Summary
Malignant hyperthermia susceptibility (MHS) designates individuals at risk of developing a hypermetabolic reaction triggered by halogenated anaesthetics or the depolarising neuromuscular blocking agent suxamethonium. Over the past few decades, beyond the operating theatre, myopathic manifestations impacting daily life are increasingly recognised as a prevalent phenomenon in MHS patients. At the request of the European Malignant Hyperthermia Group, we reviewed the literature and gathered the opinion of experts to define MHS-related myopathy as a distinct phenotype expressed across the adult lifespan of MHS patients unrelated to anaesthetic exposure; this serves to raise awareness about non-anaesthetic manifestations, potential therapies, and management of MHS-related myopathy. We focused on the clinical presentation, biochemical and histopathological findings, and the impact on patient well-being. The spectrum of symptoms of MHS-related myopathy encompasses muscle cramps, stiffness, myalgias, rhabdomyolysis, and weakness, with a wide age range of onset mainly during adulthood. Histopathological analysis can reveal nonspecific abnormalities suggestive of RYR1 involvement, while metabolic profiling reflects altered energy metabolism in MHS muscle. Myopathic manifestations can significantly impact patient quality of life and lead to functional limitations and socio-economic burden. While currently available therapies can provide symptomatic relief, there is a need for further research into targeted treatments addressing the underlying pathophysiology. Counselling early after establishing the MHS diagnosis, followed by multidisciplinary management involving various medical specialties, is crucial to optimise patient care.
Keywords: genetic testing, malignant hyperthermia susceptibility, muscle cramps, myopathy, rhabdomyolysis, RYR1
Editor's key points.
-
•
Malignant hyperthermia susceptible (MHS) patients are at risk of myopathic manifestations that significantly impact daily life.
-
•
The spectrum of symptoms of MHS-related myopathy unrelated to anaesthetic exposure encompasses muscle cramps, stiffness, myalgias, rhabdomyolysis, and weakness, with a wide age range of onset.
-
•
Evidence suggests RYR1 involvement, with altered energy metabolism in MHS muscle.
-
•
Although currently available therapies can provide symptomatic relief, there are a number of promising targeted therapies in the pipeline.
-
•
Newly diagnosed MHS patients should be counselled to monitor the appearance and progress of myopathic symptoms, with counselling and multidisciplinary management established early after the MHS diagnosis.
Background and definitions
The diagnosis of malignant hyperthermia susceptibility (MHS) designates patients at risk of developing a hypermetabolic reaction, triggered upon exposure to halogenated anaesthetics or a depolarizing neuromuscular blocking agent, known as a malignant hyperthermia event or crisis (MH).1 MHS is diagnosed by phenotypic characterisation of surgically excised skeletal muscle fascicles and their contracture response to halothane and caffeine in vitro. MHS predisposition can also be diagnosed by the presence of one or more pathogenic or likely pathogenic variants in the RYR1, CACNA1S, or STAC3 genes. Pathogenic or likely pathogenic variants predisposing to MH can be found in patients with a personal or family history of suspected MH under anaesthesia, but increasingly also as incidental findings on genetic investigation of other conditions.2 Here, we use the term MHS to encompass both patients characterised phenotypically as predisposed to MH (by contracture testing) and those with genetically determined increased MH risk.
MHS-related myopathy
A myopathy is a clinical disorder with symptoms caused by a primary structural or functional abnormality of skeletal muscle. The United States National Institute of Neurological Disorders and Stroke considers muscle weakness as the primary symptom of myopathy.3 Nevertheless, for MHS-related myopathy, we adopted a broader definition including symptoms such as muscle cramps, stiffness, myalgias, episodic rhabdomyolysis (e.g. exertional, virally induced, spontaneous, or without an identifiable trigger), and weakness, plausibly thought to result from the same skeletal muscle defect that predisposes an individual to develop an MH event (Fig. 1). Widespread adoption of contracture testing led to the common use of MHS to describe all patients whose muscle showed increased in vitro sensitivity to halothane or caffeine. However, because ‘abnormal’ in vitro contracture responses can also be found in patients with a range of other neuromuscular disorders,4,5 we excluded from our definition patients with ‘positive’ contracture test and primarily myopathic manifestations suggestive of another neuromuscular disorder but with no personal or family history suggestive of an MH event. Our clinical definition overlaps with that of RYR1-related myopathies (e.g. MHS patients with myopathic symptoms who harbour an RYR1 variant). However, not all RYR1-related myopathies are associated with MHS, not all RYR1 variants predispose to MH, and not all MHS patients harbour RYR1 variants.6, 7, 8
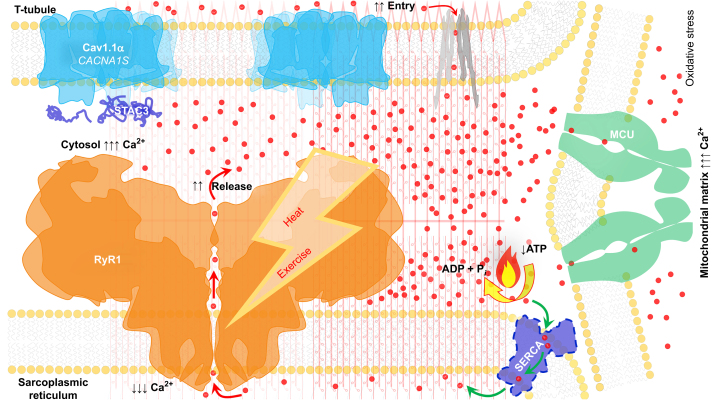
Fig 1.
Sustained RyR1 calcium leak is the mechanism underlying MHS-related myopathic manifestations exacerbated by pharmacological or environmental stimuli (e.g. heat and exercise). Downstream effects of chronic myoplasmic calcium elevation include increased mitochondrial electron transport chain activity, increased oxidative stress, and RyR1 posttranslational modifications that further impairs calcium homoeostasis. Cav1.1, voltage-dependent L-type calcium channel alpha 1S subunit encoded by the CACNA1S gene; MCU, mitochondrial calcium uniporter; MHS, malignant hyperthermia susceptibility; RyR1, skeletal muscle calcium release channel ryanodine receptor type 1; SERCA, sarcoendoplasmic reticulum calcium ATPase; red dots, calcium ions.
Our definition excludes asymptomatic individuals with incidental histopathological findings associated with MHS (e.g. cores, minicores, oxidative staining unevenness, and fibre type grouping). Although they could represent a continuum, patients with delayed diagnosis of congenital myopathies associated with MH that might not be recognised until late childhood or adult life are also beyond our scope.9 For instance, myopathic manifestations might be elicited during functional inquiry, or muscle weakness revealed by physical examination, in patients with no previous history of myopathy referred for MHS investigations following an anaesthetic reaction, who believed until then that their physical capabilities were within the normal function spectrum.10 A 10-year retrospective review at a major neuromuscular clinic in North America identified 44 patients with congenital myopathy diagnosed during adulthood, 16 of whom harboured pathogenic or likely pathogenic RYR1 variants.11 Thus, we define MHS-related myopathy as a distinct clinical entity thus far unspecified predominantly manifesting during adult life unrelated to anaesthetic exposure in patients with a primary diagnosis of MHS, in contrast to patients with primarily myopathic manifestations and structural abnormalities distinctive of other congenital and early-childhood onset myopathies associated with MH.
Clinical presentation of MHS-related myopathy
Onset
Age of onset of musculoskeletal symptoms in MHS individuals has a wide range.12 Among 12 patients harbouring RYR1 variants pathogenic or likely pathogenic for MHS who presented with recurrent rhabdomyolysis, myalgia with hyperCKaemia, or both, the median age of onset was 27 yr.13 However, in a 25-yr retrospective study involving 164 MHS patients with myopathic manifestations who received oral dantrolene for symptom relief, the median age at the start of therapy was 60 yr.14 In a series of 11 adult patients from eight unrelated families presenting between 20 and 70 yr old with axial (lumbar hyperlordosis, camptocormia, and scapula alata) or proximal muscle weakness associated with RYR1 variants, Løseth and colleagues6 identified a 40-yr-old patient with no history of exposure to anaesthetics who carried the RYR1 variant p.Val4849Ile pathogenic for MHS, corroborating previous observations of adult-onset paraspinal muscle weakness in MHS patients.15 We favour the adjective ‘adult-onset’ rather than ‘late-onset’ when referring to MHS-related myopathic manifestations.16
Clinical manifestations
Symptoms and signs of MHS-related myopathy can appear spontaneously or be triggered by identifiable stimuli (Table 1). Myalgia and muscle cramps are the most common episodic symptoms appearing at a younger age, worsening in intensity and frequency of acute exacerbations or becoming continuous later in life.13 Unlike general fatigue, characterised by a feeling of overall tiredness, muscle fatigue manifests as a decreased ability to perform over time leading to a state of exhaustion. MHS patients describe muscle weakness affecting all activities or appearing after iterative or continuous effort despite having seemingly preserved muscle strength at the start of exercise.12,14
Table 1.
Clinical manifestations of MHS-related myopathy. MHS-related myopathic manifestations can be sporadic or continuous with acute exacerbations. MHS, malignant hyperthermia susceptibility.
| Inclusion criteria |
1. MHS diagnosis confirmed by the following:
|
| Exclusion criteria |
|
MH is the hallmark syndrome with skeletal muscle manifestations triggered by specific general anaesthetic drugs.17 However, MHS individuals can also have increased risk of developing rhabdomyolysis with or without myoglobinuria, induced by exercise, heat, or both; more rarely, MHS patients may experience episodes of rhabdomyolysis with no apparent exposure to a known precipitant of rhabdomyolysis.13 A single case has been reported of a patient harbouring a likely pathogenic RYR1 variant for MHS (p.Pro4973Leu) presenting with recurrent compartment syndrome.18 A prospective cross-sectional study showed a higher proportion of patients with RYR1-related exertional rhabdomyolysis and MHS patients suffering from cramps and myalgias at rest or induced by exercise compared with healthy controls.13 The Medical Research Council Sum Score for evaluating muscle weakness,19 spirometry, and other functional measurements were normal in most patients. Three of 40 patients (7.5%) had onset of muscle weakness when they were aged in their 50s, most prominently in the proximal lower extremities, in line with previous descriptions of axial myopathy as a late manifestation of MHS.6,15
Although the association between MHS and exertional rhabdomyolysis is established, few published cases have a personal or family history suggestive of an MH event. In a report by Dlamini and colleagues,20 only one among 14 families had confirmed MH, two families harboured a RYR1 variant pathogenic for MHS (p.Gly2434Arg), and one family carried a variant likely pathogenic for MHS (p.Arg4737Gln). Eight families had RYR1 variants now categorised as benign for MHS, and three families harboured variants of uncertain significance. Viral illness, exercise in hot environments, or exercise with alcohol intake were identified as potential triggers in several individuals. Kraeva and colleagues21 reported 16 additional patients investigated for rhabdomyolysis, of which only four had a family history suggestive of MH. The report included a child presenting with rhabdomyolysis triggered by a viral infection. Moreover, a retrospective case review performed at five MH referral centres identified 41 patients with MHS confirmed by genetics or contracture testing, with a history of intense exercise or pyrexia preceding an MH reaction triggered by anaesthesia, suggestive of a clinical continuum encompassing MHS, exertional rhabdomyolysis, and heat illness.22 In another report, a survivor of a confirmed MH episode died years later from exertional heat illness.23
Although some groups have reported a high incidence of abnormal contracture test results in survivors of exertional heat illness, which also demonstrates an association with RYR1 and CACNA1S variants,24, 25, 26, 27, 28 it is difficult to estimate the risk of MHS individuals to develop exertional heat illness accurately. MHS individuals might avoid activities that pose a risk for exertional heat illness, although some suggest they compensate for increased heat production through adaptive increases in heat dissipation.29
Other symptoms in MHS patients include muscle cramps, stiffness, and myalgias that can appear spontaneously or be induced by exercise. MHS individuals can report muscle cramps or myalgia following statin medication, but there are insufficient data to assert whether these symptoms are more common with MHS compared with the general population.30,31 We did not find any reports of statin-induced rhabdomyolysis in patients with a personal or family history of MH.
On physical examination, although most MHS patients have normal strength at a younger age, some develop muscle weakness later in life.12 Muscle hypertrophy is a common finding in patients with a history of exertional rhabdomyolysis or MHS who harbour RYR1 variants, many of whom report increased muscle tone and frequent cramps. These individuals are often perceived to be physically fitter than their peers.13,20,32
Recent observations in patients and MH-susceptible animal models suggest that individuals with MHS have increased risk of developing hyperglycaemia and diabetes mellitus,33 pancreatitis,34 and bleeding abnormalities.35 Although some studies suggest plausible molecular mechanisms,36,37 more preclinical and epidemiological evidence is needed to ascertain MHS association with such disorders.
Biochemical and histopathological findings
MHS-related myopathy has no pathognomonic laboratory findings. In a cross-sectional study, 48% of patients with RYR1-related rhabdomyolysis or MHS-related myopathic symptoms had hyperCKaemia, although 81% of their muscle biopsies showed nonspecific histopathological abnormalities.12 Untargeted metabolomic profiling of skeletal muscle from 68 MHS patients and 25 non-MHS controls revealed significant accumulation of acylcarnitines, diacylglycerols, various phospholipids, phosphoenolpyruvate, histidine pathway metabolites, and oxidative stress markers, and decreased levels of monoacylglycerols, indicating a shift from carbohydrate to lipid utilisation for energy production and faster protein turnover in the MHS group.38 Metabolic impairments in MHS skeletal muscle present an attractive target for discovering diagnostic biomarkers and for developing personalised therapies to alleviate myopathic MHS manifestations.
A multicentre review of muscle biopsies from 50 European patients harbouring RYR1 variants and diagnosed with MHS (31 patients) or exertional rhabdomyolysis (19 patients) suggests MHS patients share a histopathological spectrum ranging from mild myopathic changes to cores and other features of RYR1-related congenital myopathies.39 Including some cases from a previous cohort,12 their analysis revealed histopathological features of myopathy in 90% of patients, such as increased fibre size variability, increased number of fibres with internal nuclei, and type I fibre predominance. Central cores, multi-minicores, and oxidative staining unevenness were observed in 52% of biopsies. In another study involving 46 Japanese patients referred because of personal or family history of MH, cores were seen on oxidative staining in 13 of 27 (48.1%) biopsies from MHS patients who harboured RYR1 variants.40 Hence, myopathic histopathology findings in MHS patients can suggest RYR1 involvement.
Life impact of MHS-related myopathy
MHS-related myopathic manifestations have implications across the lifespan of affected patients. An online survey involving 72 participants with RYR1-related disorders, 33 with congenital myopathy, and 39 with MHS or exertional rhabdomyolysis revealed that their functional impairments were worse compared with healthy controls, with half of them reporting severe fatigue.41 Although fatigue, pain, and physical impairments were more pronounced in patients with congenital myopathies, MHS patients with rhabdomyolysis scored higher in all relevant categories compared with healthy controls and reported that their impairments had been absent during childhood and developed only later in life. Thus, relatively mild clinical features of MHS-related myopathy are associated with fatigue and functional limitations constituting a persistent burden that adversely affects quality of life. Patients with MHS-related myopathy may face socioeconomic consequences owing to a lack of awareness of their limitations at their workplace, prompting recurrent sick leave or early retirement from physical jobs and sports.42 Therefore, MHS individuals should be counselled about the possibility of developing myopathic symptoms that might warrant follow-up after the initial diagnosis.
After MH triggered by anaesthesia, exertional heat illness and rhabdomyolysis triggered by exercise or viral infection are the main episodic acute events associated with MHS,43, 44, 45 and myalgia, cramps, muscle fatigue, and muscle weakness are the most common chronic complaints.10,14 Hot environments and strenuous physical activities involving heavy gear that impair heat exchange (e.g. ice hockey, skiing, and firefighting) appear to favour muscle injury in MHS patients, compromising performance in sports and physical professions.25,46 Despite controversy about the accuracy of in vitro contracture testing to assess the MH risk after heatstroke, it is pertinent to undertake workup for MHS and other myopathies in patients with recurrent or severe exertional heat illness.43 In a review of 166 patients referred for MHS risk assessment between 1980 and 2002 owing to exertional heat illness during military service or competitive sports, 53% had positive contracture test results.47 Accordingly, reports from MH diagnostic centres across continents indicate that half of patients referred after heatstroke or exertional rhabdomyolysis had positive contracture test results, and a third had RYR1 variants pathogenic for MHS.27,28,48,49 Compared with healthy subjects, MHS patients have increased serum free fatty acids, cortisol, lactate,50 and myoglobin,29 decreased aerobic and anaerobic capacity, and compromised oxidative ATP production after standardised physical activity.51 Hence, MHS patients are advised to avoid strenuous exercise in hot environments or when sick. Otherwise, they should implement adequate hydration, heat acclimatisation, an individualised exercise schedule, and a treatment plan for heat- or exercise-related emergencies.43 Out of concern, some countries consider MHS a disqualifying condition for military service.52,53
Therapeutic interventions and prevention
There is currently no established therapy for MHS-related myopathy. Hereunder, we discuss different drugs repurposed to alleviate myopathic symptoms in MHS patients, followed by ongoing research on candidate treatments.
Dantrolene
Dantrolene is the only specific treatment available for MH during anaesthesia. Dantrolene interferes with excitation–contraction coupling, antagonizing calcium release from the sarcoplasmic reticulum in cardiac and skeletal muscle.54 The spectrum of MHS-related myopathic manifestations is reasonably presumed to originate from chronic RYR1 dysfunction. Clinical observations suggest that oral dantrolene relieves myopathic symptoms in MHS patients by restoring myoplasmic calcium homoeostasis.55 According to a recent database review of 164 MHS patients who received oral dantrolene 25–400 mg day−1 for myopathic symptom relief, most patients adhered to therapy and reported improvement of myalgia, fatigue, rhabdomyolysis, and hyperCKaemia, especially those with personal or family history of MH.14 Three patients developed liver enzymes three to four times above the upper normal limit, which normalised after discontinuing dantrolene, but no serious adverse events were reported. Although the study provided valuable dosing and side-effect data, efficacy of oral dantrolene for symptom relief in MHS-related myopathy awaits assessment in a randomised controlled clinical trial.
Adrenergic agents
Salbutamol, a β2-adrenergic agonist used as a bronchodilator, is known to increase muscle strength.56 Salbutamol increases expression of the sarcoplasmic reticulum calcium ATPase SERCA1 (fast-twitch isoform), which can ameliorate chronic myoplasmic calcium elevation in MHS muscle and its oxidative stress consequences downstream. Functional improvements and enhanced muscle strength have been observed in patients with RYR1-related congenital myopathies after receiving daily salbutamol alone or in combination with a regimen of aerobic exercise.57,58 In contrast, carvedilol, a β-adrenergic blocker suppressing RYR2-mediated store overload-induced calcium release in cardiomyocytes, shows promising similar effects in non-muscle HEK293 cells expressing MH/CCD-associated RYR1 variants.59 Repurposing approved adrenergic agents for symptom relief in adult MHS patients presenting with muscle weakness is worthy of investigation.
N-Acetylcysteine
N-Acetylcysteine (NAC) is the rate-limiting precursor of the antioxidant glutathione. Chronic myoplasmic calcium elevation in MHS results in increased mitochondrial calcium uptake, increased electron transport chain activity, increased reactive by-products, persistent oxidative stress, and posttranslational modifications of RYR1 that further impair calcium homoeostasis.51,60,61 NAC reduced oxidative stress and improved survival of myotubes derived from RYR1-related myopathy patients, restored muscle function in relatively relaxed zebrafish,62 and rescued lipid peroxidation, improved skeletal muscle function, and decreased the number of core structures in heterozygous RYR1 Y522S mice.63,64 In a randomised double-blinded placebo-controlled trial in 33 patients with RYR1-related myopathy, a 6-month course of oral NAC neither decreased oxidative markers nor improved physical endurance on a 6-min walk test.65 It was suggested that larger doses might be required to produce a clinical benefit.
Novel therapies and future directions
Exercise interval training
Sustained RYR1 calcium leak is the mechanism underlying MHS-related myopathic manifestations.66 Although prolonged large increases in myoplasmic calcium are associated with deleterious changes, recent findings suggest that modest increases in resting myoplasmic calcium after exercise trigger mitochondrial biogenesis and improve respiratory function, leading to increased fatigue resistance. In mice, muscle adaptations induced by exercise involve upregulation of SERCA1, downregulation of STIM1, and stabilisation of RYR1–calstabin1 binding, resulting in optimised calcium homoeostasis.67 In recreationally active individuals, short bouts of vigorous exercise cause acute transient sarcoplasmic reticulum calcium leak due to RYR1 fragmentation by reactive oxygen species. RYR1 post-translational changes were associated with improved mitochondrial function.68 In healthy human muscle and in vitro models, a single session of sprint interval training triggers RYR1 protein oxidation and nitrosylation, calstabin-1 dissociation, decreased sarcoplasmic reticulum calcium content, increased mitochondrial oxidative phosphorylation proteins, super-complex formation, and enhanced NADH-linked mitochondrial respiratory capacity.69 Countering calcium leak or preventing mitochondrial calcium uptake with a 1,4-benzothiazepine derivative known as a Rycal blunts sprint interval training adaptations. Putting those findings together, in contrast to sustained myoplasmic calcium leakage observed in RYR1-related myopathies, myocyte adaptations to acute transient RYR1 calcium leak after high-intensity interval exercise in healthy subjects appear beneficial. High-intensity interval exercise stands as a time-efficient intervention seemingly adequate for the less fit to counteract the deleterious effects of physical inactivity. Whether a tighter control in calcium homoeostasis after exercise interval training is also beneficial for MHS-related myopathy patients appeals for further research.
Sodium 4-phenylbutyrate
Sodium 4-phenylbutyrate (4PBA) is a low-molecular-weight fatty acid prodrug converted to phenylbutyryl-CoA and metabolised by mitochondrial beta-oxidation to phenylacetate, a nitrogen scavenger excreted in urine after glutamine conjugation, providing a disposal pathway alternative to the urea cycle to treat hyperammonaemia.70 As a chemical chaperone, 4PBA aids trafficking and stabilisation of misfolded proteins, reducing endoplasmic reticulum and oxidative stress. Although it failed to fully restore sarcoplasmic reticulum Ca2+ homoeostasis, myopathic ryr1-I4895T knock-in mice improved their skeletal muscle function after 4PBA treatment, likely by reducing endoplasmic reticulum stress unfolded protein response, decreasing mitochondrial reactive oxygen species and muscle protein ubiquitination.71
Rycals
Rycals are benzothiazepine-derived compounds that stabilise RYRs' closed state in skeletal and cardiac muscle by restoring calstabin binding, compromised by excessive oxidative stress present in several myopathies.72,73 In August 2018, the Food and Drug Administration awarded Rycals Orphan Drug Designation for RYR1-related myopathies. S107, a RYR-stabilising Rycal molecule, decreased the excessive calcium leak and protease activity ex vivo when applied to muscle from patients with RYR1-related myopathy, providing the rationale for a clinical trial.74 No serious adverse events were reported among seven patients with RYR1-related myopathies who received daily doses of Rycal S48168 (ARM210) for 29 days in a phase 1 dose-escalation trial. Moreover, three of four participants who received the higher dose exhibited objective improvements in muscle strength and fatigue at the end of the trial.75
Compound 1: 6,7-(methylenedioxy)-1-octyl-4-quinolone-3-carboxylic acid
Compound 1 (Cpd1) is a novel oxolinic acid derivative that selectively inhibits RYR1, preventing MH and heat stroke in several MH mouse models (ryr1-R2509C, R163C, and G2435R). It has better water solubility and faster clearance than dantrolene. Cpd1 rescued animals from environmental heat illness and MH triggered by anaesthesia by reducing resting intracellular Ca2+, inhibiting Ca2+ release induced by halogenated anaesthetics, suppressing caffeine-induced skeletal muscle contracture, and reducing sarcolemmal cation influx.76
5-Aminoimidazole-4-carboxamide ribonucleoside
5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) is an analogue of adenosine monophosphate (AMP) that activates AMP-dependent protein kinase, a cellular energy sensor that protects cells against environmental heat stress by switching off biosynthetic pathways.77 AICAR decreased Ca2+ leak and reactive oxygen and nitrogen species generation in ryr1-Y524S mice. Death after heat exposure was prevented by AICAR treatment, suggesting its potential use for MHS patients with exercise or heat intolerance.78 However, the optimal route of administration and dose of AICAR that proved effective in rodents (600 mg kg−1 s.c.) would be impractical in humans.
High-throughput drug screening
High-throughput pipelines for large-scale drug screens combining various biological models (Caenorhabditis elegans worms,79 relatively relaxed zebrafish mutants,80 time-lapse Ca2+ fluorescence in HEK293 cells,81 patient-derived myotubes, and ryr1 knock-in mice82,83) were recently introduced to weigh the potential efficacy of candidate drugs to treat RYR1-related disorders. Agents with therapeutic potential identified so far include inhibitors of p38 mitogen-activated protein kinases, oxolinic acid, 9-aminoacridine, alexidine, and dantrolene.
Gene therapy
Adenovirus-mediated gene therapy is not currently feasible for RYR1-related myopathies because RYR1 exceeds the packaging capacity of recombinant adenovirus vectors, limiting the delivery of a full-length therapeutic protein.84 In vitro gene-directed therapy consisting of pseudo-exon skipping forced by a lentiviral vector restored normal sarcoplasmic reticulum calcium release in primary myoblasts obtained from a child with severe central core disease caused by two recessive RYR1 variants, resulting in inclusion of an in-frame pseudo-exon, leading to instability and minimal expression of the RYR1 protein.85
Conclusions
MHS-related myopathy is a spectrum of skeletal muscle manifestations highly prevalent among MHS patients in the absence of anaesthetics, including episodic muscle cramps, stiffness, myalgias, rhabdomyolysis, and, less frequently, muscle weakness, which can become persistent with acute exacerbations. Currently, only supportive treatments are available for MHS patients experiencing myopathic symptoms. Newly diagnosed MHS patients should be counselled to monitor the appearance and progress of myopathic symptoms. We propose that prevalence of myopathic symptoms and the infrequent occurrence of axial myopathy later in life should be part of this counselling. Limited evidence suggests that diabetes mellitus and bleeding abnormalities are also prevalent among MHS patients. Similar to patients suffering from other myopathies, MHS patients would benefit from management tailored by a multidisciplinary team involving primary care providers, neuromuscular specialists, geneticists, and physical, psychological, and occupational therapists. The potential benefits of non-pharmacological interventions such as sprint interval exercise or off-label therapy with oral dantrolene merit further consideration for randomised clinical trials. Promising emerging investigational drugs await toxicity studies and phase 1 safety and dosage trials.
Authors’ contributions
Conception: SR, HS, NV, PH, the European Malignant Hyperthermia Group
Writing of the first draft: CI, HS, NV, JR, AC, PH, SR
Revision: CI, HS, NV, LvB, HJ, JR, AC, PH, SR
Reviewed and approved the manuscript and the revision in its final form before submission: all authors.
Acknowledgements
We thank the European Malignant Hyperthermia Group, especially Klaus Glahn and Erik-Jan Kamsteeg, for their critical review and recommendations.
Declarations of interest
CI, HS, LvB, AC, PH, and SR are members of the European Malignant Hyperthermia Group (EMHG). PH is the president and member of EMHG executive committee and editor-in-chief of BJA Open. SR is the chair of advisory council of Malignant Hyperthermia Association of the United States (MHAUS). NV is a member of the European Reference Network for rare neuromuscular diseases (ERN-NMD).
Handling Editor: Hugh C Hemmings
References
- 1.Hopkins P.M. What is malignant hyperthermia susceptibility? Br J Anaesth. 2023;131:5–8. doi: 10.1016/j.bja.2023.04.014. [DOI] [PubMed] [Google Scholar]
- 2.European Malignant Hyperthermia (MH) Group Diagnostic MH mutations. https://www.emhg.org/diagnostic-mutations Available from:
- 3.National Institute of Neurological Disorders and Stroke Myopathies—NINDS catalog. https://catalog.ninds.nih.gov/health-topics/myopathy Available from.
- 4.Carpenter D., Robinson R.L., Quinnell R.J., et al. Genetic variation in RYR1 and malignant hyperthermia phenotypes. Br J Anaesth. 2009;103:538–548. doi: 10.1093/bja/aep204. [DOI] [PubMed] [Google Scholar]
- 5.Timmins M.A., Rosenberg H., Larach M.G., Sterling C., Kraeva N., Riazi S. Malignant hyperthermia testing in probands without adverse anesthetic reaction. Anesthesiology. 2015;123:548–556. doi: 10.1097/ALN.0000000000000732. [DOI] [PubMed] [Google Scholar]
- 6.Løseth S., Voermans N.C., Torbergsen T., et al. A novel late-onset axial myopathy associated with mutations in the skeletal muscle ryanodine receptor (RYR1) gene. J Neurol. 2013;260:1504–1510. doi: 10.1007/s00415-012-6817-7. [DOI] [PubMed] [Google Scholar]
- 7.Remiche G., Kadhim H., Abramowicz M., Mavroudakis N., Monnier N., Lunardi J. A novel large deletion in the RYR1 gene in a Belgian family with late-onset and recessive core myopathy. Neuromuscul Disord. 2015;25:397–402. doi: 10.1016/j.nmd.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Laughlin R.S., Niu Z., Wieben E., Milone M. RYR1 causing distal myopathy. Mol Genet Genomic Med. 2017;5:800–804. doi: 10.1002/mgg3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litman R.S., Griggs S.M., Dowling J.J., Riazi S. Malignant hyperthermia susceptibility and related diseases. Anesthesiology. 2018;128:159–167. doi: 10.1097/ALN.0000000000001877. [DOI] [PubMed] [Google Scholar]
- 10.Snoeck M., van Engelen B.G., Küsters B., et al. RYR1-related myopathies: a wide spectrum of phenotypes throughout life. Eur J Neurol. 2015;22:1094–1112. doi: 10.1111/ene.12713. [DOI] [PubMed] [Google Scholar]
- 11.Nicolau S., Liewluck T., Tracy J.A., Laughlin R.S., Milone M. Congenital myopathies in the adult neuromuscular clinic: diagnostic challenges and pitfalls. Neurol Genet. 2019;5:e341. doi: 10.1212/NXG.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Bersselaar L.R., Jungbluth H., Kruijt N., et al. Neuromuscular symptoms in patients with RYR1-related malignant hyperthermia and rhabdomyolysis. Brain Commun. 2022;4 doi: 10.1093/braincomms/fcac292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witting N., Laforêt P., Voermans N.C., et al. Phenotype and genotype of muscle ryanodine receptor rhabdomyolysis–myalgia syndrome. Acta Neurol Scand. 2018;137:452–461. doi: 10.1111/ane.12885. [DOI] [PubMed] [Google Scholar]
- 14.Ibarra Moreno C.A., Kraeva N., Zvaritch E., Jungbluth H., Voermans N.C., Riazi S. Oral dantrolene for myopathic symptoms in malignant hyperthermia-susceptible patients: a 25-year retrospective cohort study of adverse effects and tolerability. Anesth Analg. 2023;136:569–577. doi: 10.1213/ANE.0000000000006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guis S., Figarella-Branger D., Monnier N., et al. Multiminicore disease in a family susceptible to malignant hyperthermia: histology, in vitro contracture tests, and genetic characterization. Arch Neurol. 2004;61:106–113. doi: 10.1001/archneur.61.1.106. [DOI] [PubMed] [Google Scholar]
- 16.de Visser M. Late-onset myopathies: clinical features and diagnosis. Acta Myol. 2020;39:235–244. doi: 10.36185/2532-1900-027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins P.M. Malignant hyperthermia: pharmacology of triggering. Br J Anaesth. 2011;107:48–56. doi: 10.1093/bja/aer132. [DOI] [PubMed] [Google Scholar]
- 18.Famili D.T., Fernandez-Garcia M.A., Vanegas M., et al. Recurrent atraumatic compartment syndrome as a manifestation of genetic neuromuscular disease. Neuromuscul Disord. 2023;33:866–872. doi: 10.1016/j.nmd.2023.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Kleyweg R.P., van der Meché F.G., Schmitz P.I. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain–Barré syndrome. Muscle Nerve. 1991;14:1103–1109. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 20.Dlamini N., Voermans N.C., Lillis S., et al. Mutations in RYR1 are a common cause of exertional myalgia and rhabdomyolysis. Neuromuscul Disord. 2013;23:540–548. doi: 10.1016/j.nmd.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Kraeva N., Sapa A., Dowling J.J., Riazi S. Malignant hyperthermia susceptibility in patients with exertional rhabdomyolysis: a retrospective cohort study and updated systematic review. Can J Anaesth. 2017;64:736–743. doi: 10.1007/s12630-017-0865-5. [DOI] [PubMed] [Google Scholar]
- 22.Riazi S., Bersselaar L.R.V.D., Islander G., et al. Pre-operative exercise and pyrexia as modifying factors in malignant hyperthermia (MH) Neuromuscul Disord. 2022;32:628–634. doi: 10.1016/j.nmd.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Tobin J.R., Jason D.R., Challa V.R., Nelson T.E., Sambuughin N. Malignant hyperthermia and apparent heat stroke. JAMA. 2001;286:168–169. doi: 10.1001/jama.286.2.168. [DOI] [PubMed] [Google Scholar]
- 24.Gardner L., Miller D.M., Daly C., et al. Investigating the genetic susceptibility to exertional heat illness. J Med Genet. 2020;57:531–541. doi: 10.1136/jmedgenet-2019-106461. [DOI] [PubMed] [Google Scholar]
- 25.van den Bersselaar L.R., Kruijt N., Bongers C.C.W.G., et al. Comment on “Overlapping Mechanisms of Exertional Heat Stroke and Malignant Hyperthermia: Evidence vs. Conjecture”. Sports Med. 2022;52:669–672. doi: 10.1007/s40279-021-01569-9. [DOI] [PubMed] [Google Scholar]
- 26.Poussel M., Guerci P., Kaminsky P., et al. Exertional heat stroke and susceptibility to malignant hyperthermia in an athlete: evidence for a link? J Athl Train. 2015;50:1212–1214. doi: 10.4085/1062-6050-50.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roux-Buisson N., Monnier N., Sagui E., et al. Identification of variants of the ryanodine receptor type 1 in patients with exertional heat stroke and positive response to the malignant hyperthermia in vitro contracture test. Br J Anaesth. 2016;116:566–568. doi: 10.1093/bja/aew047. [DOI] [PubMed] [Google Scholar]
- 28.Sagui E., Montigon C., Abriat A., et al. Is there a link between exertional heat stroke and susceptibility to malignant hyperthermia? PLoS One. 2015;10 doi: 10.1371/journal.pone.0135496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.House C.M., Tipton M.J., Hopkins P.M., Roiz de Sa D. Thermoregulation and markers of muscle breakdown in malignant hyperthermia susceptible volunteers during an acute heat tolerance test. J Sci Med Sport. 2019;22:586–590. doi: 10.1016/j.jsams.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Haseeb M., Thompson P.D. The effect of statins on RyR and RyR-associated disease. J Appl Physiol (1985) 2021;131:661–671. doi: 10.1152/japplphysiol.01003.2020. [DOI] [PubMed] [Google Scholar]
- 31.Hedenmalm K., Granberg A.G., Dahl M.L. Statin-induced muscle toxicity and susceptibility to malignant hyperthermia and other muscle diseases: a population-based case-control study including 1st and 2nd degree relatives. Eur J Clin Pharmacol. 2015;71:117–124. doi: 10.1007/s00228-014-1776-9. [DOI] [PubMed] [Google Scholar]
- 32.van den Bersselaar L.R., van Alfen N., Kruijt N., et al. Muscle ultrasound abnormalities in individuals with RYR1-related malignant hyperthermia susceptibility. J Neuromuscul Dis. 2023;10:541–554. doi: 10.3233/JND-230018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altamirano F., Riazi S., Ibarra Moreno C.A., et al. Is malignant hyperthermia associated with hyperglycaemia? Br J Anaesth. 2019;122:e3–e5. doi: 10.1016/j.bja.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Famili D.T., Mistry A., Gerasimenko O., et al. Pancreatitis in RYR1-related disorders. Neuromuscul Disord. 2023;33:769–775. doi: 10.1016/j.nmd.2023.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Lopez R.J., Byrne S., Vukcevic M., et al. An RYR1 mutation associated with malignant hyperthermia is also associated with bleeding abnormalities. Sci Signal. 2016;9:ra68. doi: 10.1126/scisignal.aad9813. [DOI] [PubMed] [Google Scholar]
- 36.Tammineni E.R., Kraeva N., Figueroa L., et al. Intracellular calcium leak lowers glucose storage in human muscle, promoting hyperglycemia and diabetes. Elife. 2020;9 doi: 10.7554/eLife.53999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tammineni E.R., Figueroa L., Manno C., et al. Muscle calcium stress cleaves junctophilin1, unleashing a gene regulatory program predicted to correct glucose dysregulation. Elife. 2023;12 doi: 10.7554/eLife.78874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bojko B., Vasiljevic T., Boyaci E., et al. Untargeted metabolomics profiling of skeletal muscle samples from malignant hyperthermia susceptible patients. Can J Anaesth. 2021;68:761–772. doi: 10.1007/s12630-020-01895-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knuiman G.J., Küsters B., Eshuis L., et al. The histopathological spectrum of malignant hyperthermia and rhabdomyolysis due to RYR1 mutations. J Neurol. 2019;266:876–887. doi: 10.1007/s00415-019-09209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ibarra M.C.A., Wu S., Murayama K., et al. Malignant hyperthermia in Japan: mutation screening of the entire ryanodine receptor type 1 gene coding region by direct sequencing. Anesthesiology. 2006;104:1146–1154. doi: 10.1097/00000542-200606000-00008. [DOI] [PubMed] [Google Scholar]
- 41.van Ruitenbeek E., Custers J.A.E., Verhaak C., et al. Functional impairments, fatigue and quality of life in RYR1-related myopathies: a questionnaire study. Neuromuscul Disord. 2019;29:30–38. doi: 10.1016/j.nmd.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 42.The RYR1 Foundation About Cody Hodgson. https://ryr1.org/patient-stories/codys-story Available from.
- 43.Hosokawa Y., Casa D.J., Rosenberg H., et al. Round table on malignant hyperthermia in physically active populations: meeting proceedings. J Athl Train. 2017;52:377–383. doi: 10.4085/1062-6050-52.2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capacchione J.F., Sambuughin N., Bina S., Mulligan L.P., Lawson T.D., Muldoon S.M. Exertional rhabdomyolysis and malignant hyperthermia in a patient with ryanodine receptor type 1 gene, L-type calcium channel α-1 subunit gene, and calsequestrin-1 gene polymorphisms. Anesthesiology. 2010;112:239–244. doi: 10.1097/ALN.0b013e3181c29504. [DOI] [PubMed] [Google Scholar]
- 45.Zvaritch E., Gillies R., Kraeva N., Richer M., Jungbluth H., Riazi S. Fatal awake malignant hyperthermia episodes in a family with malignant hyperthermia susceptibility: a case series. Can J Anaesth. 2019;66:540–545. doi: 10.1007/s12630-019-01320-z. [DOI] [PubMed] [Google Scholar]
- 46.Potts L.E., Longwell J.J., Bedocs P., et al. Improving awareness of nonanesthesia-related malignant hyperthermia presentations: a tale of two brothers. A A Case Rep. 2014;3:23–26. doi: 10.1213/XAA.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigues G., da Silva H.C.A. Elaboração De Manual De Orientações Sobre Hipertermia Maligna Para Pacientes. Rev Neurocienc. 2021;29:1–59. [Google Scholar]
- 48.van den Bersselaar L.R., Hellblom A., Gashi M., et al. Referral indications for malignant hyperthermia susceptibility diagnostics in patients without adverse anesthetic events in the era of next-generation sequencing. Anesthesiology. 2022;136:940–953. doi: 10.1097/ALN.0000000000004199. [DOI] [PubMed] [Google Scholar]
- 49.Wappler F., Fiege M., Steinfath M., et al. Evidence for susceptibility to malignant hyperthermia in patients with exercise-induced rhabdomyolysis. Anesthesiology. 2001;94:95–100. doi: 10.1097/00000542-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Campbell I.T., Ellis F.R., Evans R.T., Mortimer M.G. Studies of body temperatures, blood lactate, cortisol and free fatty acid levels during exercise in human subjects susceptible to malignant hyperpyrexia. Acta Anaesthesiol Scand. 1983;27:349–355. doi: 10.1111/j.1399-6576.1983.tb01966.x. [DOI] [PubMed] [Google Scholar]
- 51.Thompson S.J., Riazi S., Kraeva N., et al. Skeletal muscle metabolic dysfunction in patients with malignant hyperthermia susceptibility. Anesth Analg. 2017;125:434–441. doi: 10.1213/ANE.0000000000002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacquot C., Stieglitz P. Conduite à tenir devant un sujet susceptible de présenter une hyperthermie maligne en cas d'anesthésie et dans la vie quotidienne. Ann Fr Anesth Reanim. 1989;8:417–426. doi: 10.1016/s0750-7658(89)80008-5. [DOI] [PubMed] [Google Scholar]
- 53.Lee M.A., McGlinch E.B., McGlinch M.C., Capacchione J.F. Malignant hyperthermia susceptibility and fitness for duty. Mil Med. 2017;182:e1854–e1857. doi: 10.7205/MILMED-D-16-00186. [DOI] [PubMed] [Google Scholar]
- 54.Meyler M.J., Wesseling H., Agoston S. The effects of dantrolene sodium on cardiac and skeletal muscle in rats. Eur J Pharmacol. 1976;39:127–131. doi: 10.1016/0014-2999(76)90120-5. [DOI] [PubMed] [Google Scholar]
- 55.Jungbluth H., Dowling J.J., Ferreiro A., Muntoni F. 182nd ENMC International Workshop: RYR1-related myopathies, 15–17th April 2011, Naarden, The Netherlands. Neuromuscul Disord. 2012;22:453–462. doi: 10.1016/j.nmd.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Hostrup M., Kalsen A., Onslev J., et al. Mechanisms underlying enhancements in muscle force and power output during maximal cycle ergometer exercise induced by chronic β2-adrenergic stimulation in men. J Appl Physiol (1985) 2015;119:475–486. doi: 10.1152/japplphysiol.00319.2015. [DOI] [PubMed] [Google Scholar]
- 57.Messina S., Hartley L., Main M., et al. Pilot trial of salbutamol in central core and multi-minicore diseases. Neuropediatrics. 2004;35:262–266. doi: 10.1055/s-2004-821173. [DOI] [PubMed] [Google Scholar]
- 58.Schreuder L.T., Nijhuis-van der Sanden M.W., de Hair A., et al. Successful use of albuterol in a patient with central core disease and mitochondrial dysfunction. J Inherit Metab Dis. 2010;33(Suppl. 3):S205–S209. doi: 10.1007/s10545-010-9085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W., Koop A., Liu Y., et al. Reduced threshold for store overload-induced Ca2+ release is a common defect of RyR1 mutations associated with malignant hyperthermia and central core disease. Biochem J. 2017;474:2749–2761. doi: 10.1042/BCJ20170282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aracena P., Tang W., Hamilton S.L., Hidalgo C. Effects of S-glutathionylation and S-nitrosylation on calmodulin binding to triads and FKBP12 binding to type 1 calcium release channels. Antioxid Redox Signal. 2005;7:870–881. doi: 10.1089/ars.2005.7.870. [DOI] [PubMed] [Google Scholar]
- 61.Zissimopoulos S., Docrat N., Lai F.A. Redox sensitivity of the ryanodine receptor interaction with FK506-binding protein. J Biol Chem. 2007;282:6976–6983. doi: 10.1074/jbc.M607590200. [DOI] [PubMed] [Google Scholar]
- 62.Dowling J.J., Arbogast S., Hur J., et al. Oxidative stress and successful antioxidant treatment in models of RYR1-related myopathy. Brain. 2012;135:1115–1127. doi: 10.1093/brain/aws036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Durham W.J., Aracena-Parks P., Long C., et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knock-in mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michelucci A., De Marco A., Guarnier F.A., Protasi F., Boncompagni S. Antioxidant treatment reduces formation of structural cores and improves muscle function in RYR1Y522S/WT mice. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/6792694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Todd J.J., Lawal T.A., Witherspoon J.W., et al. Randomized controlled trial of N-acetylcysteine therapy for RYR1-related myopathies. Neurology. 2020;94:e1434–e1444. doi: 10.1212/WNL.0000000000008872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Figueroa L., Kraeva N., Manno C., Toro S., Ríos E., Riazi S. Abnormal calcium signalling and the caffeine-halothane contracture test. Br J Anaesth. 2019;122:32–41. doi: 10.1016/j.bja.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ivarsson N., Mattsson C.M., Cheng A.J., et al. SR Ca2+ leak in skeletal muscle fibers acts as an intracellular signal to increase fatigue resistance. J Gen Physiol. 2019;151:567–577. doi: 10.1085/jgp.201812152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Place N., Ivarsson N., Venckunas T., et al. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc Natl Acad Sci U S A. 2015;112:15492–15497. doi: 10.1073/pnas.1507176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanou N., Dridi H., Reiken S., et al. Acute RyR1 Ca2+ leak enhances NADH-linked mitochondrial respiratory capacity. Nat Commun. 2021;12:7219. doi: 10.1038/s41467-021-27422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perlmutter D.H. Chemical chaperones: a pharmacological strategy for disorders of protein folding and trafficking. Pediatr Res. 2002;52:832–836. doi: 10.1203/00006450-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Lee C.S., Hanna A.D., Wang H., et al. A chemical chaperone improves muscle function in mice with a RyR1 mutation. Nat Commun. 2017;8 doi: 10.1038/ncomms14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kushnir A., Marks A.R. Ryanodine receptor patents. Recent Pat Biotechnol. 2012;6:157–166. doi: 10.2174/1872208311206030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crunkhorn S. Fixing the leak. Nat Rev Drug Discov. 2009;8:277. doi: 10.1038/nrd2857. [DOI] [PubMed] [Google Scholar]
- 74.Kushnir A., Todd J.J., Witherspoon J.W., et al. Intracellular calcium leak as a therapeutic target for RYR1-related myopathies. Acta Neuropathol. 2020;139:1089–1104. doi: 10.1007/s00401-020-02150-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Todd J.J., Lawal T.A., Chrismer I.C., et al. Rycal S48168 (ARM210) for RYR1-related myopathies: a phase one, open-label, dose-escalation trial. eClinicalMedicine. 2024;68 doi: 10.1016/j.eclinm.2024.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamazawa T., Kobayashi T., Kurebayashi N., et al. A novel RyR1-selective inhibitor prevents and rescues sudden death in mouse models of malignant hyperthermia and heat stroke. Nat Commun. 2021;12:4293. doi: 10.1038/s41467-021-24644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corton J.M., Gillespie J.G., Hawley S.A., Hardie D.G. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 78.Lanner J.T., Georgiou D.K., Dagnino-Acosta A., et al. AICAR prevents heat-induced sudden death in RyR1 mutant mice independent of AMPK activation. Nat Med. 2012;18:244–251. doi: 10.1038/nm.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Volpatti J.R., Endo Y., Knox J., et al. Identification of drug modifiers for RYR1-related myopathy using a multi-species discovery pipeline. Elife. 2020;9 doi: 10.7554/eLife.52946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirata H., Watanabe T., Hatakeyama J., et al. Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease. Development. 2007;134:2771–2781. doi: 10.1242/dev.004531. [DOI] [PubMed] [Google Scholar]
- 81.Murayama T., Kurebayashi N., Ishigami-Yuasa M., et al. Efficient high-throughput screening by endoplasmic reticulum Ca2+ measurement to identify inhibitors of ryanodine receptor Ca2+-release channels. Mol Pharmacol. 2018;94:722–730. doi: 10.1124/mol.117.111468. [DOI] [PubMed] [Google Scholar]
- 82.Lawal T.A., Todd J.J., Meilleur K.G. Ryanodine receptor 1-related myopathies: diagnostic and therapeutic approaches. Neurotherapeutics. 2018;15:885–899. doi: 10.1007/s13311-018-00677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beaufils M., Travard L., Rendu J., Marty I. Therapies for RYR1-related myopathies: where we stand and the perspectives. Curr Pharm Des. 2022;28:15–25. doi: 10.2174/1389201022666210910102516. [DOI] [PubMed] [Google Scholar]
- 84.Chamberlain K., Riyad J.M., Weber T. Expressing transgenes that exceed the packaging capacity of adeno-associated virus capsids. Hum Gene Ther Methods. 2016;27:1–12. doi: 10.1089/hgtb.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rendu J., Brocard J., Denarier E., et al. Exon skipping as a therapeutic strategy applied to an RYR1 mutation with pseudo-exon inclusion causing a severe core myopathy. Hum Gene Ther. 2013;24:702–713. doi: 10.1089/hum.2013.052. [DOI] [PMC free article] [PubMed] [Google Scholar]