Abstract
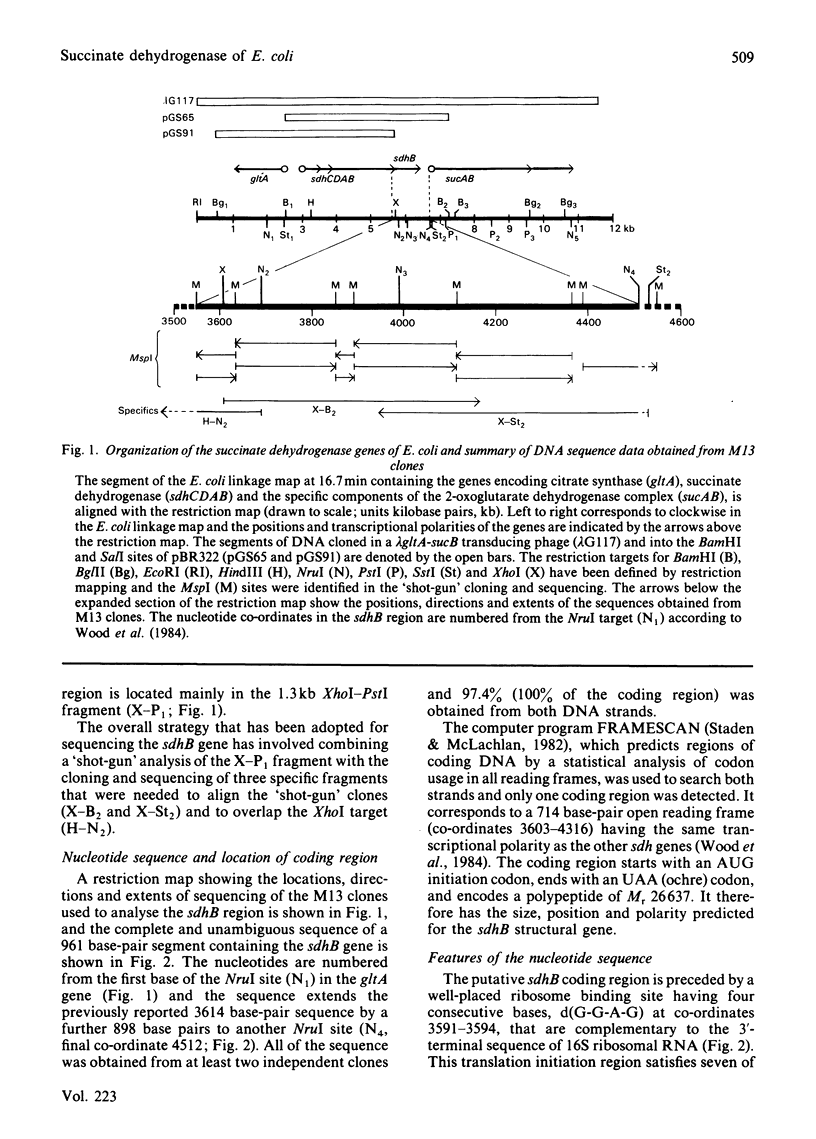
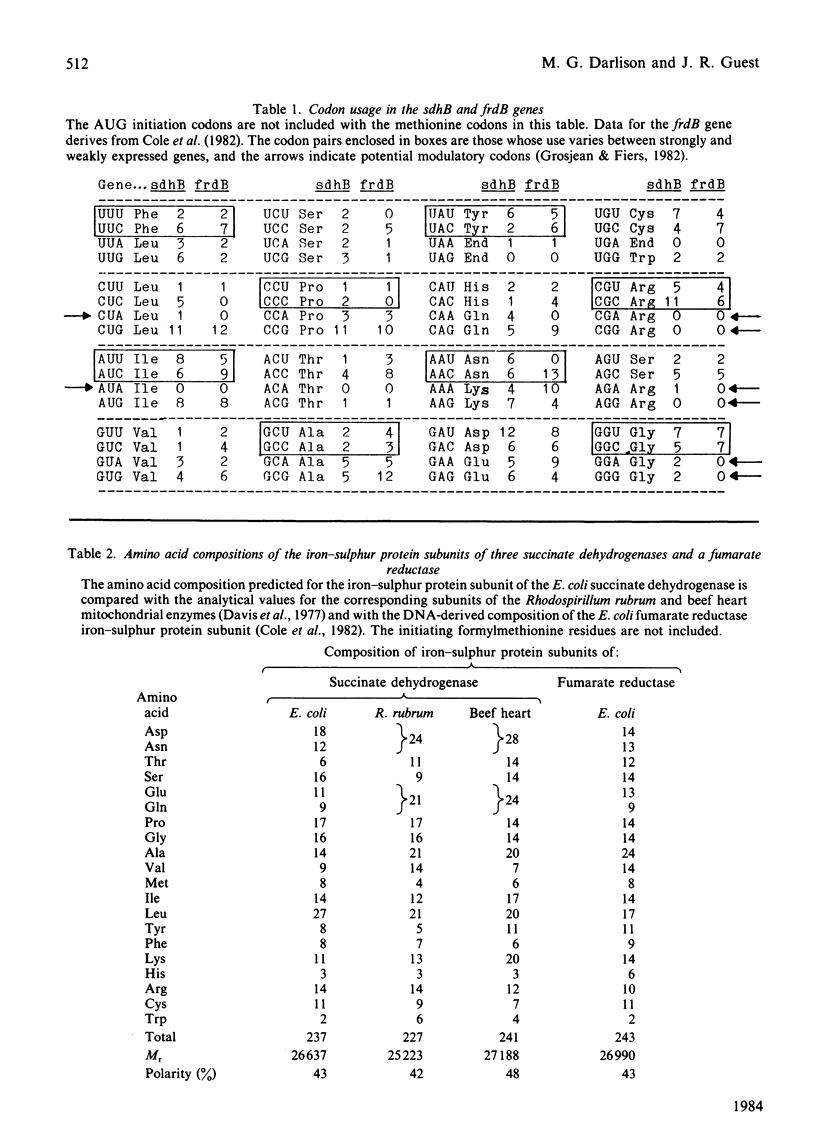
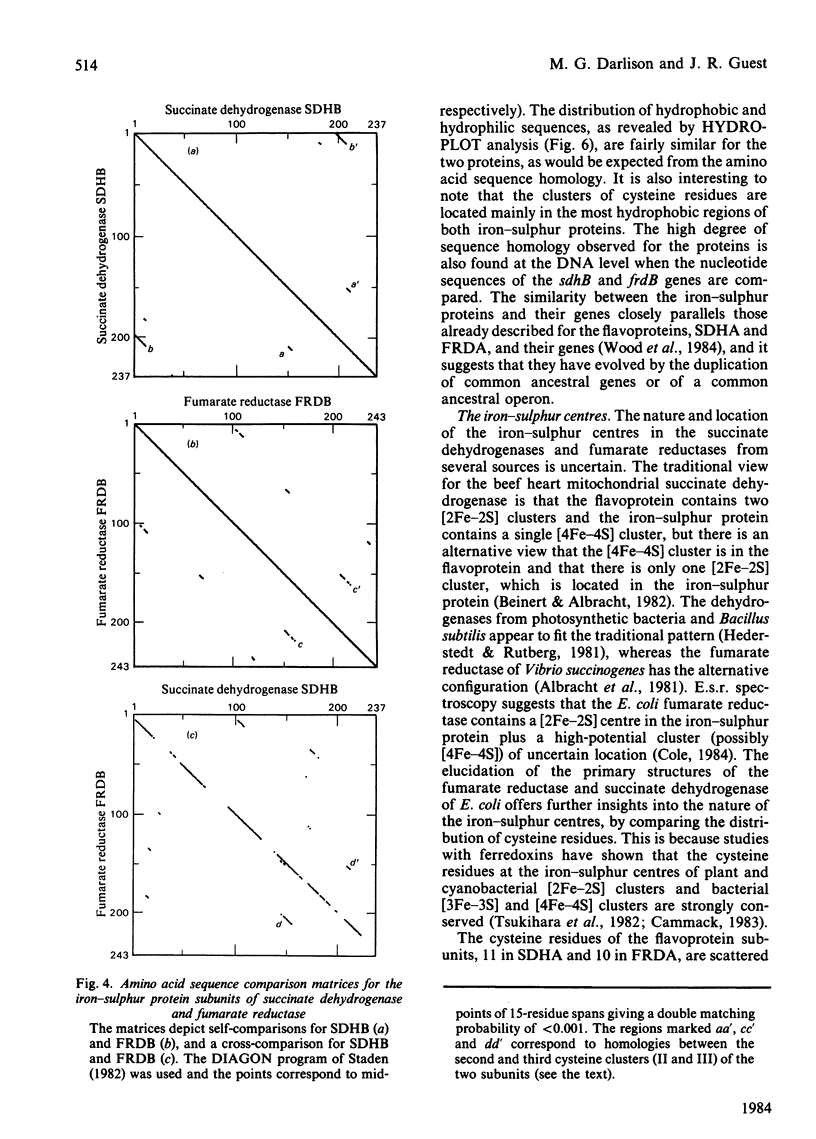
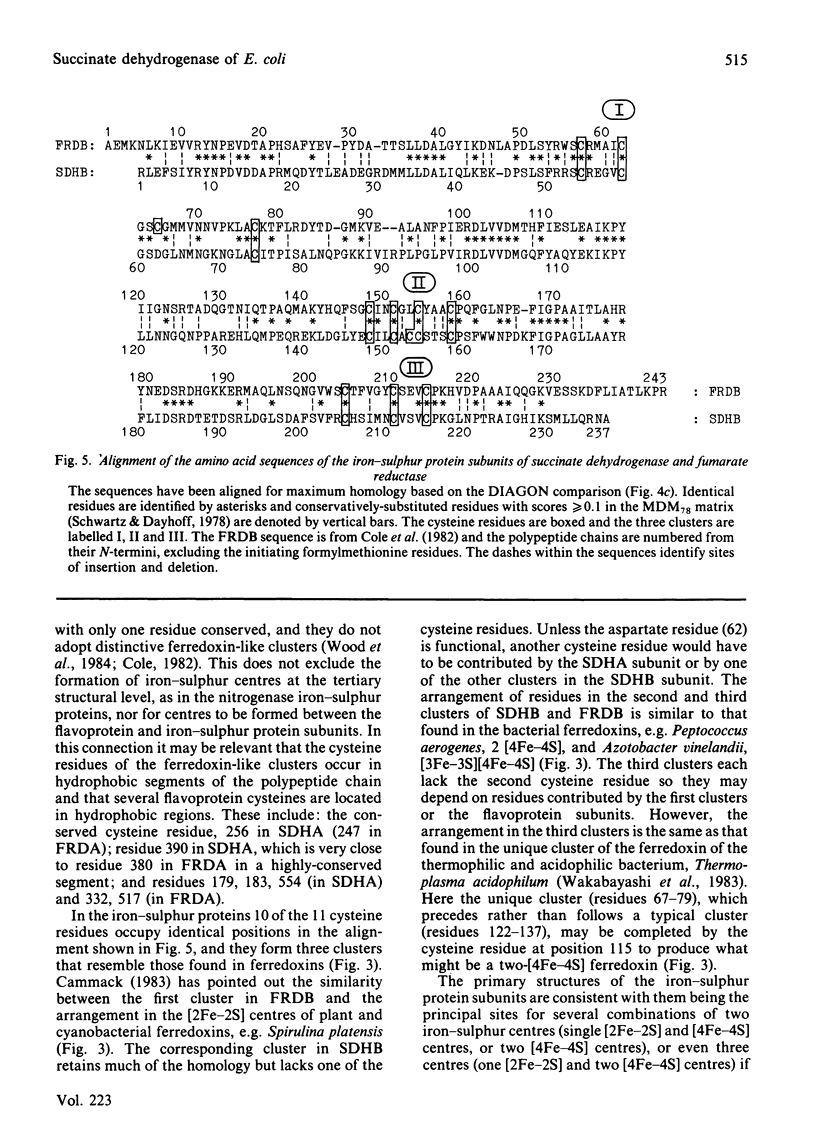
The nucleotide sequence of a 961 base-pair segment of DNA containing the sdhB gene, which encodes the iron-sulphur protein subunit of the E. coli succinate dehydrogenase, has been determined. The sdhB structural gene comprises 711 base pairs (237 codons, excluding the translational initiator and terminator). It is separated by a 15 base-pair intergenic region from the preceding flavoprotein gene (sdhA) and is the distal gene of an operon that also includes genes (sdhC and D) encoding two hydrophobic subunits, sdhCDAB. The distal end of the sdh operon is linked to the 2-oxoglutarate dehydrogenase gene (sucA) by a complex region of dyad symmetry that is homologous with several potential intercistronic regulatory elements or transcriptional attenuators. The sdhB structural gene encodes a polypeptide of Mr26637 that is strikingly homologous with the iron-sulphur protein subunit of fumarate reductase (38% identity, increasing to 58% when conservative changes are included). Both subunits contain 11 cysteine residues, 10 being conserved in three clusters resembling those found in ferredoxins. This work completes the sequence of a 9897 base-pair segment of DNA containing seven tricarboxylic acid cycle genes encoding three enzymes or enzyme complexes, citrate synthase (gltA), succinate dehydrogenase (sdh), and the 2-oxoglutarate dehydrogenase complex (suc), that are organized thus: gltA-sdhCDAB-sucAB.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albracht S. P., Unden G., Kröger A. Iron-sulphur clusters in fumarate reductase from Vibrio succinogenes. Biochim Biophys Acta. 1981 Oct 13;661(2):295–302. doi: 10.1016/0005-2744(81)90018-8. [DOI] [PubMed] [Google Scholar]
- Atkins J. F. Is UAA or UGA part of the recognition signal for ribosomal initiation? Nucleic Acids Res. 1979 Oct 25;7(4):1035–1041. doi: 10.1093/nar/7.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H., Albracht S. P. New insights, ideas and unanswered questions concerning iron-sulfur clusters in mitochondria. Biochim Biophys Acta. 1982 Dec 31;683(3-4):245–277. doi: 10.1016/0304-4173(82)90003-9. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Grundström T., Jaurin B., Robinson J. J., Weiner J. H. Location and nucleotide sequence of frdB, the gene coding for the iron-sulphur protein subunit of the fumarate reductase of Escherichia coli. Eur J Biochem. 1982 Aug;126(1):211–216. doi: 10.1111/j.1432-1033.1982.tb06768.x. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Guest J. R. Amplification of fumarate reductase synthesis with lambdafrdA transducing phages and orientation of frdA gene expression. Mol Gen Genet. 1980;179(2):377–385. doi: 10.1007/BF00425468. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Guest J. R. Genetic and physical characterization of lambda transducing phages (lambda frdA) containing the fumarate reductase gene of Escherichia coli K12. Mol Gen Genet. 1980;178(2):409–418. doi: 10.1007/BF00270492. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Guest J. R. Molecular genetic aspects of the succinate: fumarate oxidoreductases of Escherichia coli. Biochem Soc Trans. 1982 Dec;10(6):473–475. doi: 10.1042/bst0100473. [DOI] [PubMed] [Google Scholar]
- Cole S. T. Molecular and genetic aspects of the fumarate reductase of Escherichia coli. Biochem Soc Trans. 1984 Apr;12(2):237–238. doi: 10.1042/bst0120237. [DOI] [PubMed] [Google Scholar]
- Cole S. T. Nucleotide sequence coding for the flavoprotein subunit of the fumarate reductase of Escherichia coli. Eur J Biochem. 1982 Mar 1;122(3):479–484. doi: 10.1111/j.1432-1033.1982.tb06462.x. [DOI] [PubMed] [Google Scholar]
- Darlison M. G., Spencer M. E., Guest J. R. Nucleotide sequence of the sucA gene encoding the 2-oxoglutarate dehydrogenase of Escherichia coli K12. Eur J Biochem. 1984 Jun 1;141(2):351–359. doi: 10.1111/j.1432-1033.1984.tb08199.x. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y., Crawford I. P., Baltscheffsky H. Purification, molecular properties, and amino acid composition of the subunits of Rhodospirillum rubrum succinate dehydrogenase. Arch Biochem Biophys. 1977 Apr 30;180(2):459–464. doi: 10.1016/0003-9861(77)90060-1. [DOI] [PubMed] [Google Scholar]
- Ghosh D., O'Donnell S., Furey W., Jr, Robbins A. H., Stout C. D. Iron-sulfur clusters and protein structure of Azotobacter ferredoxin at 2.0 A resolution. J Mol Biol. 1982 Jun 15;158(1):73–109. doi: 10.1016/0022-2836(82)90451-x. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Grundström T., Jaurin B. Overlap between ampC and frd operons on the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1111–1115. doi: 10.1073/pnas.79.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R., Roberts R. E., Stephens P. E. Hybrid plasmids containing the pyruvate dehydrogenase complex genes and gene-DNA relationships in the 2 to 3 minute region of the Escherichia coli chromosome. J Gen Microbiol. 1983 Mar;129(3):671–680. doi: 10.1099/00221287-129-3-671. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Rutberg L. Succinate dehydrogenase--a comparative review. Microbiol Rev. 1981 Dec;45(4):542–555. doi: 10.1128/mr.45.4.542-555.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F., Barnes W. M., Clement J. M., Hofnung M. A novel intercistronic regulatory element of prokaryotic operons. Nature. 1982 Aug 19;298(5876):760–762. doi: 10.1038/298760a0. [DOI] [PubMed] [Google Scholar]
- Hull E. P., Spencer M. E., Wood D., Guest J. R. Nucleotide sequence of the promoter region of the citrate synthase gene (gltA) of Escherichia coli. FEBS Lett. 1983 Jun 13;156(2):366–370. doi: 10.1016/0014-5793(83)80530-4. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Kranz R. G., Gennis R. B. Immunochemical analysis of the membrane-bound succinate dehydrogenase of Escherichia coli. FEBS Lett. 1982 Jun 1;142(1):81–87. doi: 10.1016/0014-5793(82)80224-x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Kühn S., Fritz H. J., Starlinger P. Close vicinity of IS1 integration sites in the leader sequence of the gal operon of E. coli. Mol Gen Genet. 1979 Jan 2;167(3):235–241. doi: 10.1007/BF00267414. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Recovery of DNA from gels. Methods Enzymol. 1980;65(1):371–380. doi: 10.1016/s0076-6879(80)65048-4. [DOI] [PubMed] [Google Scholar]
- Spencer M. E., Darlison M. G., Stephens P. E., Duckenfield I. K., Guest J. R. Nucleotide sequence of the sucB gene encoding the dihydrolipoamide succinyltransferase of Escherichia coli K12 and homology with the corresponding acetyltransferase. Eur J Biochem. 1984 Jun 1;141(2):361–374. doi: 10.1111/j.1432-1033.1984.tb08200.x. [DOI] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Isolation and properties of fumarate reductase mutants of Escherichia coli. J Bacteriol. 1973 May;114(2):563–570. doi: 10.1128/jb.114.2.563-570.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Molecular cloning of four tricarboxylic acid cyclic genes of Escherichia coli. J Bacteriol. 1982 Aug;151(2):542–552. doi: 10.1128/jb.151.2.542-552.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Proteins of the inner membrane of Escherichia coli: changes in composition associated with anaerobic growth and fumarate reductase amber mutation. J Bacteriol. 1974 Mar;117(3):954–959. doi: 10.1128/jb.117.3.954-959.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Proteins of the inner membrane of Escherichia coli: identification of succinate dehydrogenase by polyacrylamide gel electrophoresis with sdh amber mutants. J Bacteriol. 1974 Mar;117(3):947–953. doi: 10.1128/jb.117.3.947-953.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. A strategy of DNA sequencing employing computer programs. Nucleic Acids Res. 1979 Jun 11;6(7):2601–2610. doi: 10.1093/nar/6.7.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R., McLachlan A. D. Codon preference and its use in identifying protein coding regions in long DNA sequences. Nucleic Acids Res. 1982 Jan 11;10(1):141–156. doi: 10.1093/nar/10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P. E., Darlison M. G., Lewis H. M., Guest J. R. The pyruvate dehydrogenase complex of Escherichia coli K12. Nucleotide sequence encoding the dihydrolipoamide acetyltransferase component. Eur J Biochem. 1983 Jul 1;133(3):481–489. doi: 10.1111/j.1432-1033.1983.tb07490.x. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T. The complete amino acid sequence of the Spirulina platensis ferredoxin. Biochem Biophys Res Commun. 1976 Apr 5;69(3):759–765. doi: 10.1016/0006-291x(76)90940-2. [DOI] [PubMed] [Google Scholar]
- Tsukihara T., Kobayashi M., Nakamura M., Katsube Y., Fukuyama K., Hase T., Wada K., Matsubara H. Structure-function relationship of [2Fe-2S] ferredoxins and design of a model molecule. Biosystems. 1982;15(3):243–257. doi: 10.1016/0303-2647(82)90009-0. [DOI] [PubMed] [Google Scholar]
- Tsunoda J. N., Yasunobu K. T., Whiteley H. R. Non-heme iron proteins. IX. The amino acid sequence of ferredoxin from Micrococcus aerogenes. J Biol Chem. 1968 Dec 10;243(23):6262–6272. [PubMed] [Google Scholar]
- Unden G., Hackenberg H., Kröger A. Isolation and functional aspects of the fumarate reductase involved in the phosphorylative electron transport of Vibrio succinogenes. Biochim Biophys Acta. 1980 Jul 8;591(2):275–288. doi: 10.1016/0005-2728(80)90159-0. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P., Hammer-Jespersen K., Boetius F., Svendsen I. Structure and function of the intercistronic regulatory deoC-deoA element of Escherichia coli K-12. EMBO J. 1984 Jan;3(1):179–183. doi: 10.1002/j.1460-2075.1984.tb01781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


