted by cytokines levels in the acute phase of COVID-19, we performed GLMM. We performed a regression model for each verbal and visuospatial episodic memory raw score measured at 6–9 months and 12–15 months with log TNFα, log IL-6 and log IL-1β levels as predictors and gender, age and educational level as covariates.
Despite the hypothesis-driven nature of this study and the fact that we only investigated a limited number of variables (inflammatory and cognitive) related to our hypotheses, we applied a correction for the false discovery rate (FDR) in order to limit statistical error on the prediction analyzes.
Supplementary Information
Acknowledgements
The present research was supported by Swiss National Science Foundation (SNSF) funding to JAP (PI) and FA (Co-PI) (grant no. 220041).
Abbreviations
- COVID-19
Coronavirus disease 2019
- FDR
False discovery rate
- GLMM
Generalized linear mixed models
- ICU
Intensive care unit
- IL
Interleukin
- Log
Logarithmic
- PCR
Polymerase chain reaction
- RLRI
Free/cued recall paradigm
- RT-PCR
Reverse transcription polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- TNFα
Tumor necrosis factor alpha
Author contributions
A. Nuber-Champier: Contributed to the writing, analysis and examination of the patients in the study. G. Breville: Contributed to the writing and examination of the cytokines in the study. P. Voruz: Contributed to the writing and examination of the patients in the study. I. Jacot de Alcântara: Contributed to the writing and examination of the patients in the study. A. Cionca: Contributed to the proofreading. G. Allali: Contributed to the neurological expertise, writing and proofreading of the analyzes and interpretations. P.H. Lalive: Contributed to the neuro-immunological expertise, writing and proofreading of the analyzes and interpretations. L. Benzakour: Contributed to the psychiatric expertise and proofreading. K.-O. Lövblad: Contributed to the expertise in neuroimaging and proofreading. O. Braillard: Contributed to the coordination of the patients and proofreading. M. Nehme: Contributed to the coordination of the patients and proofreading. M. Coen: Contributed to the coordination of the patients and proofreading. J. Serratrice: Contributed to the coordination of the patients and proofreading. J.-L Reny: Contributed to the coordination of the patients and proofreading. J. Pugin: Contributed to the coordination of the patients and proofreading. I. Guessous: Contributed to patient coordination, statistical epidemiology and proofreading. B.N. Landis: Contributed to the coordination of the patients and proofreading. F. Assal: Contributed to the writing of the overall project, proofreading and scientific direction. J.A. Péron: Contributed to the writing of the overall project, proofreading and scientific direction.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request. In the near future, the datasets generated and/or analyzed in the course of this study are available in the repository of Yareta, [https://yareta.unige.ch/home/search?search=search%3Dcovid%2520cog].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72421-z.
References
- 1.Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol.1, 1–14 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomasson, M. et al. Markers of limbic system damage following SARS-CoV-2 infection. Brain Commun.5, fcad177 (2023). [DOI] [PMC free article] [PubMed]
- 3.Voruz, P. et al. Frequency of abnormally low neuropsychological scores in post-COVID-19 syndrome: the Geneva COVID-COG cohort. Arch. Clin. Neuropsychol. (2022). [DOI] [PMC free article] [PubMed]
- 4.Soriano, J. B., Murthy, S., Marshall, J. C., Relan, P. & Diaz, J. V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet. Infect. Dis22, e102–e107 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceban, F. et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun.101, 93–135 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taquet, M. et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry9, 815–827 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díez-Cirarda, M. et al. Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain146, 2142–2152 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartung, T. J. et al. Fatigue and cognitive impairment after COVID-19: A prospective multicentre study. EClinicalMedicine53 (2022). [DOI] [PMC free article] [PubMed]
- 9.Voruz, P. et al. Long COVID neuropsychological deficits after severe, moderate, or mild infection. Clin. Transl. Neurosci.6, 9 (2022). [Google Scholar]
- 10.Morioka, S. et al. Epidemiology of post-COVID conditions beyond 1 year: A cross-sectional study. Public Health216, 39–44 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Davis, H. E. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine38 (2021). [DOI] [PMC free article] [PubMed]
- 12.Monje, M. & Iwasaki, A. The neurobiology of long COVID. Neuron (2022). [DOI] [PMC free article] [PubMed]
- 13.Greene, C. et al. Blood–brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment. Nat. Neurosci.27, 421–432 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein, J. et al. Distinguishing features of Long COVID identified through immune profiling. Nature 1–3 (2023). [DOI] [PMC free article] [PubMed]
- 15.Nuber-Champier, A. et al. Monocytosis in the acute phase of SARS-CoV-2 infection predicts the presence of anosognosia for cognitive deficits in the chronic phase. Brain Behav. Immun. Health26, 100511 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etter, M. M. et al. Severe Neuro-COVID is associated with peripheral immune signatures, autoimmunity and neurodegeneration: A prospective cross-sectional study. Nat. Commun.13, 6777 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soung, A. L. et al. COVID-19 induces CNS cytokine expression and loss of hippocampal neurogenesis. Brain145, 4193–4201 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAfoose, J. & Baune, B. Evidence for a cytokine model of cognitive function. Neurosci. Biobehav. Rev.33, 355–366 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Nuber-Champier, A. et al. Acute TNFα levels predict cognitive impairment 6–9 months after COVID-19 infection. Psychoneuroendocrinology 106104 (2023). [DOI] [PMC free article] [PubMed]
- 20.Voruz, P. et al. Functional connectivity underlying cognitive and psychiatric symptoms in post-COVID-19 syndrome: Is anosognosia a key determinant? Brain Commun. 4, fcac057 (2022). [DOI] [PMC free article] [PubMed]
- 21.Zorzo, C., Solares, L., Mendez, M. & Mendez-Lopez, M. Hippocampal alterations after SARS-CoV-2 infection: A systematic review. Behav. Brain Res. 114662 (2023). [DOI] [PubMed]
- 22.Voruz, P. et al. Brain functional connectivity alterations associated with neuropsychological performance 6–9 months following SARS-CoV-2 infection. Hum. Brain Mapp.1, 1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toniolo, S., Di Lorenzo, F., Scarioni, M., Frederiksen, K. S. & Nobili, F. Is the frontal lobe the primary target of SARS-CoV-2?. J. Alzheimer’s Dis.81, 75–81 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Douaud, G. et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature604, 697–707 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosp, J. A. et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain144, 1263–1276 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarifkar, P., Peinkhofer, C., Benros, M. E. & Kondziella, D. Frequency of neurological diseases after COVID-19, influenza A/B and bacterial pneumonia. Front. Neurol.13, 1276 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine, K. S. et al. Virus exposure and neurodegenerative disease risk across national biobanks. Neuron1, 1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva, N. M. L., Barros-Aragão, F. G., De Felice, F. G. & Ferreira, S. T. Inflammation at the crossroads of COVID-19, cognitive deficits and depression. Neuropharmacology209, 109023 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz, M. & Cahalon, L. The vicious cycle governing the brain–immune system relationship in neurodegenerative diseases. Curr. Opin. Immunol.76, 102182 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Dursun, E. et al. The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s disease, mild cognitive impairment or Parkinson’s disease. J. Neuroimmunol.283, 50–57 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Trapero, I. & Cauli, O. Interleukin 6 and cognitive dysfunction. Metab. Brain Dis.29, 593–608 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Taquet, M. et al. Acute blood biomarker profiles predict cognitive deficits 6 and 12 months after COVID-19 hospitalization. Nat. Med.1, 1–11 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damiano, R. F. et al. Cognitive impairment in long-COVID and its association with persistent dysregulation in inflammatory markers. Front. Immunol.14, 1174020 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeidman, P. & Maguire, E. A. Anterior hippocampus: The anatomy of perception, imagination and episodic memory. Nat. Rev. Neurosci.17, 173–182 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altmann, D. M., Whettlock, E. M., Liu, S., Arachchillage, D. J. & Boyton, R. J. The immunology of long COVID. Nat. Rev. Immunol.23, 618–634 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Nottet, H. S. & Gendelman, H. E. Unraveling the neuroimmune mechanisms for the HIV-1-associated cognitive/motor complex. Immunology today16, 441–448 (1995). [DOI] [PubMed] [Google Scholar]
- 37.Maier, S. F. & Watkins, L. R. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol. Rev.105, 83 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Magaki, S., Mueller, C., Dickson, C. & Kirsch, W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp. Gerontol.42, 233–240 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frontera, J. A. et al. Trajectories of inflammatory markers and post-COVID-19 cognitive symptoms: A secondary analysis of the CONTAIN COVID-19 randomized trial. Neurol. Neuroimmunol. Neuroinflamm.11, e200227 (2024). [DOI] [PMC free article] [PubMed]
- 40.Bermejo, P. et al. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer’s disease. Immunol. Lett.117, 198–202 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Eustache, F., Viard, A. & Desgranges, B. The MNESIS model: Memory systems and processes, identity and future thinking. Neuropsychologia87, 96–109 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Grober, E. & Buschke, H. Genuine memory deficits in dementia. Dev. Neuropsychol.3, 13–36 (1987). [Google Scholar]
- 43.Meyers, J. E. & Meyers, K. R. Rey Complex Figure Test and recognition trial professional manual. (Psychological Assessment Resources, 1995).
- 44.Nuber-Champier, A. et al. Monocytosis in the acute phase of SARS-CoV-2 infection predicts the presence of anosognosia for cognitive deficits in the chronic phase. Brain Behav. Immunity Health26, 1. 10.1016/j.bbih.2022.100511 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sb, C. H., Browner, W., Grady, D., Newman, T. & Gaertner, R. Designing clinical research: An epidemiologic approach (2013).
- 46.Genser, B., Cooper, P. J., Yazdanbakhsh, M., Barreto, M. L. & Rodrigues, L. C. A guide to modern statistical analysis of immunological data. BMC Immunol.8, 1–15 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Winter, J. C., Gosling, S. D. & Potter, J. Comparing the Pearson and Spearman correlation coefficients across distributions and sample sizes: A tutorial using simulations and empirical data. Psychol. Methods21, 273 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request. In the near future, the datasets generated and/or analyzed in the course of this study are available in the repository of Yareta, [https://yareta.unige.ch/home/search?search=search%3Dcovid%2520cog].