Abstract
Objective:
Treatment-resistant depression (TRD) affects around 20-30% of people with major depressive disorder. In 2019, esketamine nasal spray was approved for TRD by both the US Food and Drug Administration and the European Medicines Agency. While its clinical efficacy and safety are proven, the mechanisms underlying its antidepressant effect remain unclear. The use of metabolomics may allow understanding the metabolic effects of esketamine and predicting biological features associated with clinical response in TRD. Nonetheless, there is a lack of studies exploring the predictive value of metabolomics. The Resistant Depression Response to Esketamine Assessing Metabolomics (ReDREAM) project aims at identifying metabolic biosignatures that may represent novel correlates of response to esketamine treatment.
Study Design:
This is the protocol of an observational, prospective study.
Methods:
We plan to select 60 people with TRD from 3 clinical sites in Italy. The participants will be administered with esketamine nasal spray, following standard clinical practice, twice a week for 4 weeks (“induction phase”), then once a week for 4 additional weeks (“maintenance phase”). We will test the correlations between baseline metabolic profile and depressive symptom improvement at study endpoints (weeks 4 and 8) and we will explore the likelihood of different metabolic phenotypes between responders and non-responders.
Expected results:
An involvement of energy metabolism, amino acid metabolism, urea cycle, and nitric oxide synthesis in response to treatment with esketamine nasal spray is hypothesized.
Conclusion:
Unbiased data from untargeted metabolomics associated with clinical changes after esketamine treatment may contribute to define new paradigms for precision psychiatry-oriented, personalized care of TRD.
Keywords: Biomarkers, esketamine, major depressive disorder, metabolomics
Main Points
The mechanisms underlying the antidepressant effects of esketamine and the differences between responders and non-responders remain unclear.
Metabolomics may help shed light on candidate biomarkers involved in the clinical response to esketamine in treatment-resistant depression.
Sixty participants with treatment-resistant depression will be treated with esketamine nasal spray for 8 weeks.
A baseline metabolomics assessment and longitudinal psychometric evaluations will be performed.
Correlations between baseline metabolic profile and depressive symptom improvement, as well as possible metabolome differences between responders and non-responders, will be explored.
Introduction
Major depressive disorder (MDD) is a severe and often recurrent mental disorder, significantly affecting people’s psychosocial functioning and quality of life.1 Systematic evidence suggests a wide variety of approaches to treat MDD, including pharmacological, biological non-pharmacological, and psychological interventions.1,2 Nonetheless, a substantial proportion of subjects does not sufficiently benefit from standard treatments.3 Treatment-resistant depression (TRD), most commonly defined as MDD not responding to a minimum of 2 prior treatments with adequate dose and duration, is relatively common, involving up to 30% of subjects from this clinical population.4,5 In recent years, several innovative pharmacological agents, targeting biological pathways other than monoaminergic neurotransmission, such as the glutamatergic system, have been proposed.3 Among these, the N-methyl-d-aspartate receptor (NMDAR) antagonist ketamine and its S (+) enantiomer esketamine have been identified as some of the most promising pharmacological agents for the management of TRD due to their rapid antidepressant and antisuicidal effects.3,6-8 In 2019, esketamine nasal spray was approved as a new supplemental drug for the management of TRD—augmenting an oral selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) antidepressant—by both the United States (US) Food and Drug Administration (FDA) and the European Medicines Agency (EMA).9 While its clinical efficacy and safety are established,4 the relevant mechanisms underlying the rapid decrease of depressive symptoms induced by esketamine, as well as the clinical and biological differences between responders and non-responders, remain unclear.8,10
Nonetheless, in recent years, advances in metabolomics research have shown its potential to study candidate biomarkers possibly involved in response to ketamine and esketamine.11-14 Metabolomics, i.e., the global analysis of all metabolites found within a specific biological sample through a simultaneous measurement of numerous small molecules (molecular weight < 1500 Da),15 provides an overview of the individual metabolic phenotype.16 Metabolomics is emerging as a powerful tool in research on severe mental disorders and may help gain additional insight into the pathophysiology of depression, the mechanism of action of different drugs, and the between-subjects differences in response to treatment.11 Along with a more appropriate understanding of esketamine biological mechanisms, metabolomics may thus help predict individual metabolic features of clinical response to esketamine.10 Nonetheless, studies using metabolomics assessments of people with TRD treated with ketamine and esketamine, besides being limited to a single ketamine or esketamine infusion, and to very short follow-up periods, have mainly focused on longitudinal metabolome changes11-13 or post-administration metabolome differences.14 Added clinical value would be provided by the identification of baseline metabolomics predictors, which may provide guidance on subjects who would benefit from treatment with esketamine the most. Finding biosignatures that can predict response to esketamine may ultimately contribute to defining new paradigms for precision psychiatry-oriented personalized care of TRD.5
Aims of the Project
The main aim of this project is to explore whether the metabolic phenotype can predict clinical response to treatment with intranasal esketamine, prescribed following standard clinical practice, in people suffering from TRD. Consistently, we will test the correlation between the baseline metabolome and longitudinal changes in depressive symptoms, also exploring possible metabolome differences between responders and non-responders. Our study will help identify a subset of subjects with TRD who are likely to benefit from treatment with esketamine, thus assessing biosignatures predicting individual antidepressant response.
Materials and Methods
We will conduct a prospective observational study following “STrengthening the Reporting of OBservational studies in Epidemiology (STROBE)” guidelines.17
Ethics and Dissemination
The study protocol (version 1.1 dated October 24, 2023) has been approved by “Comitato Etico Territoriale Lombardia 3” (Milan, Italy) ethics committee on 13 December 2023 (approval number 3910_S_N).
All participants will receive detailed oral and written information on the nature of the study and the use of data (with relevant information on privacy protection) and will be required to sign an informed consent form prior to entering the study. The Declaration of Helsinki’s ethical principles for Medical Research involving human subjects will be followed. Results will be disseminated via conference presentations and peer-reviewed publications.
Inclusion and Exclusion Criteria
We plan to select 60 participants with TRD, aged between 18 and 64 years, requiring treatment with esketamine nasal spray according to standard clinical practice. These participants will be consecutively recruited, during an 18-month index period, at the Psychiatric Inpatient and Outpatient Services of the 3 Italian university clinics involved in the project (Università degli Studi di Milano-Bicocca – UNIMIB, Università degli Studi “Magna Graecia” di Catanzaro – UNICZ, and Università degli Studi di Trieste – UNITS), and followed up for 8 weeks. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)18 criteria will be used to make a diagnosis of MDD: fully trained consultant psychiatrists will administer the Structured Clinical Interview for DSM-5 (SCID-5)19 to confirm the diagnosis as well as to assess possible comorbid conditions.
Consistently with established evidence,5 we will define TRD as a condition in which all the following criteria are met: i) a diagnosis of MDD, as defined by DSM-5 criteria;18 ii) a current major depressive episode, as defined by DSM-5 criteria;18 iii) a current Montgomery-Åsberg Depression Rating Scale (MADRS)20 score ≥ 20, indicating at least moderate severity;21 iv) the failure of 2 or more trials with pharmacological agents, approved by the EMA for the treatment of depression, at an adequate dose for a total of at least 6 weeks, as assessed by the Massachusetts General Hospital (MGH) Antidepressant Treatment Response Questionnaire (ATRQ).22
We will exclude: i) people presenting contraindicated conditions as reported by the prescribing information for esketamine nasal spray; ii) people with a history of treatment with ketamine or esketamine; iii) people with documented active SARS-CoV-2 infection; iv) pregnant or breastfeeding women; v) people with a DSM-5 diagnosis of alcohol or substance use disorders within the previous 3 months; vi) people with a current MDD with DSM-5-defined mixed or psychotic features; vii) people with a current or prior diagnosis of a psychotic disorder; viii) people with a current comorbid post-traumatic stress disorder or obsessive-compulsive disorder; ix) people with a diagnosis of dementia or cognitive impairment.
Esketamine Nasal Spray Administration Procedures
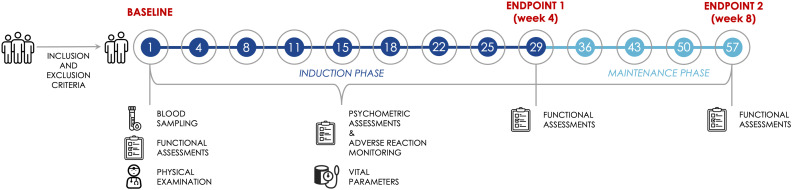
Participants will be treated with esketamine nasal spray, in addition to standard antidepressants, according to standard clinical practice: twice a week for the first 4 weeks (“induction phase”), then once a week for the following 4 weeks (“maintenance phase”). Participants will fast for at least 2 hours before administration and avoid drinking liquids for at least 30 minutes prior to administration as well as during the observation period. Esketamine nasal spray will be self-administered by the participant under the supervision of a physician. Each nasal spray device delivers 2 sprays containing a total of 28 mg of esketamine. Patients will be administered 56 mg (2 devices) on Day 1 and 56 or 84 mg (2 or 3 devices), based on tolerability as per the clinical judgment of the prescribing physician, at each subsequent administration up to week 8. A 5-minute rest is required between the use of each device. Participants will then be monitored for the subsequent 2 hours for possible adverse events. Vital parameters will be checked 40 minutes and 2 hours after the administration of the last device.
During the 8-week study period, participants will be treated with standard oral antidepressant treatment as appropriate, according to the judgment of the prescribing physician.
Adverse reactions will be monitored throughout the study using the Antidepressant Side-Effect Checklist (ASEC),23 which measures the occurrence and severity of 21 potential adverse events of antidepressant drugs, along with clinical observation for at least 2 hours after administration. Any reasons for treatment discontinuation (including the occurrence of side effects or serious adverse effects) will be recorded.
Data Collection and Assessments
Data will be collected and anonymously included in an appropriate dataset, not allowing patient identification, following standard ethical requirements. Standard socio-demographic characteristics (e.g., age, gender, ethnicity, education, employment, housing, marital status, ongoing medical treatments), along with clinical information, will be retrieved from interviews, electronic charts, and clinical records.
A thorough psychometric assessment and side effects evaluation will be carried out using several appropriate tools. All of the following scales will be administered at each drug administration session both before and after esketamine nasal spray administration to evaluate the severity of symptoms at baseline and follow-up visits:
the MADRS20 will be used to measure depressive symptoms;
the Young Mania Rating Scale (YMRS)24 will be used to assess possible emerging manic symptoms;
the 4-item positive symptom subscale from the Brief Psychiatric Rating Scale (BPRS+)25 will be used to assess possible emerging psychotic symptoms;
the Generalized Anxiety Disorder 7-item (GAD-7) Scale26 will be used to measure anxiety symptoms;
the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S)27 will be used to assess the level of sedation;
the Clinician Administered Dissociative States Scale (CADSS)28 will be used to measure possible symptoms of esketamine-induced dissociation;
the Columbia–Suicide Severity Rating Scale (C-SSRS)29 will be used to assess suicidal behaviors;
the Clinical Global Impression - Severity and Improvement (CGI-S and CGI-I)30 will be used to measure the global severity and improvement of the illness based on the participant’s history, psychosocial circumstances, symptoms, behavior, and the impact of the symptoms on functioning.
Moreover, functional evaluations will be conducted at baseline as well as at the end of the induction phase (week 4) and the end of the maintenance phase (week 8) using:
the EuroQol - 5 Dimension - 3 Level (EQ-5D-3L)31 to measure the reported quality of life;
the Global Assessment of Functioning (GAF), published for the first time within the DSM-IV-TR,32 to subjectively assess social, occupational, and psychological functioning;
the Short Blessed Test (SBT)33 to evaluate orientation, registration, and attention.
The psychometric and functional assessment schedule during the 8-week observation period is reported in Table 1.
Table 1.
Psychometric and Functional Assessment Schedule
| Baseline | Endpoint 1 |
Endpoint 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 |
Day 4 |
Day 8 |
Day 11 |
Day 15 |
Day 18 |
Day 22 |
Day 25 |
Day 29 |
Day 36 |
Day 43 |
Day 50 |
Day 57 |
||
| Psychometric assessments | MADRSb | X | X | X | X | X | X | X | X | X | X | X | X | X |
| YMRSa | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| BPRS+a | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| GAD-7b | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| MOAA/Sa | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| CADSSa | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| C-SSRSa | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| CGI-Sb | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| CGI-Ib | X§ | X | X | X | X | X | X | X | X | X | X | X | X | |
| Functional assessments | EQ-5D-3Lc | X | X | X | ||||||||||
| GAFc | X | X | X | |||||||||||
| SBTc | X | X | X | |||||||||||
BPRS+ = BPRS 4-item positive symptom subscale; CADSS = Clinician Administered Dissociative States Scale; CGI-I = Clinical Global Impression - Improvement; CGI-S = Clinical Global Impression - Severity; C-SSRS = Columbia–Suicide Severity Rating Scale; EQ-5D-3L = EuroQol - 5 Dimension - 3 Level; GAD-7 = Generalized Anxiety Disorder 7-item; GAF = Global Assessment of Functioning; MADRS = Montgomery-Åsberg Depression Rating Scale; MOAA/S = Modified Observer’s Assessment of Alertness/Sedation; SBT = Short Blessed Test; YMRS = Young Mania Rating Scale.
aat each visit, before administration, after 40 minutes, and after 2 hours.
bat each visit, before administration and after 2 hours.
cat each visit, before administration.
§Only after 2 hours.
Physical Examination
Height and weight will be used to estimate Body Mass Index (BMI) at each visit, while hip circumference and waist circumference will be measured to calculate waist-to-hip ratio (WHR). Resting blood pressure, heart rate, and blood oxygen saturation will be monitored before and after each administration. Routine blood tests and an electrocardiogram (ECG) will be performed before starting the treatment with esketamine nasal spray as per standard clinical practice.
Blood Sampling and Metabolomics Assessment
Peripheral venous blood samples for metabolomics assessments will be collected at baseline (Day 1), before the first administration of esketamine nasal spray. Blood will be drawn in the morning after an overnight fast, using vacutainer blood collection tubes with serum clot activator. To ensure the reliability and accuracy of measurements, trained personnel will perform standardized pre-analytical processing immediately after the drawing. All vacutainers will be inverted 5 times immediately after blood drawing to mix the clot activator with the whole blood. Next, tubes will stand for 30 minutes at room temperature, then they will be centrifuged for 15 minutes at 2000 × g at room temperature to separate serum from blood cells. Following this, microtubes containing 150 μL aliquots of serum will be prepared, labeled, and stored at −80 °C at each center until metabolomics analyses. Temperatures will be monitored throughout the entire procedures.15
Serum aliquots will be analyzed by the UNIMIB Mass Spectrometry Platform – Proteomics and Metabolomics to perform untargeted metabolomics assessments. Serum guarantees higher sensitivity for biomarker discovery studies due to the higher concentration of metabolites.15 Metabolic profiling will be completed on serum samples coupling liquid chromatography (LC) with high-resolution mass spectrometry (MS) by using the 6546 LC-Q/TOF (Agilent, Santa Clara, CA, USA) mass spectrometer. The LC-MS-based metabolomics workflow will be implemented by focusing on small polar metabolites and using hydrophilic interaction liquid chromatography (HILIC) as a chromatographic strategy.34
A flow diagram of study procedures and the schedule of clinical, psychometric, functional, and physical assessments is shown in Figure 1.
Figure 1.
Flow diagram of study procedures and assessment schedule.
Statistical Analysis Plan
Standard statistics will be computed for descriptive purposes. With the aim of exploring the potential relationship between the metabolomic profile and clinical response to treatment, response is defined as a reduction of ≥ 50% in MADRS scores from baseline to endpoint,5 considering the 2 study endpoints, i.e., the end of the induction phase (week 4) and the end of the maintenance phase (week 8). In particular, we will carry out 2 sets of analyses. First, we will perform a correlation analysis (on the basis of parametric and non-parametric standards) to test whether longitudinal changes in MADRS scores (i.e., post- vs. pre-treatment with esketamine) are correlated with the multidimensional metabolome profile at baseline, considering relevant covariates such as age, gender, BMI, and scores in other psychometric scales. Second, the potential predictive value of metabolomic patterns for treatment response (responders vs. non-responders) will be examined also by exploiting machine learning algorithms by comparing different classifiers (e.g., generalized linear model/logistic regression, random forest, support vector machines) to test classification models.
Due to possible attrition bias, both per-protocol analyses (differences between responders and non-responders including only those participants who completed treatment with esketamine during the observation period) and intention-to-treat analyses (differences between responders and non-responders considering all recruited participants) based on the last reported observation (LOCF) will be considered. However, given some intrinsic limitations, if the assumptions do not seem plausible in the relevant clinical context, the analysis will be supplemented with available-case methods as opposed to ad-hoc imputation through the use of mixed models. Given the high-dimensional and possibly multicollinear nature of untargeted datasets, the threshold for statistical significance will be set at P < .01. In addition, in order to account for multiple testing and to limit the likelihood of false positives, the False Discovery Rate (FDR) correction will also be computed through the use of the Benjamini-Hochberg adjustment method.35
All analyses will be carried out using Stata statistical software package release 18 for Windows (StataCorp 2023, College Station, TX, USA) and the Python programming language release 3.9 (Python Software Foundation. Python Language Reference. Available at http://www.python.org).
Expected Results
The main results to be achieved encompass the identification of possible correlations between several metabolic pathways and MADRS score improvement, as well as metabolomics patterns in esketamine responders compared with non-responders. Consistently with the existing literature in this field, we hypothesize an involvement of energy metabolism (with changes in the levels of compounds involved in the tricarboxylic acid cycle, glycolysis, and the pentose phosphate pathway, as well as in the concentrations of energy equivalents), amino acid metabolism, the urea cycle, and nitric oxide synthesis (with a possible role of the bioavailability of arginine) in the response to esketamine nasal spray.11-13
Discussion
To date, the only study that has tried to relate the baseline metabolome of subjects with TRD to the response to esketamine treatment did not find any baseline metabolite to be associated with treatment response.13 Nonetheless, studies assessing pre-/post-treatment differences in the metabolome of people with TRD have speculated on the involvement of a number of metabolic pathways,11-13 suggesting a possible influence of treatment with esketamine on mitochondrial and cellular energy production, membrane homeostasis, neurotransmission, and signalling.36
This is the first independent, non-sponsored study to use metabolomics methods to investigate biological mechanisms underlying the antidepressant action of esketamine and to assess predictors of response to treatment. Untargeted metabolomics, representing an unbiased source of information, will allow us to capture a broad spectrum of metabolites that might help identify potential biomarkers or pathways associated with response to treatment with esketamine. Thorough psychometric and functional assessments will serve as clinical complements to metabolomics analyses and treatment follow-up, helping to integrate biological and clinical data. Unlike previous metabolomics studies in people with TRD administered with ketamine and esketamine, which were limited to a single ketamine or esketamine intravenous infusion and very short follow-up periods,36 our research will benefit from prolonged treatment with esketamine nasal spray and a long follow-up period, allowing for several clinical assessments at different time points as well as the evaluation of correlates of sustained treatment response.
However, some limitations should be acknowledged. Indeed, our study design does not include healthy controls, who would have provided additional information in comparison to participants with TRD. Second, the use of a single-point laboratory assessment will limit the use of metabolomics data as predictors of treatment response, not allowing for a more comprehensive study of dynamic processes and potential changes in the metabolome influenced by treatment with esketamine. Moreover, the piloting nature of our study and the insufficient prior knowledge on the topic prevent us from relying on solid hypotheses and may make it challenging to interpret results or draw meaningful conclusions. Nonetheless, our findings may serve as a foundation for informing and designing future hypothesis-driven studies.
The ReDREAM study may thus help clarify individual differences in biological patterns that distinguish people with TRD who respond to esketamine nasal spray from those who do not, ultimately shedding ultimately light on the mechanisms of the antidepressant action of esketamine. This would contribute to the rapid identification of people with TRD for whom treatment with esketamine would be helpful, moving towards a personalized management of TRD and leading to improved treatment patterns for this vulnerable population.
Acknowledgments:
We thank Tommaso Callovini (Catholic University of the Sacred Heart) for his contribution to the development of this study protocol. We also thank Paolo Fabbrini, Stefano Pastori, and Davide Zenoni (Nord-Milano Community Health and Social Care Trust) for sharing their expertise, resources, and technical assistance.
Availability of Data and Materials:
Not applicable.
Funding Statement
This study is funded by the Italian Ministry of Universities and Research (MUR) as a Relevant Research of National Interest (PRIN – Progetto di Rilevante Interesse Nazionale) – 2022 call – Prot. 2022C7AL7F.
Footnotes
Ethics Committee Approval: This study has been approved by “Comitato Etico Territoriale Lombardia 3” (Milan, Italy) ethics committee (approval number: 3910_S_N, date: 13 December 2023).
Informed Consent: Written informed consent will be obtained from all participants prior to their participation in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – F.B., D.C.; Design – F.B., D.C.; Supervision – F.B., U.A., P.D.F., G.C.; Resources – F.B., D.C., I.R., R.d.F., R.Z.C., G.P., U.A., P.D.F.; Materials – F.B., D.C., I.R., R.d.F., R.Z.C.; Data Collection and/or Processing – D.C., I.R., C.C., R.d.F., R.Z.C.; Analysis and/or Interpretation – C.C., U.A., P.D.F., G.C.; Literature Search – F.B., D.C.; Writing – F.B., D.C.; Critical Review – I.R., C.C., R.d.F., R.Z.C., G.P., U.A., P.D.F., G.C.
Declaration of Interests: Francesco Bartoli is Editor-in-Chief of Alpha Psychiatry and was not involved in the processing of this article. In the last three years, he has received consultant fees by IQVIA Solutions Italy and Edra S.p.A., and carried out paid editorial activities for Elsevier and AVES. In the last three years Renato de Filippis has received speaker fee from Janssen, Angelini, Lundbeck, and Rovi, and travel support from Rovi, Otsuka, Lundbeck, and Janssen. In the last three years Umberto Albert received speaker fee from Angelini Pharma, Janssen Pharmaceutica, OM Pharma Suisse, Lundbeck, Fidia, and Boehringer. The other authors declare no conflicts of interest.
References
- 1. Rosson S, de Filippis R, Croatto G, et al. Brain stimulation and other biological non-pharmacological interventions in mental disorders: an umbrella review. Neurosci Biobehav Rev. 2022;139:104743. ( 10.1016/j.neubiorev.2022.104743) [DOI] [PubMed] [Google Scholar]
- 2. Maj M, Stein DJ, Parker G, et al. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry. 2020;19(3):269 293. ( 10.1002/wps.20771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsugiyama LE, Moraes RCM, Moraes YAC, Francis-Oliveira J. Promising new pharmacological targets for depression: the search for efficacy. Drug Discov Today. 2023;28(12):103804. ( 10.1016/j.drudis.2023.103804) [DOI] [PubMed] [Google Scholar]
- 4. Maina G, Adami M, Ascione G, et al. Nationwide consensus on the clinical management of treatment-resistant depression in Italy: a Delphi panel. Ann Gen Psychiatry. 2023;22(1):48. ( 10.1186/s12991-023-00478-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McIntyre RS, Alsuwaidan M, Baune BT, et al. Treatment-resistant depression: definition, prevalence, detection, management, and investigational interventions. World Psychiatry. 2023;22(3):394 412. ( 10.1002/wps.21120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bartoli F, Wlkinson ST. Ketamine and esketamine for suicidal ideation: recent progress and practical issues. Aust N Z J Psychiatry. 2020;54(2):206 207. ( 10.1177/0004867419894064) [DOI] [PubMed] [Google Scholar]
- 7. Bartoli F, Clerici M, Carrà G. Antidepressant response and dissociative effects after ketamine treatment: two sides of the same coin? J Clin Psychiatry. 2017;78(9):e1318. ( 10.4088/JCP.17lr11789) [DOI] [PubMed] [Google Scholar]
- 8. Bartoli F, Riboldi I, Crocamo C, Di Brita C, Clerici M, Carrà G. Ketamine as a rapid-acting agent for suicidal ideation: A meta-analysis. Neurosci Biobehav Rev. 2017;77:232 236. ( 10.1016/j.neubiorev.2017.03.010) [DOI] [PubMed] [Google Scholar]
- 9. Mahase E. Esketamine is approved in Europe for treating resistant major depressive disorder. BMJ. 2019;367:l7069. ( 10.1136/bmj.l7069) [DOI] [PubMed] [Google Scholar]
- 10. Estrade I, Petit AC, Sylvestre V, et al. Early effects predict trajectories of response to esketamine in treatment-resistant depression. J Affect Disord. 2023;342:166 176. ( 10.1016/j.jad.2023.09.030) [DOI] [PubMed] [Google Scholar]
- 11. Singh B, MahmoudianDehkordi S, Voort JLV, et al. Metabolomic signatures of intravenous racemic ketamine associated remission in treatment-resistant depression: A pilot hypothesis generating study. Psychiatry Res. 2022;314:114655. ( 10.1016/j.psychres.2022.114655) [DOI] [PubMed] [Google Scholar]
- 12. Moaddel R, Shardell M, Khadeer M, et al. Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacol (Berl). 2018;235(10):3017 3030. ( 10.1007/s00213-018-4992-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rotroff DM, Corum DG, Motsinger-Reif A, et al. Metabolomic signatures of drug response phenotypes for ketamine and esketamine in subjects with refractory major depressive disorder: new mechanistic insights for rapid acting antidepressants. Transl Psychiatry. 2016;6(9):e894. ( 10.1038/tp.2016.145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villaseñor A, Ramamoorthy A, Silva dos Santos M, et al. A pilot study of plasma metabolomic patterns from patients treated with ketamine for bipolar depression: evidence for a response-related difference in mitochondrial networks. Br J Pharmacol. 2014;171(8):2230 2242. ( 10.1111/bph.12494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paglia G, Del Greco FM, Sigurdsson BB, et al. Influence of collection tubes during quantitative targeted metabolomics studies in human blood samples. Clin Chim Acta. 2018;486:320 328. ( 10.1016/j.cca.2018.08.014) [DOI] [PubMed] [Google Scholar]
- 16. Paglia G, Stocchero M, Cacciatore S, et al. Unbiased metabolomic investigation of Alzheimer’s disease brain points to dysregulation of mitochondrial aspartate metabolism. J Proteome Res. 2016;15(2):608 618. ( 10.1021/acs.jproteome.5b01020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453 1457. ( 10.1016/S0140-6736(07)61602-X) [DOI] [PubMed] [Google Scholar]
- 18. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington: American Psychiatric Association; 2013. [Google Scholar]
- 19. First MB, Williams JBW, Karg RS, Spitzer RL. Structured clinical interview for DSM-5 – Research version (SCID-5 for DSM-5, Research version; SCID-5-RV). American Psychiatric Association; 2015. [Google Scholar]
- 20. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382 389. ( 10.1192/bjp.134.4.382) [DOI] [PubMed] [Google Scholar]
- 21. Herrmann N, Black SE, Lawrence J, Szekely C, Szalai JP. The Sunnybrook Stroke Study: a prospective study of depressive symptoms and functional outcome. Stroke. 1998;29(3):618 624. ( 10.1161/01.str.29.3.618) [DOI] [PubMed] [Google Scholar]
- 22. Chandler GM, Iosifescu DV, Pollack MH, Targum SD, Fava M. Research: validation of the Massachusetts General Hospital antidepressant treatment history questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322 325. ( 10.1111/j.1755-5949.2009.00102.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uher R, Farmer A, Henigsberg N, et al. Adverse reactions to antidepressants [published correction appears in Br J Psychiatry. 2010 May;196(5):417]. Br J Psychiatry. 2009;195(3):202 210. ( 10.1192/bjp.bp.108.061960) [DOI] [PubMed] [Google Scholar]
- 24. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429 435. ( 10.1192/bjp.133.5.429) [DOI] [PubMed] [Google Scholar]
- 25. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):799 812. ( 10.2466/pr0.1962.10.3.799) [DOI] [Google Scholar]
- 26. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092 1097. ( 10.1001/archinte.166.10.1092) [DOI] [PubMed] [Google Scholar]
- 27. Kim TK, Niklewski PJ, Martin JF, Obara S, Egan TD. Enhancing a sedation score to include truly noxious stimulation: the Extended Observer’s Assessment of Alertness and Sedation (EOAA/S). Br J Anaesth. 2015;115(4):569 577. ( 10.1093/bja/aev306) [DOI] [PubMed] [Google Scholar]
- 28. Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress. 1998;11(1):125 136. ( 10.1023/A:1024465317902) [DOI] [PubMed] [Google Scholar]
- 29. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266 1277. ( 10.1176/appi.ajp.2011.10111704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28 37. [PMC free article] [PubMed] [Google Scholar]
- 31. Scalone L, Cortesi PA, Mantovani LG, Ciampichini R, Cesana G. Reference Eq-5d-3l and Eq-5d-5l data from the Italian general population. Value Health. 2014;17(7):A514 A515. ( 10.1016/j.jval.2014.08.1591) [DOI] [PubMed] [Google Scholar]
- 32. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev. Arlington: American Psychiatric Association; 2000. [Google Scholar]
- 33. Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140(6):734 739. ( 10.1176/ajp.140.6.734) [DOI] [PubMed] [Google Scholar]
- 34. Dünnwald T, Paglia G, Weiss G, et al. High intensity concentric-eccentric exercise under hypoxia changes the blood metabolome of trained athletes. Front Physiol. 2022;13:904618. ( 10.3389/fphys.2022.904618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289 300. ( 10.1111/j.2517-6161.1995.tb02031.x) [DOI] [Google Scholar]
- 36. Cavaleri D, Riboldi I, Crocamo C, Paglia G, Carrà G, Bartoli F. Evidence from preclinical and clinical metabolomics studies on the antidepressant effects of ketamine and esketamine. Neurosci Lett. 2024;831:137791. ( 10.1016/j.neulet.2024.137791) [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.



 Content of this journal is licensed under a
Content of this journal is licensed under a