Abstract
Background
The combined effects of metformin and epigallocatechin-3-gallate (EGCG) on cortisol, 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), and blood glucose levels have not been investigated. This study evaluated the effectiveness of combining EGCG with metformin in regulating those levels in a rat model of diet-induced diabetes and obesity.
Methods
Thirty diabetic and obese rats on a high-fat diet were treated daily for 28 days with EGCG (100 mg/kg of body weight/day), metformin (200 mg/kg of body weight/day), or both. Control groups comprised lean rats, untreated obese diabetic rats, and metformin-only-treated rats. Blood samples were collected to measure cortisol and fasting blood glucose (FBG) levels and liver tissue samples were examined for 11β-HSD1 levels.
Results
Rats receiving combination therapy had significantly reduced cortisol levels (from 36.70±15.13 to 31.25±7.10 ng/mL) compared with the untreated obese diabetic rats but not the rats receiving monotherapy. Rats receiving combination therapy and EGCG monotherapy had significantly lower 11β-HSD1 levels compared with the untreated obese diabetic rats (92.68±10.82 and 93.74±18.11 ng/L vs. 120.66±14.00 ng/L). Combination therapy and metformin monotherapy significantly reduced FBG levels (440.83±133.39 to 140.50±7.36 mg/dL and 480.67±86.32 to 214.17±102.78 mg/dL, respectively) by approximately 68.1% and 55.4% compared with rats receiving EGCG monotherapy and untreated obese diabetic rats.
Conclusion
Combining EGCG with metformin exhibited synergistic effects compared with monotherapy for managing diabetes, leading to improved outcomes in reduction of baseline cortisol levels along with reduction in 11β-HSD1 and blood glucose levels.
Keywords: Epigallocatechin gallate, Hydrocortisone, 11-Beta-hydroxysteroid dehydrogenase type 1, Diabetes mellitus, Obesity, Blood glucose
INTRODUCTION
A factor contributing to the development of type 2 diabetes mellitus (T2DM) is the increased levels of cortisol in individuals with obesity. Subjects who are obese tend to have higher cortisol levels compared with individuals with a normal body mass index.1 An important function of cortisol is to activate gluconeogenesis in the liver, contributing to hyperglycemia.2 The excess cortisol contributes to elevated blood glucose levels and insulin resistance.3 The enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) is a key cortisol regulator in tissues and is responsible for converting inactive cortisone into its active form, cortisol.4 Therefore, targeting the 11β-HSD1 enzyme to reduce cortisol levels has become an important therapeutic approach in managing diabetes mellitus.5
Among the various studies in which the therapeutic effects of 11β-HSD1 inhibitor were investigated, green tea extract has been a subject of interest due to its potential benefits in obesity and diabetes management. Green tea extract exhibits anti-inflammatory properties, aids in weight reduction, helps control glucose levels, and improves lipid metabolism.6 One specific component in green tea extract is epigallocatechin-3-gallate (EGCG), which is the most abundant catechin present in green tea.7 Although limited, existing studies in which EGCG was evaluated as an 11β-HSD1 inhibitor have shown promising results. EGCG shows the strongest inhibitory activity against the 11β-HSD1 enzyme compared with other catechins.8,9 Results of in vitro studies have indicated that EGCG inhibits cortisol production by inducing a shift from nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) to nicotinamide adenine dinucleotide phosphate (NADP), effectively hindering 11β-HSD1 activity.8
Metformin remains the first-line therapy for patients diagnosed with T2DM10 and has proven effective in preventing the onset of T2DM in high-risk individuals.11 Metformin exerts its effects by inhibiting glucose production in the liver, promoting glucose utilization by tissues, reducing glucose absorption in the intestines, and increasing glucose uptake.12 However, long-term monotherapy with metformin becomes less effective as the disease progresses, necessitating combination therapy to achieve optimal glycemic control. Combining metformin with other antidiabetic agents has been shown to improve metabolic parameters and blood glucose control more effectively than metformin monotherapy.13 In a previous study, results indicated that metformin effectively reduces the level of glucose 6-phosphate (G6P)14 that is utilized by hexose-6-phosphate dehydrogenase (H6PD) in the endoplasmic reticulum (ER) to generate NADPH, a cofactor used by 11β-HSD1 in converting cortisone to cortisol.15,16 In another study with different findings, metformin increased cortisol regeneration. Therefore, combining metformin with 11β-HSD1 inhibitors may yield better outcomes in terms of glycemic control for individuals with T2DM.17
To date, the combined effects of metformin and EGCG on cortisol, 11β-HSD1, and blood glucose levels have not been investigated. Hence, this research aimed to evaluate the effectiveness of this combination therapy.
METHODS
The animal handling procedures in this study followed the guidelines from the Institutional Review Board and Institutional Animal Care and Use Committee (IACUC) of Diponegoro University. The Health Research Ethics Committee of the Faculty of Medicine at Diponegoro University approved all procedures of this study (No. 110/EC/H/FNGUNDIP/IX/2022).
Chemicals
Metformin was purchased from Bernofarm Pharmaceutical Company. EGCG (98% purity) was purchased from Arisun ChemPharm Co. Ltd. All other chemicals were obtained from standard sources with the highest purity available.
Animals
Sample size for animal experiments was based on the World Health Organization General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine.18 Thirty male Sprague Dawley rats, 10 weeks of age and weighing between 175 and 200 g, were purchased from Kemuning (CV Dunia Kaca). Using only male rats in this study minimized biases associated with the hormonal cycles of female rats, ensuring a more consistent experimental environment. Because the research on gender-related similarities in outcomes for diabetes and obesity is limited, this approach aids in controlling hormonal variability, enabling focused investigations.
The rats were allowed to acclimate to their new environment for one week, during which the animals had continuous access to water, were fed the standard diet BR 594 (containing 21% protein, 5% fiber, and 5% fat), and were housed under controlled conditions with a 10-hour light and 14-hour dark cycle (7:00 AM to 5:00 PM).
Experiment protocols
After the acclimation period, the rats were randomly divided into five groups for the experiment (n=6). Throughout the study, all rats had continuous access to water. The first group, the lean group, received standard diet BR 594. The other four groups were fed a high-fat (HF) diet composed of 60% standard diet BR 594, 20% cow fat, and 20% egg yolk. This diet was maintained for 7 weeks to induce obesity, which was confirmed using the Lee index formula.
The weight and nose-anus length of each rat was assessed before and after the 7-week preparation period. Using the formula ∛(body weight in g/nose-anus length in cm)×1,000, the Lee obesity index was calculated. A Lee index result >300 was indicative of obesity.19
Before starting the experiments, diabetes was induced in the HF diet-fed rats by intravenous injection of streptozotocin (STZ) at a dose of 40 mg/kg under ether anesthesia, administered through the tail vein. The STZ was dissolved in citrate buffer (pH 4.5). Diabetes induction was confirmed by measuring fasting blood glucose (FBG) levels, which had to exceed 200 mg/dL at three days after STZ injection. Cortisol levels were measured in all five groups before the initiation of treatment.
The confirmed diabetic rats were subjected to various treatment regimens with an HF diet for 28 days. The four treatment groups were as follows: the diabetes and obesity baseline was considered the negative control (NC) group, in which rats received an oral gavage of saline solution at a concentration of 0.9% body weight/volume; and the positive control (PC) group included rats receiving metformin monotherapy administered orally once a day at a dosage of 200 mg/kg of body weight. The EGCG group included rats treated with EGCG monotherapy, receiving an oral dose of EGCG once daily at 100 mg/kg of body weight; and the combination therapy group included rats administered both metformin and EGCG, with respective doses of 200 mg/kg of body weight and 100 mg/kg of body weight once a day via oral gavage.
After 28 days of treatment, FBG and cortisol levels were measured before the rats were sacrificed for liver extraction at days 29 and 30, respectively.
Measurement of metabolic parameters
The FBG measurements were performed before and after the experiments by collecting blood samples from the tail vein after a fasting period. The procedure involved cutting a small portion of the rat’s tail, discarding the first drop of blood, and applying subsequent drops to the Glucose Nesco Multicheck 1 Glucose Test Strips (Bioptik Technology Inc.). The test strips were then inserted into the Nesco GCU-Nesco Multicheck 3 in 1 (GCU/N-01; Bioptik Technology Inc.).
Serum cortisol levels were measured using the rat cortisol enzyme-linked immunosorbent assay (ELISA) kit (PT Bioenzy; Catalog No. BZ-08188280-EB) in a 96-well plate format. Blood samples for cortisol analysis were collected from the orbital sinus of the rats in the morning, specifically between 7:00 AM and 8:00 AM, following the methods used in previous studies.20,21 Pre- and post-treatment samples were collected to measure cortisol levels, with a 1-day interval after the pre- and post-test FBG examinations to minimize potential biases.
To measure the 11β-HSD1 level, the rat 11β-HSD1 ELISA kit (PT Bioenzy; Catalog No. BZ-08188291-EB) was utilized in a 96-well plate format. The measurements were conducted on liver tissues adapted from previous research.22 The rats were euthanized using chloroform inhalation before the liver samples were collected.
Statistical analysis
Results were analyzed using IBM SPSS Statistic version 27 (IBM Co.), and data were expressed as mean±standard deviation. Statistical analyses were performed using analysis of variance (ANOVA) followed by post hoc Tukey’s HSD test. Paired sample t-test and Wilcoxon test were used to compare the variables before and after the treatment. Groups of data were considered significantly different if P<0.05.
RESULTS
All rats in the NC, PC, EGCG, and combination therapy groups showed a Lee index >300 and FBG >200 mg/dL, confirming successful obesity and diabetes induction before the start of the treatment. Rat mortalities did not occur during the entire study period.
Cortisol
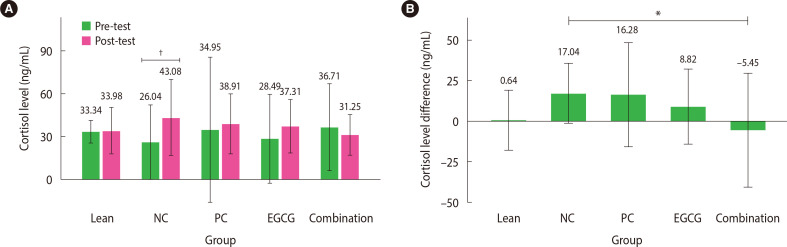
A paired t-test was performed for each group to compare the cortisol levels before and after the treatment. The results revealed a significant increase in cortisol levels of approximately 65% in the NC group (from 26.04±12.99 to 43.08±13.33 ng/mL; P<0.01; 95% confidence interval [CI], –26.72 to –7.36). However, significant differences were not observed in the remaining groups, including the combination therapy group, despite a decrease in cortisol levels (from 36.71±15.13 ng/mL to 31.25±7.10; 95% CI, –12.93 to 23.84) (Fig. 1A). The parametric one-way ANOVA test was performed for differences (pre- and post-treatment) in cortisol level data while excluding outliers in the PC group (n=5). The results indicated significant differences in the groups (P<0.05). Further post hoc Tukey’s analysis revealed a significant reduction in cortisol levels in the combination therapy group compared with elevated cortisol levels in the NC group (−5.45±17.52 ng/mL vs. 17.04±9.22 ng/mL; P<0.05; 95% CI, –44.66 to –0.34) (Fig. 1B). Significant difference was not found in cortisol levels between the other therapy groups (PC and EGCG) and the NC group. In addition, significant cortisol level differences were not observed among the therapy groups (PC, EGCG, and combination).
Figure 1.
Mean cortisol level. (A) The mean cortisol levels before and after the test are reported as mean±2 standard deviation (SD; n=6 rats). (B) The mean difference in cortisol levels between pre- and post-test without outliers is presented as mean±2 SD (n=6 rats; except for n in the positive control group=5 rats). *P<0.05; †P<0.01. NC, negative control; PC, positive control; EGCG, epigallocatechin-3-gallate.
11β-HSD1
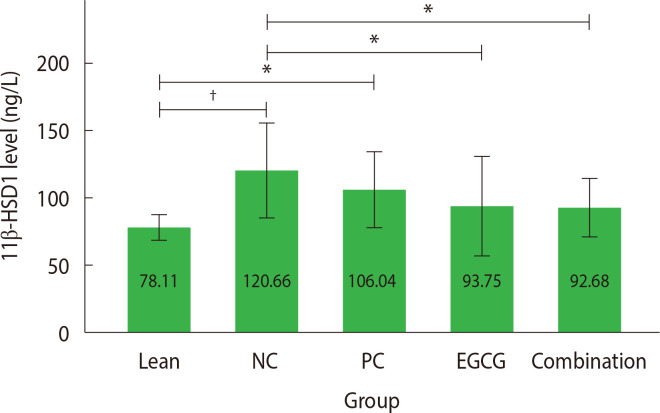
The analysis of 11β-HSD1 levels was performed on liver tissue after sacrificing all rats in the groups, resulting in data available only from the post-test. The parametric one-way ANOVA test results indicated significant differences between groups (P<0.05).
The post hoc Tukey’s test (Fig. 2) showed significantly higher 11β-HSD1 levels after the 28-day treatment in both the NC (P<0.001; 95% CI, −66.17 to −18.93) and the PC groups (P<0.05; 95% CI, −51.55 to −4.31) compared with the lean group (120.66±17.54 and 106.04±14.00 ng/L vs. 78.11±4.63 ng/L) although the mean value in the PC group was lower than in the NC group. Significant differences were not found between the EGCG and combination therapy groups compared with the lean group (P>0.05; 95% CI [−39.25 to 7.98] and [−38.19 to 9.05] respectively). In contrast, the combination therapy and EGCG groups showed significantly lower 11β-HSD1 levels compared with the NC group (92.68±10.82 and 93.74±18.11 ng/L vs. 120.66±14.00 ng/L; P<0.05; 95% CI [4.36 to 51.60] and [3.29 to 50.54] respectively). Significant differences were not observed between the PC and NC groups (P>0.05; 95% CI, −8.99 to 38.24). Significant differences were also not found among the treatment groups (PC, EGCG, and combination therapy).
Figure 2.
Mean 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) level. The mean 11β-HSD1 levels were analyzed and presented as mean±2 standard deviation (n=6 rats). *P<0.05; †P<0.001. NC, negative control; PC, positive control; EGCG, epigallocatechin-3-gallate.
FBG
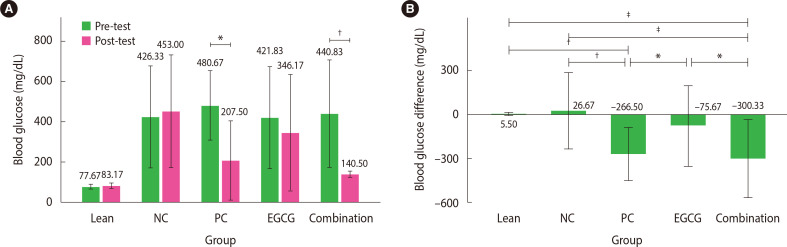
The paired t-test conducted in each group to compare pre-test and post-test FBG levels revealed significant differences in the combination therapy group (P<0.01; 95% CI, 160.10 to 440.57). In the PC group, the Wilcoxon test showed similar results (P<0.05) (Fig. 3A). However, the other groups did not show significant differences. Furthermore, the parametric one-way ANOVA on the FBG data indicated groups with significant differences (P<0.05).
Figure 3.
Mean fasting blood glucose (FBG) level. (A) Mean FBG levels were compared pre- and post-test in each group. The values are presented as mean±2 standard deviation (SD; n=6 rats). (B) The mean difference in FBG levels pre- and post-test was analyzed. The values are presented as mean±2 SD (n=6 rats). *P<0.05; †P<0.01; ‡P<0.001. NC, negative control; PC, positive control; EGCG, epigallocatechin-3-gallate.
The combination therapy group showed a significantly reduced (68.1%) FBG (440.83±133.39 to 140.50±7.36 mg/dL) compared with the NC group (P<0.001; 95% CI, −515.85 to −138.15) and the EGCG group (P<0.05, 95% CI, −413.52 to −35.81) based on the post hoc Tukey’s test (Fig. 3B). In addition, the PC group showed a significant (55.4%) decrease (480.67±86.32 to 214.17±102.78 mg/dL) in FBG compared with the EGCG group (P<0.05; 95% CI, −379.69 to −1.98) and the NC group (P<0.01; 95% CI, −482.02 to −104.31). Significant difference was not observed in the reduction of FBG between the PC and combination therapy groups. However, although the FBG decreased by 17.9% in the EGCG group, the difference was not significant compared with the other therapy groups (P>0.05).
DISCUSSION
EGCG reduces insulin resistance23 and inhibits cortisol production by shifting from NADPH to NADP+ in the ER lumen, inhibiting 11β-HSD1 activity and converting cortisone to cortisol.8 Metformin improves glycemic control through various mechanisms.12 However, as the disease progresses, monotherapy loses effectiveness after a few years, necessitating combination therapy to maintain glycemic targets.10 In the present study, the effectiveness of combining EGCG and metformin in reducing cortisol, 11β-HSD1, and blood glucose levels in Sprague Dawley rats with obesity and diabetes compared with monotherapy was demonstrated.
The serum cortisol levels stimulated by adrenocorticotropic hormone (ACTH) secretion in the adenohypophysis after stimulation of hypothalamic corticotropin-releasing factor were analyzed.24 The cortisol present in circulation results from both adrenal gland secretion and the regeneration of cortisol in tissues through the action of 11β-HSD1 in the liver and adipose tissues.25,26 To align with previous studies in which T2DM was predicted based on evening cortisol levels in humans,27 serum samples were collected in the morning from rats, considering their nocturnal nature. The NC group showed a significant increase in cortisol levels, consistent with previous findings of elevated serum cortisol in untreated diabetes.28,29 This increase can be attributed to hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis, impaired negative feedback, and elevated blood glucose levels leading to cortisol hypersecretion.29,30
The combination therapy of metformin and EGCG demonstrated superior outcomes in reducing cortisol levels compared with monotherapy. Metformin improves glycemic control through multiple mechanisms, including inhibiting gluconeogenesis, stimulating glycolysis in the liver, reducing glucose absorption in the intestines, and enhancing glucose uptake in tissues.12 In addition, metformin activates AMP-activated protein kinase (AMPK), which suppresses proopiomelanocortin (POMC) and ACTH expression in the pituitary by inhibiting liver X receptor α (LXRα) phosphorylation, resulting in reduced cortisol levels.31 EGCG can also restore HPA axis function by regulating extracellular signal-regulated kinase 1/2 (ERK1/2) signaling and reducing cortisol levels by inhibiting ACTH and corticotropin-releasing hormone (CRH) under stress conditions.32,33 These processes indicate the synergistic action of metformin, activating AMPK and suppressing ACTH expression, along with EGCG regulating ERK1/2 signaling to inhibit ACTH increase, can lead to more effective reduction of cortisol levels compared with individual treatment approaches.
The 11β-HSD1 enzyme is highly abundant in metabolically active tissues, particularly in the liver and adipose tissues, where it converts cortisone to cortisol.2,4 In the present study, the 11β-HSD1 level in the liver was analyzed. The NC group exhibited elevated 11β-HSD1 levels compared with the lean group, consistent with previous research indicating increased 11β-HSD1 in individuals with obesity and metabolic syndrome.4,34 Both the combination therapy and EGCG monotherapy significantly reduced 11β-HSD1 levels. These findings are in agreement with previous studies in which EGCG was shown to inhibit cortisol production by inducing a shift from NADPH to NADP+ in the ER lumen, inhibiting 11β-HSD1 activity.8,9 In addition, EGCG hinders the conversion of cortisone to cortisol in intact microsomal vesicles by oxidizing NADPH8 and directly binding to the active site of 11β-HSD1, forming hydrogen bonds that compete with the binding of respective substrates and/or cofactors.9 Metformin does not affect the conversion of cortisone to cortisol in tissues.17 However, metformin was suggested to potentially reduce G6P levels,14 triggering the glucose-6-phosphate translocase (G6PT)-H6PD-11β-HSD1 catalytic triad in tissues.8,15 The combined effects of metformin and EGCG on these triad enzymes led to reduced 11β-HSD1 levels in the present study.
Both combination therapy and metformin monotherapy significantly reduced FBG levels. Metformin, a first-line anti-T2DM drug,10 effectively improved glycemic control35 by inhibiting gluconeogenesis, promoting glycolysis in the liver, reducing glucose absorption in the intestines, and enhancing glucose uptake in tissues.12 A decrease in FBG levels was also observed in the EGCG group, although statistical significance was not reached compared with the other therapy groups. This finding is in agreement with previous studies in which stronger anti-hyperglycemic effects of metformin were demonstrated.36,37 EGCG enhances glycogen synthesis, improves insulin signaling pathways, reduces insulin resistance by increasing lipid oxidation, and stimulates glucose uptake in skeletal muscle, enhancing insulin responsiveness and maximizing glucose uptake.23,38 The combination therapy of metformin and EGCG demonstrated superior effectiveness in reducing FBG levels, as previously shown in studies where EGCG was combined with other diabetes therapies.39
Although the results of the present study provide valuable insights into the efficacy of EGCG and metformin combination therapy on cortisol, 11β-HSD1, and blood glucose levels in obese and diabetic rats, limitations exist. Notably, male rats were exclusively utilized to mitigate bias, resulting in a modest sample size. Furthermore, due to financial constraints encountered during the study, evaluation of the homeostatic model assessment of insulin resistance (HOMA-IR) and lipid profiles was not performed. Due to the primary focus on cortisol and 11β-HSD1, resources were primarily directed toward these parameters, relegating the analysis of HOMA-IR and lipid profiles to a secondary consideration.
In previous studies, combining HOMA-IR assessment with lipid profile examination was shown to enable early metabolic syndrome detection.40 HOMA-IR, indicating insulin resistance, aids in understanding therapy response in diabetes and obesity. Integrating lipid profile parameters provides valuable insights due to the obesity-insulin resistance link. For future research, including both sexes and examining a broader array of parameters would be beneficial to facilitate a more comprehensive understanding of the effects of EGCG and metformin combination therapy on diabetes and obesity.
In summary, combining EGCG and metformin showed synergistic effects, resulting in better reduction of cortisol levels from baseline along with 11β-HSD1 and blood glucose levels compared with using either treatment alone. These findings support the potential of combination therapies for managing diabetes and related complications.
ACKNOWLEDGMENTS
The authors are grateful to the following individuals for their invaluable contributions to this project: Dr. Hardian for his insightful guidance and expertise in data analysis, Mrs. Debi Anatiasara for her invaluable assistance in the data collection process, and Mr. Sumardi for his generous support and assistance in animal care.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: DMA, NSW, and NM; acquisition of data: DMA; analysis and interpretation of data: DMA, MM, NS, and NM; drafting of the manuscript: DMA; critical revision of the manuscript: NSW, MM, NS, and NM; statistical analysis: DMA and NM; and study supervision: NM.
REFERENCES
- 1.Ganesan N, Priya J, Devi G. Estimation of cortisol in type II diabetes mellitus among South Indian population. Drug Invent Today. 2019;12:2074. [Google Scholar]
- 2.Almeida C, Monteiro C, Silvestre S. Inhibitors of 11β-hydroxy-steroid dehydrogenase type 1 as potential drugs for type 2 diabetes mellitus: a systematic review of clinical and in vivo preclinical studies. Sci Pharm. 2021;89:5. doi: 10.3390/scipharm89010005. [DOI] [Google Scholar]
- 3.Dias JP, Joseph JJ, Kluwe B, Zhao S, Shardell M, Seeman T, et al. The longitudinal association of changes in diurnal cortisol features with fasting glucose: MESA. Psychoneuroendocrinology. 2020;119:104698. doi: 10.1016/j.psyneuen.2020.104698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dammann C, Stapelfeld C, Maser E. Expression and activity of the cortisol-activating enzyme 11β-hydroxysteroid dehydrogenase type 1 is tissue and species-specific. Chem Biol Interact. 2019;303:57–61. doi: 10.1016/j.cbi.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Scheen AJ. Targeting the metabolic pathway of cortisol as a potential therapy of type 2 diabetes. Med Mal Metab. 2016;10:725–31. doi: 10.1016/S1957-2557(16)30211-5. [DOI] [Google Scholar]
- 6.Asbaghi O, Fouladvand F, Gonzalez MJ, Aghamohammadi V, Choghakhori R, Abbasnezhad A. The effect of green tea on C-reactive protein and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Complement Ther Med. 2019;46:210–6. doi: 10.1016/j.ctim.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Legeay S, Rodier M, Fillon L, Faure S, Clere N. Epigallocatechin gallate: a review of its beneficial properties to prevent metabolic syndrome. Nutrients. 2015;7:5443–68. doi: 10.3390/nu7075230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szelényi P, Révész K, Konta L, Tüttõ A, Mandl J, Kereszturi É, et al. Inhibition of microsomal cortisol production by (-)-epigallocatechin-3-gallate through a redox shift in the endoplasmic reticulum: a potential new target for treating obesity-related diseases. Biofactors. 2013;39:534–41. doi: 10.1002/biof.1095. [DOI] [PubMed] [Google Scholar]
- 9.Hintzpeter J, Stapelfeld C, Loerz C, Martin HJ, Maser E. Green tea and one of its constituents, epigallocatechine-3-gallate, are potent inhibitors of human 11β-hydroxysteroid dehydrogenase type 1. PLoS One. 2014;9:e84468. doi: 10.1371/journal.pone.0084468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Professional Practice Committee, author. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S125–43. doi: 10.2337/dc22-S009. [DOI] [PubMed] [Google Scholar]
- 11.Hostalek U, Campbell I. Metformin for diabetes prevention: update of the evidence base. Curr Med Res Opin. 2021;37:1705–17. doi: 10.1080/03007995.2021.1955667. [DOI] [PubMed] [Google Scholar]
- 12.Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother. 2020;131:110708. doi: 10.1016/j.biopha.2020.110708. [DOI] [PubMed] [Google Scholar]
- 13.Singh AK, Singh R, Chakraborty PP. Diabetes monotherapies versus metformin-based combination therapy for the treatment of type 2 diabetes. Int J Gen Med. 2021;14:3833–48. doi: 10.2147/IJGM.S295459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moonira T, Chachra SS, Ford BE, Marin S, Alshawi A, Adam-Primus NS, et al. Metformin lowers glucose 6-phosphate in hepatocytes by activation of glycolysis downstream of glucose phosphorylation. J Biol Chem. 2020;295:3330–46. doi: 10.1074/jbc.RA120.012533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czegle I, Csala M, Mandl J, Benedetti A, Karádi I, Bánhegyi G. G6PT-H6PDH-11βHSD1 triad in the liver and its implication in the pathomechanism of the metabolic syndrome. World J Hepatol. 2012;4:129–38. doi: 10.4254/wjh.v4.i4.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker EA, Ahmed A, Lavery GG, Tomlinson JW, Kim SY, Cooper MS, et al. 11Beta-hydroxysteroid dehydrogenase type 1 regulation by intracellular glucose 6-phosphate provides evidence for a novel link between glucose metabolism and hypothalamo-pituitary-adrenal axis function. J Biol Chem. 2007;282:27030–6. doi: 10.1074/jbc.M704144200. [DOI] [PubMed] [Google Scholar]
- 17.Anderson AJ, Andrew R, Homer NZ, Jones GC, Smith K, Livingstone DE, et al. Metformin increases cortisol regeneration by 11βHSD1 in obese men with and without type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101:3787–93. doi: 10.1210/jc.2016-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization, author. General guidelines for methodologies on research and evaluation of traditional medicine. World Health Organization; 2000. [Google Scholar]
- 19.Okechukwu GN, Ofobuike NB, Akunna GG, Nwafo JA, Ibegbu AO. Morphometric changes due to β carotene on Wistar rats fed dietary fat. J Middle East N Afr Sci. 2018;4:16–20. doi: 10.12816/0047907. [DOI] [Google Scholar]
- 20.Radahmadi M, Shadan F, Karimian SM, Sadr SS, Nasimi A. Effects of stress on exacerbation of diabetes mellitus, serum glucose and cortisol levels and body weight in rats. Pathophysiology. 2006;13:51–5. doi: 10.1016/j.pathophys.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Vélez-Marín M, Hurtado Salazar A, Uribe-Velásquez LF. Plasma cortisol activity in rats under conditions of chronic stress supplemented with resveratrol. Colomb Med (Cali) 2012;43:221–5. doi: 10.25100/cm.v43i3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupczyk D, Studzińska R, Bilski R, Woźniak A. Application of ELISA technique and human microsomes in the search for 11β-hydroxysteroid dehydrogenase inhibitors. Biomed Res Int. 2019;2019:5747436. doi: 10.1155/2019/5747436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casanova E, Salvadó J, Crescenti A, Gibert-Ramos A. Epigallocatechin gallate modulates muscle homeostasis in type 2 diabetes and obesity by targeting energetic and redox pathways: a narrative review. Int J Mol Sci. 2019;20:532. doi: 10.3390/ijms20030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kontoangelos K, Papageorgiou CC, Raptis AE, Tsiotra P, Lambadiari V, Papadimitriou GN, et al. Homocysteine, cortisol, diabetes mellitus, and psychopathology. J Diabetes Res. 2015;2015:354923. doi: 10.1155/2015/354923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harno E, White A. Will treating diabetes with 11β-HSD1 inhibitors affect the HPA axis? Trends Endocrinol Metab. 2010;21:619–27. doi: 10.1016/j.tem.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci. 2018;12:127. doi: 10.3389/fnbeh.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackett RA, Kivimäki M, Kumari M, Steptoe A. Diurnal cortisol patterns, future diabetes, and impaired glucose metabolism in the Whitehall II cohort study. J Clin Endocrinol Metab. 2016;101:619–25. doi: 10.1210/jc.2015-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosili P, Mkhize BC, Ngubane P, Sibiya N, Khathi A. The dysregulation of the hypothalamic-pituitary-adrenal axis in diet-induced prediabetic male Sprague Dawley rats. Nutr Metab (Lond) 2020;17:104. doi: 10.1186/s12986-020-00532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Si MW, Yang MK, Fu XD. Effect of hypothalamic-pituitary-adrenal axis alterations on glucose and lipid metabolism in diabetic rats. Genet Mol Res. 2015;14:9562–70. doi: 10.4238/2015.August.14.19. [DOI] [PubMed] [Google Scholar]
- 30.Diz-Chaves Y, Gil-Lozano M, Toba L, Fandiño J, Ogando H, González-Matías LC, et al. Stressing diabetes?: the hidden links between insulinotropic peptides and the HPA axis. J Endocrinol. 2016;230:R77–94. doi: 10.1530/JOE-16-0118. [DOI] [PubMed] [Google Scholar]
- 31.Cho K, Chung JY, Cho SK, Shin HW, Jang IJ, Park JW, et al. Antihyperglycemic mechanism of metformin occurs via the AMPK/LXRα/POMC pathway. Sci Rep. 2015;5:8145. doi: 10.1038/srep08145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao X, Liu F, Jin H, Li R, Wang Y, Zhang W, et al. Involvement of PKCα and ERK1/2 signaling pathways in EGCG's protection against stress-induced neural injuries in Wistar rats. Neuroscience. 2017;346:226–37. doi: 10.1016/j.neuroscience.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee B, Shim I, Lee H, Hahm DH. Effects of epigallocatechin gallate on behavioral and cognitive impairments, hypothalamic-pituitary-adrenal axis dysfunction, and alternations in hippocampal BDNF expression under single prolonged stress. J Med Food. 2018;21:979–89. doi: 10.1089/jmf.2017.4161. [DOI] [PubMed] [Google Scholar]
- 34.Kuo T, McQueen A, Chen TC, Wang JC. Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med Biol. 2015;872:99–126. doi: 10.1007/978-1-4939-2895-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sivitz WI, Phillips LS, Wexler DJ, Fortmann SP, Camp AW, Tiktin M, et al. Optimization of metformin in the GRADE cohort: effect on glycemia and body weight. Diabetes Care. 2020;43:940–7. doi: 10.2337/dc19-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hadi S, Alipour M, Aghamohammadi V, Shahemi S, Ghafouri-Taleghani F, Pourjavidi N, et al. Improvement in fasting blood sugar, anthropometric measurement and hs-CRP after consumption of epigallocatechin-3-gallate (EGCG) in patients with type 2 diabetes mellitus. Nutr Food Sci. 2020;50:348–59. doi: 10.1108/NFS-04-2019-0126. [DOI] [Google Scholar]
- 37.Zhu T, Li M, Zhu M, Liu X, Huang K, Li W, et al. Epigallocatechin-3-gallate alleviates type 2 diabetes mellitus via β-cell function improvement and insulin resistance reduction. Iran J Basic Med Sci. 2022;25:483–8. doi: 10.22038/IJBMS.2022.58591.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potenza MA, Iacobazzi D, Sgarra L, Montagnani M. The intrinsic virtues of EGCG, an extremely good cell guardian, on prevention and treatment of diabesity complications. Molecules. 2020;25:3061. doi: 10.3390/molecules25133061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pathak NM, Millar PJB, Pathak V, Flatt PR, Gault VA. Beneficial metabolic effects of dietary epigallocatechin gallate alone and in combination with exendin-4 in high fat diabetic mice. Mol Cell Endocrinol. 2018;460:200–8. doi: 10.1016/j.mce.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Endukuru CK, Gaur GS, Yerrabelli D, Sahoo J, Vairappan B. Cut-off values and clinical utility of surrogate markers for insulin resistance and beta-cell function to identify metabolic syndrome and its components among southern Indian adults. J Obes Metab Syndr. 2020;29:281–91. doi: 10.7570/jomes20071. [DOI] [PMC free article] [PubMed] [Google Scholar]