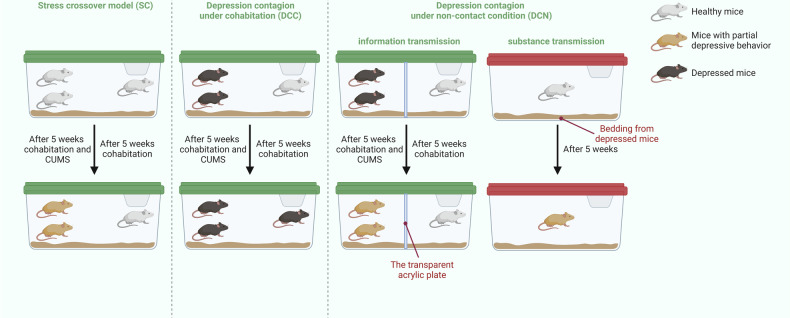
Abstract
Depression is a significant mental health issue with extensive economic implications, and recent studies suggest it may be transmitted between individuals. However, the mechanisms of this contagion remain unclear, and the social buffering effect has been understudied. This research employs three rodent models, including stress crossover, cohabitation-induced, and non-contact induced depression contagion models, to explore these mechanisms. Here, we report that that naive mice cohabiting with depressed mice showed increased corticosterone levels and depressive behaviors, unlike those with stressed mice, who did not exhibit these changes and even mitigated desperation in stressed mice. Non-contact cohabitation did not produce significant behavioral differences, but exposure to bedding from depressed mice reduced sucrose preference in naive mice. This study introduces reliable models of depression contagion, suggesting it operates independently of stress transmission. The interplay between depression contagion and social buffering may vary in different contexts. These findings provide new insights into the mechanisms of depression contagion and potential strategies for preventing depressive disorders.
Subject terms: Psychology, Physiology, Depression
Introduction
Depression is a prevalent mental disorder that imposes a significant economic burden on the society and families [1]. It consistently ranks among the primary causes of mental disability worldwide and its incidence rate has been increasing dramatically [2–5]. Although the reasons underlying the increased incidence of depression have not been clearly defined, social environment, psychological stress, economic conditions are generally believed to be involved, such as social aging and declining income [6, 7]. However, these reasons are not sufficient to explain the surge in depressive disorder. Recently, an emerging perspective holds that depression may spread among the population akin to infectious diseases [8, 9]. Evidences supporting the contagion of depression have been reported in some particular populations, including roommates [10], classmates [9] and partners [11]. Conversely, contrasting findings have also emerged in other populations [12]. The above findings suggest that the mechanisms and processes underlying depression contagion remain elusive.
The primary manifestation of depression lies in the prolonged presence of a depressive mood [13]. Since Elaine Hatfield [14] coined emotional contagion in 1993, researchers have been trying to explain the specific mechanisms of this emotional “match” process and have proposed different theories [15]. Both intraspecific and cross-species empirical studies had found that receiving social cues from the demonstrator individuals contributed to similar emotional states of observers in social contact progress [16–18]. However, current researches limited in immediate emotional states (such as anxiety and fear), little research focused on long-term mood states (like depression) [19, 20]. Thus, the mechanisms involved in depression contagion remain speculative based on transmission of immediate emotional states, incorporating visual mimicry [21], odor [16], intimate contact [22], and vocal cues [23]. Given the capacity of stress transmission to induce immediate synaptic changes [23] and long-term effects on physiological stress states [24], it is plausible to posit an contagion basis for depression as well. Whether the mechanisms and consequence of depression contagion are different from that of the former in behavioral and biologic dimension are needed to be consider. Matthew Boyko et al. [25] established an animal model of depression contagion and demonstrated cohabitation with depressed rats can leads to depressive behaviors of healthy individuals. The reliable animal model lays the foundation for further research on the underlying mechanisms of contagion depression. Furthermore, investigating the potential of healthy individuals to alleviate symptoms in those with depression is a promising avenue for further exploration.
In present study, we attempted to remold the experimental mouse models of depression contagion. To be more specific, we designed a series of experiments to seek for evidence, exploring the specific mechanisms how demonstrators elicit behavioral and physical changes in observers (Fig. 1). As mentioned above, emotional contagion involves diverse sensory pathways, we examined the factors of vision and bedding environment separately. Generally speaking, we hypothesized that depressive symptoms in individuals can cumulatively increase the likelihood of developing depressive behaviors in healthy individuals, and explored specific pathways of depression contagion for first time, which could help excavate potential mechanisms of depression contagion. Simultaneously, we have observed that depression contagion and social buffering exert distinct effects under varying circumstances, thereby holding significant implications for the prevention and treatment of depression.
Fig. 1. Schematic diagram illustrating the experimental findings.
Created in BioRender. BioRender.com/s47q887.
Material and methods
Ethics statement and animals
All experiments were conducted in accordance with experiments norms and approved by the Ethical Committee of Naval Medical University. Minimizing suffering and the numbers of animals used as much as possible was considered discreetly. Adult (200–220 g in the 6 week) male wild-type C57BL/6 mice were used in all experiments. All mice bought from Animal Center of Naval Medical University (Shanghai, China) were housed in a standard animal room (22 ± 2 °C, lights on from 7 AM to 7 PM), with food and water available ad libitum. The sample size in this study was primarily based on previous research designs (6–12 subjects per group).
Treatment
Chronic unpredictable mild stress (CUMS)
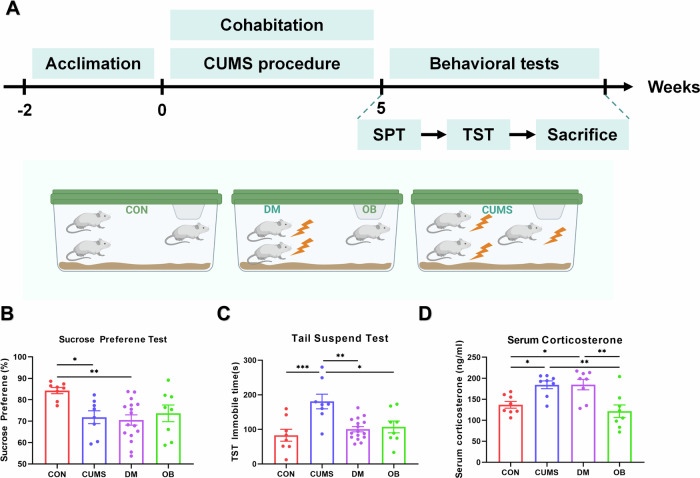
Male mice were caged individually and subjected to the chronic unpredictable mild stress (CUMS) protocol. The CUMS paradigm was adapted from our past research, involving exposure to a series of mildly intense stressors in random order. The stressors included 45° cage inclination for 12–15 h, reversal of the light–dark cycle for 24 h, restraint for 2–4 h, food or water deprivation for 24 h, 45 °C dry-heat stress for 5–15 min, bedding deprivations for 12–20 h, damp bedding for 12–18 h, cage vibration for 20–60 min, and swimming at 4 °C for 3–6 min. Mice were exposed to one of the above stressors daily in a random order. After 5-week CUMS, the paradigm was stopped. The timing of the processing schedules for CUMS and behavioral tests was shown in Fig. 2A. CUMS procedure of DCC and DCN were conducted as described above. The behavioral tests were carried out after CUMS. And the mice were sacrificed immediately after all behavioral tests.
Fig. 2. Stressed mice could not trigger stress transmission to healthy mice after cohabitation.
Four groups were cohoused or intervened for five weeks according to the experimental plan. Behavioral and corticosterone tests were conducted after the scheduled time (A). The sucrose preference percentage of CUMS and DM was significantly reduced by chronic stress (B), while the immobility time of CUMS in the tail suspension test (C) and serum CORT levels (D) of CUMS and DM was increased. However, no significant difference was observed between CON and OB mice (B–D). (Group DM represented demonstrator mice who underwent the chronic unpredictable mild stress procedure, while Group OB consisted of naive mice cohabiting with DM mice. *p < 0.05, **p < 0.01, ***p < 0.001, n(DM) = 16, n(Other) = 8) (Created in BioRender. BioRender.com/g71g470).
Stress crossover model (SC)
After two weeks of adaption to the new environment and the sucrose solution (2%, weight/volume), thirty-two mice were randomly divided into four groups: CON group, CUMS group, demonstrator (DM) group and observer (OB) group. Twenty-four mice subjected to the CUMS protocol were randomly divided into two groups: one group (DM, n = 16) cohabited with naive mice (OB, n = 8) at a ratio of 2:1, while the other group served as a positive control (CUMS, n = 8). The CON group consisted of untreated naive mice (n = 8). That is, two DM mice were cohoused with a single OB mice, and each three CON/CUMS mice cohabited in the same cage. The stress crossover model lasted for five weeks. After each day’s stress program, DM mice returned to the same cage as OB mice, cohabiting for no less than 12 hours per day.
Depression contagion under cohabitation (DCC)
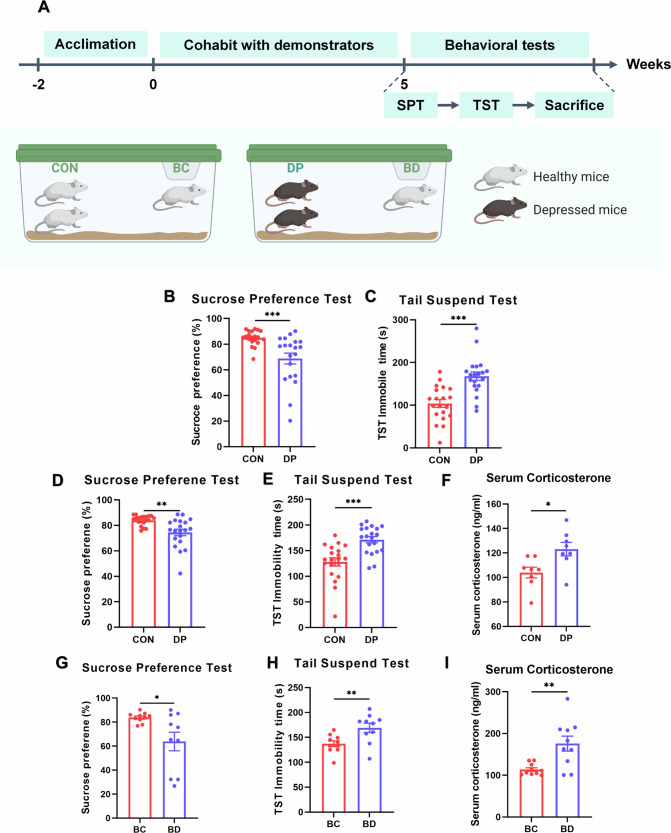
Forty C57BL/6 male mice were housed and randomly divided into two groups: DP group and CON group. After 5-week CUMS modeling, DP group performed depressive behaviors and CON group did not receive any intervention in the duration. Every two mice from DP or CON group cohabited with a single naive mouse aged eight weeks for five weeks. The naive mice were divided into two groups: Group BC represented naive mice cohabiting with CON mice, while group BD represented naive mice cohabiting with DP mice. Ten naive mice were included in group BD and group BD, respectively. To maintain depression-like behavior in depressed mice, the chronic unpredictable mild stress procedure was still performed. After each day’s stress program, DP mice returned to the same cage as the paired naive mice, cohabiting for no less than 12 hours per day.
Depression contagion under non-contact condition (DCN)
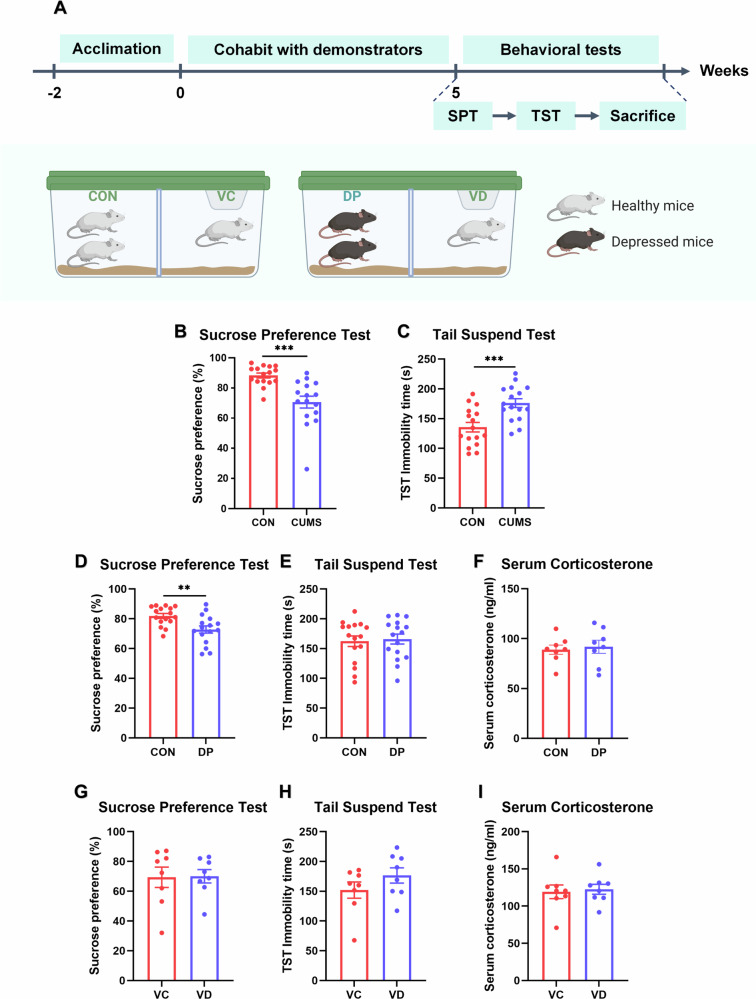
We further explored the possible pathways of depression contagion. The transmission of information and substance was taken into consideration, leading to the design of two corresponding models. The treatment protocols for the demonstrator mice were identical to those used for the DCC. DP mice were subjected to chronic unpredictable mild stress to develop and maintain depressive behaviors, while CON mice received no intervention. Both of them served as demonstrators for subsequent experiments. On the one hand, sixteen of DP mice and sixteen of CON mice cohabited with naive mice at a ratio of 2 to 1, respectively. A transparent acrylic plate was inserted in the middle of each cage to separate the demonstrators from the observers. This separation allowed for unobstructed visual and auditory information transmission. On the other hand, the beddings of remaining DP and CON mice were collected and subsequently provided to eight-week-old naive mice, with weekly replacements. Both non-contact conditions lasted for five weeks.
Behavioral tests
Sucrose preference test (SPT)
Sucrose preference test was used to evaluate the depressive-like behaviors. Before measuring, mice were presented with two bottles of 2% (weight/volume) sucrose solution for the first week. The following week, mice were presented with two bottles choice: one bottle containing 1% sucrose solution, while the other containing tap water. Positions of two bottles were exchanged every day to eliminate the learning effect. According to previous literature, mice were individually housed, deprived of food and water for 18 h, and then provided with two bottles of the same size: one captaining 2% sucrose solution and another one containing clean tap water. The bottles were placed alternately to avoid bias. Fluid consumptions were evaluated after 1 h test. Sucrose preference was calculated as the percentage of sucrose solution consumption out of total liquid consumption.
Tail suspension test (TST)
Tail suspension test is widely applied to evaluate the despair behavior in rodent model. Each mouse was suspended upside down by the end of its tail on the hook of a standard apparatus, PHM-300 tail suspension chamber (MED Associates Inc, St. Albans, VT). A 1.5 cm plastic tube was attached to the tail of the mouse to avoid mice climbing. After a 1-min period of adaption to the apparatus, the immobility time during the last 5-min suspension was recorded and analyzed using the software (Tail Suspension SOF-821, Med Associates Inc.) with a threshold of 0.5. 75% ethanol was used to clean smell of inner walls and diminish influence between each trial.
Plasma collection and analysis
After the behavioral tests, all animals were immediately euthanized under general anesthesia with isoflurane to collect blood. All the procedures took place at night. Blood samples were coagulated for 30 min at room temperature before centrifuged at 4000 rpm, 4 °C for 20 min. Then the supernatant serum was collected and kept at −80 °C. Mice serum sample were removed from −80 °C and melted at room temperature. CORT levels in serum were measured with mouse Corticosterone ELISA kit (Elabscience, Shanghai, China) according to the manufacturer’s instructions.
Statistical analysis
The data were presented as MEAN ± SEM and analyzed with GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Significant differences among groups in SC were mainly assessed by one-way ANOVA followed by the Tukey test for post-hoc comparisons. Independent sample t-test was applied in DCC and DCN. Differences considered statistically significant at p < 0.05.
Results
A series of experiments was conducted to investigate the conditions and underlying mechanisms of depression contagion. The subsequent section provides all details of results and Fig. 1 showed a comprehensive overview of the findings from this study.
Stressed mice could not trigger stress transmission to healthy mice after cohabitation
Stress crossover model procedure was illustrated in Fig. 2A. Group DM represented demonstrator mice who underwent the chronic unpredictable mild stress procedure, while Group OB consisted of observer mice cohabiting with DM mice. Both CUMS and DM mice were subjected to a five-week period of chronic unpredictable mild stress. Following each stress session, when the CUMS mice were housed individually, the DM mice were reintroduced into cages with matched OB mice.
After a five-week period, the sucrose preference scores of DM and CUMS mice were found to be significantly lower compared to those of CON mice (F(3,36) = 4.556, p = 0.0083; Tukey’s test: CON vs. CUMS, p = 0.0383; CON vs. DM, p = 0.0053; CON vs. OB, p = 0.0966; Fig. 2B). In the tail suspend test, it was observed that immobile times were higher in CUMS mice when compared to other groups (F(3,36) = 7.747, p = 0.0004; Tukey’s test: CON vs. CUMS, p = 0.0006; CUMS vs. DM, p = 0.0011; CUMS vs. OB, p = 0.0115; CON vs. OB, p = 0.7075; Fig. 2C). Furthermore, both serum corticosterone levels in CUMS and DM mice were found to be elevated as compared to the other two groups (F(3,28) = 8.054, p = 0.0005; Tukey’s test: CON vs. CUMS, p = 0.0318; CON vs. DM, p = 0.0300; CUMS vs. OB, p = 0.0032; DM vs. OB, p = 0.0030; CON vs. OB, p = 0.7861; Fig. 2D). No significant differences were observed between CON and OB mice across all tests conducted.
Depressed mice contribute to depressive behaviors in naive mice after cohabitation
Although stress was not transmitted between mice experiencing CUMS and cohabiting naive mice, it remained uncertain whether CUMS-induced depression-like behaviors could be transferred between mice. To explore this question, we set up DCC model including two demonstrator groups and two observer groups. The demonstrator group consisted of DP mice that underwent a 5-week CUMS procedure to exhibit depressive behaviors, while CON mice did not receive any intervention. The observer groups were respectively housed with them. Group BC represented naive mice cohabiting with CON mice, while group BD represented naive mice cohabiting with DP mice. The experimental procedure is illustrated in Fig. 3A. After five weeks of chronic stress exposure, the sucrose preference scores of DP mice were significantly lower than those of CON mice (t(38) = 3.637, p = 0.0008, Fig. 3B), and the immobile times in the tail suspension test for DP mice were significantly higher than those for CON mice (t(38) = 4.725, p < 0.0001, Fig. 3C).
Fig. 3. Depressed mice contribute to depressive behaviors in naive mice after cohabitation.
Two groups of naive mice were cohoused with two groups of demonstrators for five weeks, respectively. Behavioral and corticosterone tests were conducted after the scheduled time (A). After 5-week CUMS, the sucrose preference percentage of DP was lower than that of CON (B), and the immobility time in the tail suspension test of DP was also higher (C). Following a 5-week cohabitation, depressive behaviors persisted and stress levels increased in the DP mice (D–F). Naive mice cohabiting with DP mice showed similar behavioral and physiological changes (G–I). (Group BC represented naive mice cohabiting with CON mice, while group BD represented naive mice cohabiting with DP mice. *p < 0.05, **p < 0.01, ***p < 0.001, n(DP&CON) = 20, n(BC&BD) = 10) (Created in BioRender. BioRender.com/k05d903).
Following a five-week period of cohabitation, the sucrose preference scores of DP mice were still significantly lower than those of CON mice (t(38) = 3.530, p = 0.0011, Fig. 3D), and the immobile times in the tail suspension test for DP mice were significantly higher than those for CON mice (t(38) = 4.320, p = 0.0001, Fig. 3E). The sucrose preference scores of BD mice were found to be significantly lower compared to BC mice (t(18) = 2.533, p = 0.0208, Fig. 3G). Additionally, the TST immobile times of BD mice were observed to be higher than those of BC mice (t(18) = 2.907, p = 0.0094, Fig. 3H). Furthermore, serum corticosterone levels in DP mice were higher than those in CON mice (t(14) = 2.734, p = 0.0162, Fig. 3F), and similar difference in serum corticosterone level occurred between BD and BC mice (t(18) = 3.388, p = 0.0033, Fig. 3I).
The transplantation of the bedding from depressed mice mediated depression contagion without stress contagion
The aforementioned findings demonstrated the potential transmission of depression from depressed mice to naive observers. However, the complexity of social interactions during cohabitation introduced unpredictable factors, making it challenging to establish a clear link between these confounding variables and underlying mechanisms. In order to investigate the possible sensory pathways involved in depression contagion, we considered narrowing the sensory range and devised two sub-experiments of DCN model to examine the impact of non-contact information cues and bedding substance cues on depression contagion.
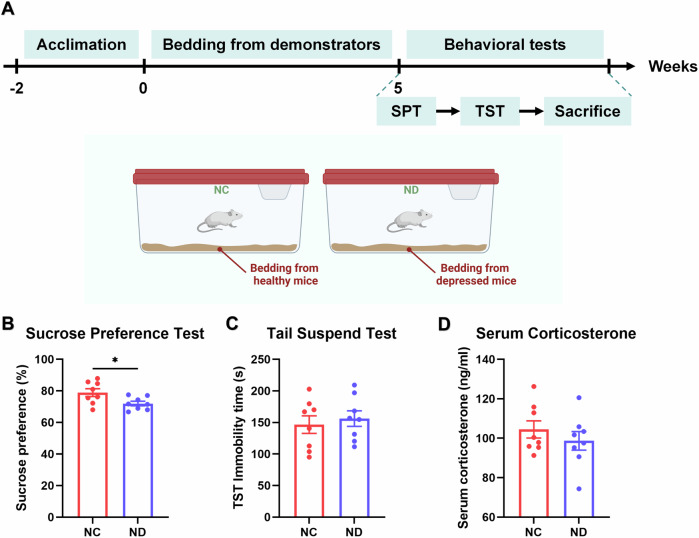
In the first sub-experiment, a transparent acrylic plate was inserted in the middle of each cage to separate the demonstrators from the observers based on the model of depression contagion under cohabitation. This separation allowed for unobstructed visual and auditory information transmission. The experimental procedure was presented in Fig. 4A. Group VC represented naive mice lived in the same cages with healthy mice (CON), while Group VD represents naive mice cohoused with depressed mice (DP). In another sub-experiment, bedding derived from matched demonstrators was used for naive mice, as shown as Fig. 5A. Group NC consisted of naive mice housed in bedding from healthy mice, while Group ND comprised those housed in bedding from depressed mice. Following a five-week chronic unpredictable mild stress procedure, DP mice exhibited depressive behaviors (SPT: t(30) = 4.184, p = 0.0002, Fig. 4B; TST: t(30) = 3.720, p = 0.0008, Fig. 4C). Subsequently, these DP mice were utilized as demonstrators alongside CON mice in the following experiments.
Fig. 4. Non-contact cohabitation failed to induce depression contagion between depressed and naïve mice.
Two groups of naive mice were cohoused with two groups of demonstrators for five weeks, with a transparent acrylic board separating them. Behavioral and corticosterone tests were conducted after the scheduled time (A). Before cohabitation, the sucrose preference percentage of DP decreased significantly (B), while TST immobility time increased (C). However, no significant difference was observed between CON and DP mice in TST immobility time and serum corticosterone after 5-week cohabitation (E, F). Sucrose preference percentage remained lower in DP than in CON (D). Moreover, no difference was found between VD and VC in behavior tests and serum corticosterone level (G–I). (Group VC represented naive mice lived in the same cages with healthy mice, while Group VD represents naive mice cohoused with depressed mice. *p < 0.05, **p < 0.01, ***p < 0.001, n(DP&CON) = 16, n(VD&VC) = 8) (Created in BioRender. BioRender.com/p75t345).
Fig. 5. The transplantation of the bedding mediated depression contagion without stress transmission.
The two groups of naive mice received bedding from depressed mice and healthy mice, respectively. Behavioral and corticosterone tests were conducted after the scheduled time (A). After a duration of 5 weeks, the sucrose preference percentage in ND was found to be lower compared to that in NC (B), but no differences were found in the tail suspension test (C) and serum corticosterone levels (D). (Group NC consisted of naive mice housed in bedding from healthy mice, while Group ND comprised those housed in bedding from depressed mice. *p < 0.05, **p < 0.01, ***p < 0.001, n = 8) (Created in BioRender. BioRender.com/a24n235).
After a duration of five weeks, there were no significant differences observed between the VC and VD groups in all conducted tests (SPT: t(14) = 0.0764, p = 0.9401, Fig. 4G; TST: t(14) = 0.7153, p = 0.4836, Fig. 4H; serum corticosterone: t(14) = 0.2909, p = 0.7754, Fig. 4I). However, both desperation behavior and serum corticosterone level of DP mice decreased (SPT: t(30) = 3.124, p = 0.0039, Fig. 4D; TST: t(30) = 0.2885, p = 0.7749, Fig. 4E; serum corticosterone: t(14) = 0.3704, p = 0.7166, Fig. 4F). Moreover, although there were no significant differences in TST immobile times and serum corticosterone levels between ND and NC groups (TST: t(14) = 0.5179, p = 0.6126, Fig. 5C; serum corticosterone: t(14) = 0.9061, p = 0.3802, Fig. 5D), the former exhibited lower SPT scores compared to the latter group (t(14) = 2.4120, p = 0.0302, Fig. 5B).
Discussion
In this study, we demonstrated that healthy mice exhibited depressive-like behaviors only when cohabiting with depressed mice, rather than stress-induced mice undergoing CUMS procedure. Interestingly, transplantation of bedding environment induced depressive behaviors in naive mice without elevating stress levels. Conversely, despite previous reports on facial expression recognition and emotional features (e.g., intensity, valence, and persistence) in mice, the non-contact cohabitation which allowed for visual and auditory information transmission did not exhibit depression contagion during the same period [26]. This is the first exploration of potential pathways for depression contagion which differs from stress contagion according to the results of behavioral and serum corticosterone tests.
The results of SC revealed that the transmission of CUMS-induced stress state and depressive behavior from stressed mice to healthy cohabitants was not observed, which contradicts previous studies [27]. This discrepancy may be attributed to variations in rodent species selection, experimental protocols, particularly the stress procedures applied. Previous investigations on stress contagion primarily relied on exposure to short-term and intense stressors (such as repeated multiple inescapable plantar electrical stimulation) to induce a stressed state in demonstrators. These studies predominantly focused on investigating stress rather than depression [24]. The utilization of traditional chronic unpredictable mild stress was deemed more effective in replicating the real-life environment associated with depression onset. Additionally, it should be noted that in our study, the mice housed together were unfamiliar to each other, as familiarity could potentially interfere the impact of emotional contagion [28]. Meanwhile, DCC revealed that both the stress-induced status and depressive behaviors induced by CUMS could be transmitted from depressed mice to naive mice, indicating that depression-like behaviors in demonstrators are essential for depression contagion. However, it should be noted that the increased serum corticosterone levels in naive mice fail to provide conclusive evidence for CUMS-induced stress contagion when cohabitation, as depressed cohabitants can be considered a novel stressor for naive mice.
The findings of DCN suggest that the bedding environment, rather than the visual or auditory pathway, played a more prominent role in facilitating depression contagion. Interestingly, it was observed that inducing depression contagion through bedding failed to result in an elevation of stress levels. The current evidence suggests that substance transmission serves as the foundation for depression contagion. Due to limitations in manipulability, present researches has been unable to fully examine the impact of direct physical contact on depression contagion. The previous studies related to stress contagion considered intimate contact was more likely necessary for stress contagion and subsequent development of depressive behaviors. However, the daily close proximity between mice inevitably entails the exchange of material information. The exchange of material information primarily involves the oronasal sensory pathway, and this process may be facilitated by sniffing specific informative odors or ingesting feces from depressed mice. Evidence showed the gut microbiota composition of depressed patients was different from that of healthy controls, which was transplanted into rodents to induce depressive-like behaviors [29]. Future investigations should focus on monitoring changes in cage odors and gut microbiota among depression infected mice. Existing research suggested that emotional contagion primarily occurred through the transmission of social information, with pheromones serving as key carriers of such information within species. Various odors, from predator scents to those produced by stressed individuals, could induce stress responses in odor receivers, triggering anxiety or fear [30]. The neuroendocrine mechanisms primarily engage two hypothalamic peptides (Corticotropin releasing hormone and oxytocin, CRH and OT) [31] and several empathy-related brain regions (Insular Cortex, Nucleus Accumbens, Ventral Tegmental Area, and Amygdala) [32]. A critical brain area linking olfactory information to emotional processing is the amygdalo-piriform transition area, where chemogenetic activation of CRH neurons stimulates an increase in stress hormones [33]. In contrast to existing studies, our findings indicate that under a rearing environment with bedding from depressed mice, the levels of cortical corticosterone (a stable hormone reflecting stress levels in the CUMS model) remain unchanged, yet depressive-like behaviors still emerge. This suggests that under conditions lacking social interaction, depression contagion operates independently of stress contagion. Given the temporal relationship between stress and depression (where early stress can evolve into later depression), conducting multi-time point assessments will more effectively elucidate the similarities and connections between stress and depression contagion. Our model can also be utilized to investigate the neurobiological processes of depression in the absence of stress influences.
Moreover, healthy cohabitants may serve as a protective factor for stressed mice. In the model of SC, following a five-week CUMS procedure, CUMS mice developed depressive behaviors; however, DM mice only displayed a decrease in sugar preference scores without an increase in desperation behavior. This finding suggests that cohabitation with a healthy individual may mitigate the impact of stress on an individual’s emotional state. Consolation behavior has been recognized as a bidirectional mechanism linking stress contagion and social buffering [31, 34]. Meanwhile, subsequent experiments showed that non-contact cohabitation also alleviated depressive-like behavior among DP mice. However, due to the lack of positive control group, the possibility of spontaneous regression of depressive behaviors cannot be completely excluded, although the intensity of CUMS was not reduced during cohabitation. These findings suggest that depression contagion necessitates material exchange, whereas social buffering may be achieved solely through perceived social support. As a pair of confrontation forces, the relative dominance between depression contagion and social buffering varies across different circumstances. The utilization of the adaptive transition under different conditions offers a novel avenue for the prevention and treatment of depression.
Current research primarily focuses on the contagion of stress and immediate emotional states (e.g., anxiety and fear). Correspondingly, widely employed models like the witness stress model and stress crossover model are utilized to investigate intricate mechanisms. The witness stress model enables observers to experience vicarious stress induced by witnessing others experiencing physical stress events (e.g., foot shock, defeat, restraint, etc.) in adjacent compartments, resulting in similar behavioral phenotypes (e.g., freezing) to those demonstrators experiencing direct stress [24]. Stress crossover model refers to observer perform stress response and significant stressful behaviors after demonstrator experienced stress and returned to the cage [35]. In contrast, depression contagion models are in their infancy and lack of empirical research. Numerous studies are required to validate the efficacy of current model and explore its underlying mechanisms. Depression contagion, the long-term emotional contagion, encounters the challenge of intricate interfering factors. By alleviating these confounding variables, this study elucidated and refined the depression contagion pathway, offering a novel direction and practical foundation for investigating the molecular mechanism underlying depression contagion.
Several limitations persisted in the present study. Firstly, the duration of contagion may not be optimal, as it was determined based on the previous study protocol and coincided with that of CUMS procedure [25]. Considering the consistent durations of contagion and CUMS, as well as the possibility that the effect of contagion might be weaker than experiencing chronic stress, it is plausible that the five-week period may not suffice to induce depression contagion; thus, absolutely ruling out the non-contact pathway’s involvement in depression contagion was to be discussed. In future experiments, we can design varying duration intervals and observe the mice’s performance at different time points to construct a dose-effect curve. Additionally, in DCN, only differences in SPT scores were detected among the mice, which may be attributed to an insufficient duration of contagion. Furthermore, not all experiments included a positive control group (only in SC) to investigate changes in stressed or depressed demonstrator mice before and after cohabitation. It could provide strict supplementary evidences regarding social buffering. Considering the impact of female hormone fluctuations on emotional states, existing studies on emotional contagion have primarily focused on male subjects. A study highlighted the influence of gender differences and indicated that male and female rodents exhibit variations in specific emotional expression behaviors and neural circuit activation [36]. Due to the lack of evidence regarding both the phenotypes and mechanisms of depression contagion, this study still selected males as subjects to standardize the model. Future research should address susceptibility differences in depression contagion related to gender and explore the underlying neurophysiological mechanisms.
In summary, our research has successfully developed the specific model of depression contagion, marking a novel advancement in understanding the mechanisms underlying this phenomenon. Additionally, we have distinguished between the effects of depression contagion and stress contagion, demonstrating that depression can be transmitted independently from stress. These findings contribute to the current knowledge on depression contagion and hold promise for developing innovative therapeutic strategies for preventing and treating contagious depression. Furthermore, by documenting the existence of contagion effect in mice, we provide valuable insights and extend the potential direction of pathway related to emotional contagion.
Conclusion
Collectively, this study presents novel insights into the mechanisms underlying depression contagion. Mere cohabitation with mice exposed to CUMS did not result in the transmission of depressive symptoms; instead, both depressed mice and bedding from them were capable of inducing depressive behavior in naïve individuals, while bedding alone could induce depression without elevating stress levels. Additionally, depression contagion and social buffering effects may act as a pair of opposing forces, with their relative dominance varying across different circumstances. These findings deepen our understanding of depression contagion and offer innovative study strategies for contagious depression.
Acknowledgements
We want to express our gratitude for the drawing materials provided by BioRender. Figures 1–5 were created in BioRender. Huang (2024) (BioRender.com/s47q887, BioRender.com/g71g470, BioRender.com/k05d903, BioRender.com/p75t345, BioRender.com/a24n235). This work was supported by grants from National Natural Science Foundation of China (32170931 and 31900827) and Natural Science Foundation of Shandong Province (ZR202212020310).
Author contributions
CLJ and YZL designed all experiments and organized all results, including the writing of the manuscript. CWH and TH planned and performed all experiments and participated in the writing of the manuscript. HZ and YLW performed biochemical analysis. JML, YMW, WJS and WW analyzed and interpreted the data. All authors read and approved the final manuscript.
Data availability
All the data during the current study have been shown in manuscript, and unprocessed data are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests. Drawing materials from BioRender are available with permission for publication.
Ethical approval
This study was approved by the Ethical Committee of Naval Medical University (Shanghai, China). Ethical approval (20230310) was acquired for the present original studies and all methods were performed in accordance with the relevant guidelines and regulations.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chen-Wei Huang, Ting Hu, Hong Zheng.
Contributor Information
Yun-Zi Liu, Email: kekeyiran@126.com.
Chun-Lei Jiang, Email: cljiang@vip.163.com.
References
- 1.Mehdi SMA, Costa AP, Svob C, Pan L, Dartora WJ, Talati A, et al. Depression and cognition are associated with lipid dysregulation in both a multigenerational study of depression and the National Health and Nutrition Examination Survey. Transl Psychiatry. 2024;14:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Anim Models Neuropsychiatr Dis. 2016;321:138–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu J, Xu X, Huang Y, Li T, Ma C, Xu G, et al. Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2021;8:981–90. [DOI] [PubMed] [Google Scholar]
- 5.Dubovsky SL, Ghosh BM, Serotte JC, Cranwell V. Psychotic depression: diagnosis, differential diagnosis, and treatment. Psychother Psychosom. 2021;90:160–77. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Yang H, Zheng P, Liu B, Guo Z, Geng S, et al. Life negative events and depressive symptoms: the China longitudinal ageing social survey. BMC Public Health. 2020;20:968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lokman JC, Bockting CL. Pathways to depressive and anxiety disorders during and after the COVID-19 pandemic. Lancet Psychiatry. 2022;9:531–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dishion TJ, Tipsord JM. Peer contagion in child and adolescent social and emotional development. Annu Rev Psychol. 2011;62:189–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L, Zhang S, Bian B, Zhou M, Bi Z. Peer effects of depression between left-behind and non-left-behind children: quasi-experimental evidence from rural China. Child Adolesc Psychiatry Ment Health. 2023;17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinn DM, Canevello A, Crocker JK. Understanding the role of depressive symptoms in academic outcomes: a longitudinal study of college roommates. PLoS ONE. 2023;18:e0286709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristensen TB, Pfeffer J, Dahl MS, Holm M, Feldhues ML. Does depression co-occur within households? The moderating effects of financial resources and job insecurity on psychological contagion. SSM Popul Health. 2022;19:101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horesh D, Hasson-Ohayon I, Harwood-Gross A. The contagion of psychopathology across different psychiatric disorders: a comparative theoretical analysis. Brain Sci. 2022;12. 10.3390/brainsci12010067. [DOI] [PMC free article] [PubMed]
- 13.Li J-M, Liu L-L, Su W-J, Wang B, Zhang T, Zhang Y, et al. Ketamine may exert antidepressant effects via suppressing NLRP3 inflammasome to upregulate AMPA receptors. Neuropharmacology. 2019;146:149–53. [DOI] [PubMed] [Google Scholar]
- 14.Hatfield E, Cacioppo JT, Rapson RL. Emotional contagion. Curr Dir Psychol Sci. 1993;2:96–100. [Google Scholar]
- 15.Heyes C. Empathy is not in our genes. Neurosci Biobehav Rev. 2018;95:499–507. [DOI] [PubMed] [Google Scholar]
- 16.Brandl HB, Pruessner JC, Farine DR. The social transmission of stress in animal collectives. Proc R Soc B Biol Sci. 2022;289:20212158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundman A-S, Van Poucke E, Svensson Holm A-C, Faresjö Å, Theodorsson E, Jensen P, et al. Long-term stress levels are synchronized in dogs and their owners. Sci Rep. 2019;9:7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adriaense JEC, Koski SE, Huber L, Lamm C. Challenges in the comparative study of empathy and related phenomena in animals. Neurosci Biobehav Rev. 2020;112:62–82. [DOI] [PubMed] [Google Scholar]
- 19.Kim S-W, Kim M, Shin H-S. Affective empathy and prosocial behavior in rodents. Curr Opin Neurobiol. 2021;68:181–9. [DOI] [PubMed] [Google Scholar]
- 20.Warren BL, Vialou VF, Iñiguez SD, Alcantara LF, Wright KN, Feng J, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paz LV, Viola TW, Milanesi BB, Sulzbach JH, Mestriner RG, Wieck A, et al. Contagious depression: automatic mimicry and the mirror neuron system—a review. Neurosci Biobehav Rev. 2022;134:104509. [DOI] [PubMed] [Google Scholar]
- 22.Qu Y, Zhang L, An S, Tai F, Qiao H. Chronic stress and stressful emotional contagion affect the empathy-like behavior of rats. Cogn Affect Behav Neurosci. 2023;23:1160–74. [DOI] [PubMed] [Google Scholar]
- 23.Sterley T-L, Baimoukhametova D, Füzesi T, Zurek AA, Daviu N, Rasiah NP, et al. Social transmission and buffering of synaptic changes after stress. Nat Neurosci. 2018;21:393–403. [DOI] [PubMed] [Google Scholar]
- 24.Warren BL, Mazei-Robison MS, Robison AJ, Iñiguez SD. Can I get a witness? Using vicarious defeat stress to study mood-related illnesses in traditionally understudied populations. Biol Psychiatry. 2020;88:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyko M, Kutz R, Grinshpun J, Zvenigorodsky V, Gruenbaum SE, Gruenbaum BF, et al. Establishment of an animal model of depression contagion. Behav Brain Res. 2015;281:358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolensek N, Gehrlach DA, Klein AS, Gogolla N. Facial expressions of emotion states and their neuronal correlates in mice. Science. 2020;368:89–94. [DOI] [PubMed] [Google Scholar]
- 27.Wang, Bin Wang H, Pan M, Jiang S, Wang Y, Zhu Y, et al. Disorders in the gut and liver are involved in depression contagion between isosexual post-stroke depression mice and the healthy cohabitors. Behav Brain Res. 2023;439:114246. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Liencres C, Juckel G, Tas C, Friebe A, Brüne M. Emotional contagion in mice: the role of familiarity. Behav Brain Res. 2014;263:16–21. [DOI] [PubMed] [Google Scholar]
- 29.Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–18. [DOI] [PubMed] [Google Scholar]
- 30.Silveira LM, Tavares LRR, Baptista-de-Souza D, Carmona IM, Carneiro de Oliveira PE, Nunes-de-Souza RL, et al. Anterior cingulate cortex, but not amygdala, modulates the anxiogenesis induced by living with conspecifics subjected to chronic restraint stress in male mice. Front Behav Neurosci. 2023;16. 10.3389/fnbeh.2022.1077368. [DOI] [PMC free article] [PubMed]
- 31.Peen NF, Duque-Wilckens N, Trainor BC. Convergent neuroendocrine mechanisms of social buffering and stress contagion. Horm Behav. 2021;129:104933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baptista-de-Souza D, Rodrigues Tavares LR, Canto-de-Souza L, Nunes-de-Souza RL, Canto-de-Souza A. Behavioral, hormonal, and neural alterations induced by social contagion for pain in mice. Neuropharmacology. 2022;203:108878. [DOI] [PubMed] [Google Scholar]
- 33.Kondoh K, Lu Z, Ye X, Olson DP, Lowell BB, Buck LB. A specific area of olfactory cortex involved in stress hormone responses to predator odours. Nature. 2016;532:103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun EK, Donovan M, Liu Y, Wang Z. Behavioral, neurochemical, and neuroimmune changes associated with social buffering and stress contagion. Neurobiol Stress. 2022;16:100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carnevali L, Montano N, Tobaldini E, Thayer JF, Sgoifo A. The contagion of social defeat stress: insights from rodent studies. Neurosci Biobehav Rev. 2020;111:12–18. [DOI] [PubMed] [Google Scholar]
- 36.Fang S, Luo Z, Wei Z, Qin Y, Zheng J, Zhang H, et al. Sexually dimorphic control of affective state processing and empathic behaviors. Neuron. 2024;112:1498–517.e8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data during the current study have been shown in manuscript, and unprocessed data are available from the corresponding author on reasonable request.