Abstract
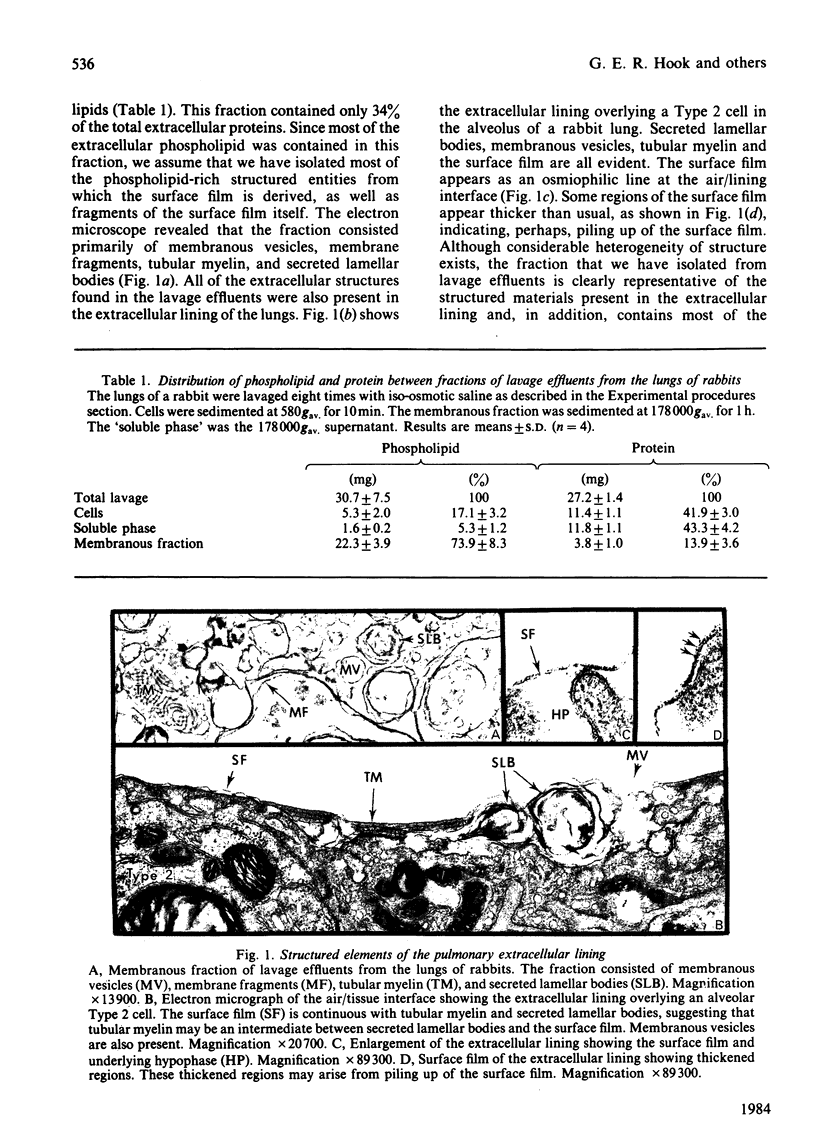
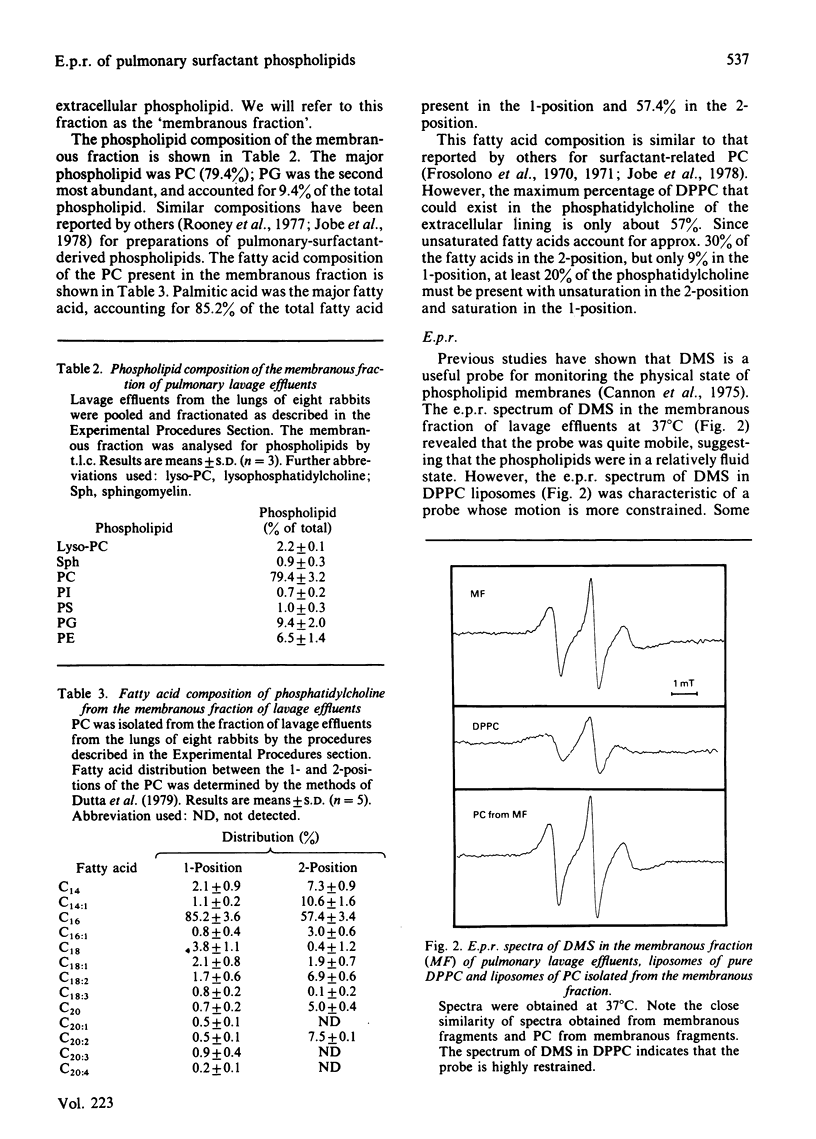
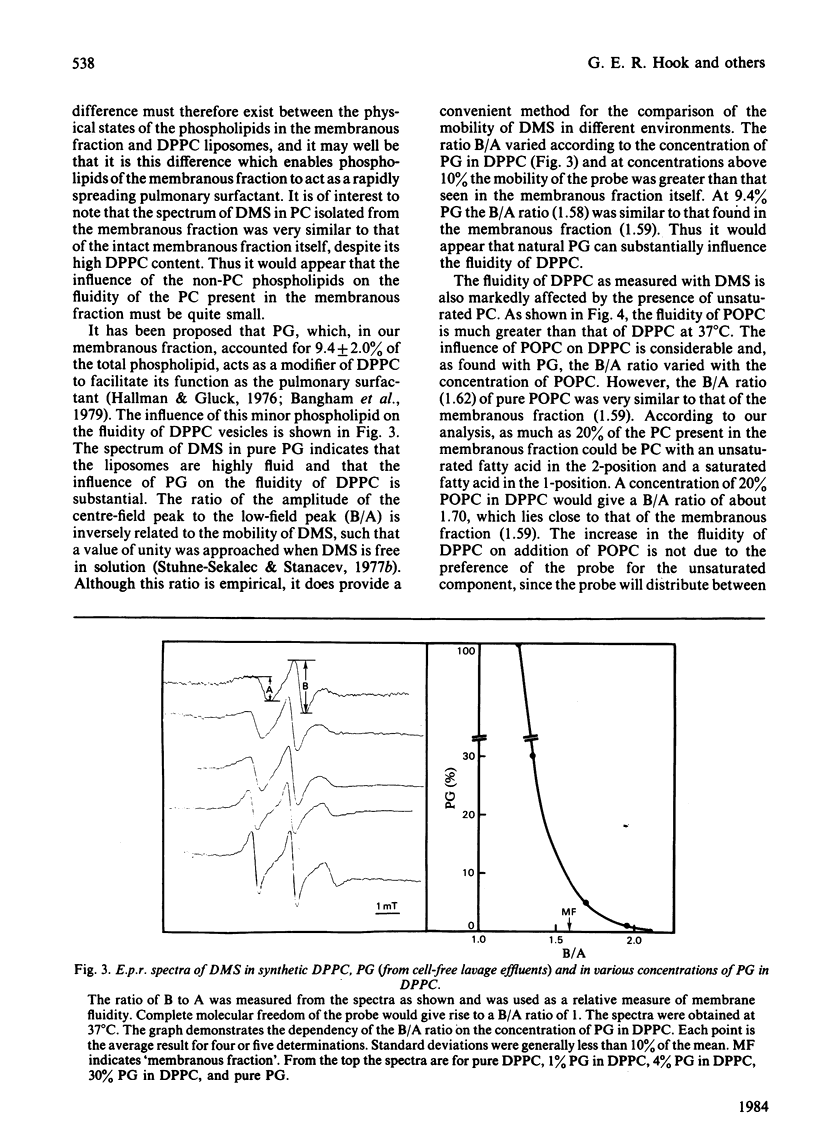
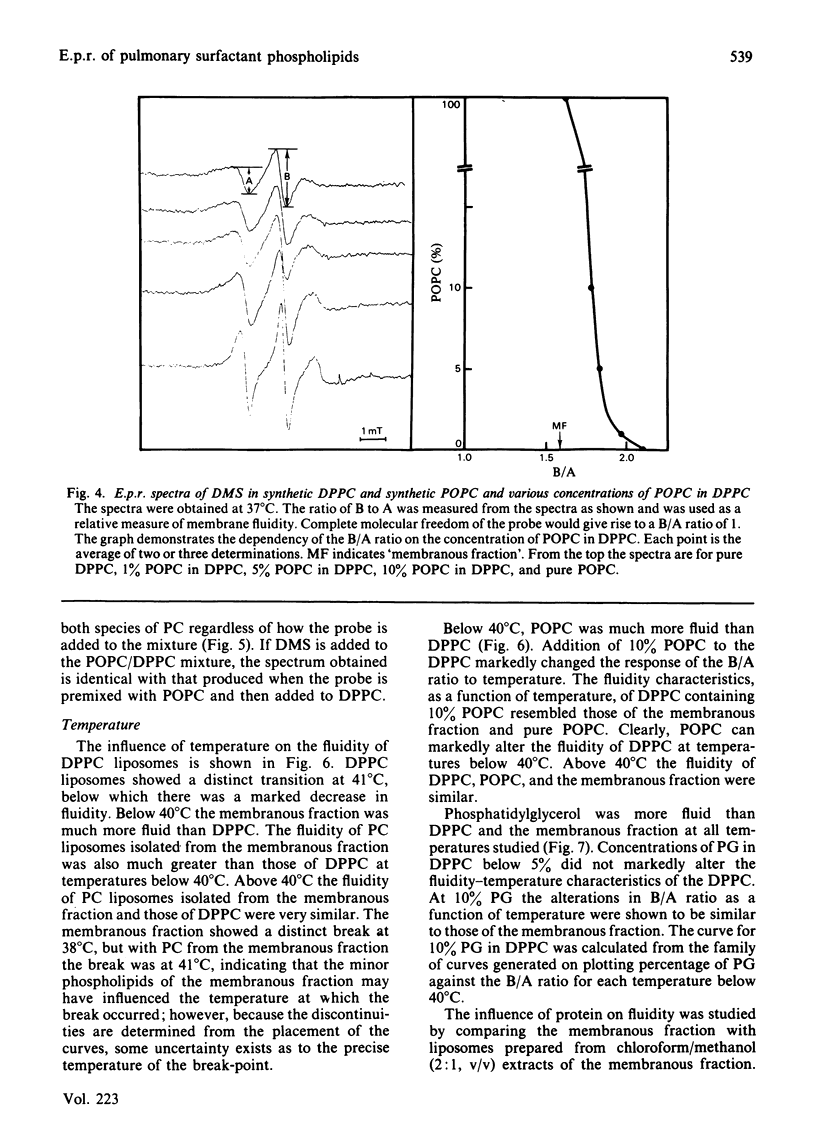
The membranous structures of the pulmonary extracellular lining were removed from the lungs of rabbits by pulmonary lavage and isolated by differential centrifugation. This membranous fraction contained 93% of the total extracellular phospholipids present in lavage effluents and consisted of membranous vesicles, membrane fragments, tubular myelin and secreted lamellar bodies. The fraction was rich in phosphatidylcholine (79.4%) containing 85.2% palmitic acid in the 1-position and 57.4% palmitic acid in the 2-position. Phosphatidylglycerol was the next most abundant phospholipid, accounting for 9.4% of the total. E.p.r. spectra, obtained by using 5-doxylmethylstearate as a probe, showed that the extracellular phospholipids of the pulmonary lining were organized into structures which were much more fluid than erythrocyte-ghost membranes. The fluidity of phosphatidylcholine isolated from the membranous fraction was similar to that of the fraction itself, indicating that the minor phospholipids had very little influence on the fluidity of the major phospholipid. At physiological temperature, the fluidity of dipalmitoyl phosphatidylcholine was relatively low, but could be markedly increased by the presence of 1-palmitoyl-2-oleoyl phosphatidylcholine or phosphatidylglycerol (10%). Protein present in the extracellular phospholipid fraction did not affect the fluidity of the fraction. These studies indicate that the unsaturated phosphatidylcholines could play a major role in determining the fluidity of the important surface-tension-lowering phospholipids such as dipalmitoyl phosphatidylcholine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askin F. B., Kuhn C. The cellular origin of pulmonary surfactant. Lab Invest. 1971 Sep;25(3):260–268. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BENSCH K., SCHAEFER K., AVERY M. E. GRANULAR PNEUMOCYTES: ELECTRON MICROSCOPIC EVIDENCE OF THEIR EXOCRINIC FUNCTION. Science. 1964 Sep 18;145(3638):1318–1319. doi: 10.1126/science.145.3638.1318-a. [DOI] [PubMed] [Google Scholar]
- BROWN E. S. ISOLATION AND ASSAY OF DIPALMITYL LECITHIN IN LUNG EXTRACTS. Am J Physiol. 1964 Aug;207:402–406. doi: 10.1152/ajplegacy.1964.207.2.402. [DOI] [PubMed] [Google Scholar]
- Bangham A. D., Morley C. J., Phillips M. C. The physical properties of an effective lung surfactant. Biochim Biophys Acta. 1979 Jun 21;573(3):552–556. doi: 10.1016/0005-2760(79)90229-7. [DOI] [PubMed] [Google Scholar]
- Cannon B., Polnaszek C. F., Butler K. W., Eriksson L. E., Smith I. C. The fluidity and organization of mitochondrial membrane lipids of the brown adipose tissue of cold-adapted rats and hamsters as determined by nitroxide spin probes. Arch Biochem Biophys. 1975 Apr;167(2):505–518. doi: 10.1016/0003-9861(75)90493-2. [DOI] [PubMed] [Google Scholar]
- Clements J. A. Functions of the alveolar lining. Am Rev Respir Dis. 1977 Jun;115(6 Pt 2):67–71. doi: 10.1164/arrd.1977.115.S.67. [DOI] [PubMed] [Google Scholar]
- Dutta J., Das A. K., Biswas A. Enzymatic reactions on thin-layer chromatographic plates. II. Phospholipase A2 hydrolysis of phosphatidylcholine and separation of the products on a single plate. J Chromatogr. 1979 May 21;173(2):379–387. doi: 10.1016/s0021-9673(00)92307-0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Frosolono M. F., Charms B. L., Pawlowski R., Slivka S. Isolation, characterization, and surface chemistry of a surface-active fraction from dog lung. J Lipid Res. 1970 Sep;11(5):439–457. [PubMed] [Google Scholar]
- Frosolono M. F., Slivka S., Charms B. L. Acyl transferase activities in dog lung microsomes. J Lipid Res. 1971 Jan;12(1):96–103. [PubMed] [Google Scholar]
- Galdston M., Shah D. O., Shinowara G. Y. Isolation and characterization of a lung lipoprotein surfactant. J Colloid Interface Sci. 1969 Feb;29(2):319–334. doi: 10.1016/0021-9797(69)90202-1. [DOI] [PubMed] [Google Scholar]
- Goerke J. Lung surfactant. Biochim Biophys Acta. 1974 Dec 16;344(3-4):241–261. doi: 10.1016/0304-4157(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Gray G. M. Chromatography of lipids. I. An improved chromatographic procedure for the quantitative isolation of the neutral ceramide-containing glycolipids from mammalian tissues. Biochim Biophys Acta. 1967 Dec 5;144(3):511–518. doi: 10.1016/0005-2760(67)90039-2. [DOI] [PubMed] [Google Scholar]
- Gómez-Fernández J. C., Goñi F. M., Bach D., Restall C., Chapman D. Protein--lipid interactions. A study of (Ca2+-Mg2+)ATPase reconstituted with synthetic phospholipids. FEBS Lett. 1979 Feb 15;98(2):224–228. doi: 10.1016/0014-5793(79)80187-8. [DOI] [PubMed] [Google Scholar]
- Hallman M., Gluck L. Phosphatidylglycerol in lung surfactant. III. Possible modifier of surfactant function. J Lipid Res. 1976 May;17(3):257–262. [PubMed] [Google Scholar]
- Hatasa K., Nakamura T. Electron microscopic observations of lung alveolar epithelial cells of normal young mice, with special reference to formation and secretion of osmiophilic lamellar bodies. Z Zellforsch Mikrosk Anat. 1965 Oct 12;68(2):266–277. doi: 10.1007/BF00342433. [DOI] [PubMed] [Google Scholar]
- Hook G. E. Extracellular hydrolases of the lung. Biochemistry. 1978 Feb 7;17(3):520–528. doi: 10.1021/bi00596a023. [DOI] [PubMed] [Google Scholar]
- Jobe A., Kirkpatrick E., Gluck L. Labeling of phospholipids in the surfactant and subcellular fractions of rabbit lung. J Biol Chem. 1978 Jun 10;253(11):3810–3816. [PubMed] [Google Scholar]
- Kikkawa Y. Morphology of alveolar lining layer. Anat Rec. 1970 Aug;167(4):389–400. doi: 10.1002/ar.1091670403. [DOI] [PubMed] [Google Scholar]
- King R. J., Clements J. A. Surface active materials from dog lung. II. Composition and physiological correlations. Am J Physiol. 1972 Sep;223(3):715–726. doi: 10.1152/ajplegacy.1972.223.3.715. [DOI] [PubMed] [Google Scholar]
- King R. J., Macbeth M. C. Physicochemical properties of dipalmitoyl phosphatidylcholine after interaction with an apolipoprotein of pulmonary surfactant. Biochim Biophys Acta. 1979 Oct 19;557(1):86–101. doi: 10.1016/0005-2736(79)90092-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T. Freeze-fracture study of alveolar lining layer in adult rat lungs. J Ultrastruct Res. 1979 Oct;69(1):86–97. doi: 10.1016/s0022-5320(79)80044-1. [DOI] [PubMed] [Google Scholar]
- Meban C. Effect of lipids and other substances on the adsorption of dipalmitoyl phosphatidylcholine. Pediatr Res. 1981 Jul;15(7):1029–1031. doi: 10.1203/00006450-198107000-00010. [DOI] [PubMed] [Google Scholar]
- Metcalfe I. L., Enhorning G., Possmayer F. Pulmonary surfactant-associated proteins: their role in the expression of surface activity. J Appl Physiol Respir Environ Exerc Physiol. 1980 Jul;49(1):34–41. doi: 10.1152/jappl.1980.49.1.34. [DOI] [PubMed] [Google Scholar]
- PATTLE R. E. SURFACE LINING OF LUNG ALVEOLI. Physiol Rev. 1965 Jan;45:48–79. doi: 10.1152/physrev.1965.45.1.48. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney S. A., Nardone L. L., Shapiro D. L., Motoyama E. K., Gobran L., Zaehringer N. The phospholipids of rabbit type II alveolar epithelial cells: comparison with lung lavage, lung tissue, alveolar macrophages, and a human alveolar tumor cell line. Lipids. 1977 May;12(5):438–442. doi: 10.1007/BF02533629. [DOI] [PubMed] [Google Scholar]
- Shelley S. A., L'Heureux M. V., Balis J. U. Characterization of lung surfactant: factors promoting formation of artifactual lipid-protein complexes. J Lipid Res. 1975 May;16(3):224–234. [PubMed] [Google Scholar]
- Skipski V. P., Barclay M., Reichman E. S., Good J. J. Separation of acidic phospholipids by one-dimensional thin-layer chromatography. Biochim Biophys Acta. 1967 Feb 14;137(1):80–89. doi: 10.1016/0005-2760(67)90010-0. [DOI] [PubMed] [Google Scholar]
- Steim J. M. Isolation and characterization of lung surfactant. Biochem Biophys Res Commun. 1969 Feb 21;34(4):434–440. doi: 10.1016/0006-291x(69)90400-8. [DOI] [PubMed] [Google Scholar]
- Stratton C. J. The three-dimensional aspect of the mammalian lung surfactant myelin figure. Tissue Cell. 1977;9(2):285–300. doi: 10.1016/0040-8166(77)90022-2. [DOI] [PubMed] [Google Scholar]
- Stuhne-Sekalec L., Stanacev N. Z. Selective enzymatic radioactive and spin labelling of phospholipids in biological membranes: application to study of temperature-induced changes of microsomal phosphatidylinositols and mitochondrial polyglycerophosphatides. Can J Biochem. 1977 Feb;55(2):186–204. doi: 10.1139/o77-028. [DOI] [PubMed] [Google Scholar]
- Stuhne-Sekalec L., Stanacev N. Z. Temperature-induced changes in lecithin model membranes detected by novel covalent spin-labelled phospholipids. Can J Biochem. 1977 Feb;55(2):173–185. doi: 10.1139/o77-027. [DOI] [PubMed] [Google Scholar]
- Sueishi K., Tanaka K., Oda T. Immunoultrastructural study of surfactant system. Distribution of specific protein of surface active material in rabbit lung. Lab Invest. 1977 Aug;37(2):136–142. [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Gil J. Electron microscopic demonstration of an extracellular duplex lining layer of alveoli. Respir Physiol. 1968 Jan;4(1):42–57. doi: 10.1016/0034-5687(68)90006-6. [DOI] [PubMed] [Google Scholar]
- Williams M. C. Conversion of lamellar body membranes into tubular myelin in alveoli of fetal rat lungs. J Cell Biol. 1977 Feb;72(2):260–277. doi: 10.1083/jcb.72.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gier J., Mandersloot J. G., van Deenen L. L. Lipid composition and permeability of liposomes. Biochim Biophys Acta. 1968 Jun 11;150(4):666–675. doi: 10.1016/0005-2736(68)90056-4. [DOI] [PubMed] [Google Scholar]



