Abstract
Background
Metabolic syndrome (MetS) is associated with an increased risk of cardiovascular diseases. Compelling evidence supports the key role of dysfunction in the autonomic nervous system in that association, as well as mutual correlation among the components of MetS. The autonomic nervous system index (ANSI) is a percentile-ranked unitary proxy of cardiac autonomic regulation (CAR) that is designed to be free of age and sex bias, with higher values indicating better autonomic control. This study investigates CAR using the ANSI in patients with MetS.
Methods
A total of 133 patients referred to the Exercise Medicine Clinic of Istituto Auxologico Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) underwent CAR assessment using the ANSI and answered lifestyle questions in ad hoc questionnaires. The participants were retrospectively subdivided into two groups according to the presence or absence of MetS criteria.
Results
Of the subjects, 58 were diagnosed with MetS, and 75 were not (no MetS). The ANSI was significantly impaired (32.9 vs. 44.8, P<0.01) in the MetS group, and ANSI scores showed a decreasing trend (P=0.004) as the number of MetS components increased. No significant lifestyle differences were found between the groups.
Conclusion
The ANSI was significantly reduced in subjects with MetS, and, net of age and sex effects, CAR impairment became progressively more apparent as the number of MetS components increased.
Keywords: Metabolic syndrome, Cardiovascular risk factors, Autonomic nervous system, Life style
INTRODUCTION
Metabolic syndrome (MetS) is a severe public health problem worldwide and affects all age groups. MetS is a complex of interrelated risk factors, including central obesity, elevated blood pressure, high triglycerides, reduced high-density lipoprotein cholesterol (HDL-C), and increased fasting glucose.1 Several definitions of MetS have been established focusing on different components and using different cut-off values; for instance, by the World Health Organization in 1998, the National Cholesterol Education Program Adult Treatment Panel III in 2004, the International Diabetes Federation in 2005, and the Joint Interim Statement in 2009.1
Subjects with MetS are targets for preventive strategies because MetS is associated with an increased risk of cardiovascular, metabolic, and oncological diseases.2,3 The mechanisms underlying those associations and the correlations among the components of MetS are complex and multifarious and have been discussed in a comprehensive review.4 Along with immunological and hormonal alterations, compelling evidence supports the key role of autonomic nervous system (ANS) dysfunction in this setting.5,6 Cardiac autonomic regulation (CAR) is impaired in obese subjects of any age, with a dominant activity of the sympathetic system over the vagal one;7-9 this imbalance ultimately results in structural and functional abnormalities in the cardiovascular system. The relationship between obesity/insulin resistance and autonomic dysfunction appears to be bidirectional.10,11 High insulin, adipokine, and inflammatory marker levels—all linked to abdominal fat excess—contribute to sympathetic activation and baroreflex impairment, which reduce insulin sensitivity and foster both the onset and progression of organ damage.8,12,13
CAR impairment characterizes arterial hypertension in both childhood and adulthood,14,15 representing a main pathogenetic mechanism of this condition that is also evident in its early stages.16 CAR impairment is also present in more severe conditions such as coronary artery disease, cardiac failure, stroke, diabetes, and even cancer.17,18 It is generally characterized by sympathetic overactivity, and many therapeutic strategies used to treat those conditions, from the classical use of β-blocking agents to the employment of non-pharmacological approaches such as physical exercise, weight reduction, and stress management, are geared toward re-establishing ANS functionality.7,19 CAR impairment increases cardiometabolic risk, from obesity and hypertension to more severe diseases such as diabetes, coronary artery disease, stroke, and cardiac failure, suggesting that ANS plays an important pathogenetic role in those conditions. Nevertheless, it is difficult to study ANS in a clinical setting (both to reveal its possible impairment or to detect changes after pharmacological/behavioral interventions). Spectral analysis of heart rate variability (HRV) is currently the de facto standard method for assessing autonomic cardiac regulation in a clinical setting because it is simple, economical, and non‐invasive. On the other hand, the complexity of the techniques required to analyze HRV recordings, the difficulty of interpreting the variables derived from such an analysis, and the well‐known influence of age and sex on CAR are barriers to extensive use of the technique.20 Given the fundamentally unitary nature of visceral neural regulation, we previously introduced the integrated, unitary autonomic nervous system index (ANSI) as a single, composite, percentile‐ranked proxy of autonomic balance, with higher values indicating better autonomic control.21 The ANSI is, by design, free of age and sex bias and is built considering only variables derived from HRV (without the need to continuously record systolic arterial pressure [SAP]). It is correlated with baroreflex control,22 which is considered to be an important cardiac prognostic predictor, and was recently found to change progressively with changes in body mass index (BMI) in a cohort of overweight/obese subjects.23
It would be useful to be able to verify the presence of CAR impairment before a clear diagnosis of cardiometabolic disease, i.e., in subjects characterized by an initial increase in cardiometabolic risk, such as those with MetS, particularly considering the number of MetS components.24 To this aim, we investigated CAR using the ANSI in patients affected with MetS. We hypothesized that the ANSI would be lower in MetS patients, and we explored potential relationships between the ANSI and the number of MetS components.
METHODS
Ethical considerations
Informed consent was obtained from all individuals participating in this study. The study protocol followed the principles of the Declaration of Helsinki and Title 45, United States Code of Federal Regulations, Part 46, Protection of Human Subjects, Revised November 13, 2001, effective December 13, 2001. It was approved by the local institutional ethics committee (Comitato Etico Istituto Auxologico Italiano on January 25, 2022, approval n 45C201, and by the Independent Ethics Committee of Humanitas on October 13, 2015). At the time of the first clinical assessment, all participants signed an agreement for use of their anonymized data for population studies and possible publications. They acknowledged that they could not be identified via the paper, and that the authors would use fully anonymized data.
This retrospective study is based on data from the short-term HRV anonymized database of the Exercise Medicine Clinic of the University of Milan, Istituto Auxologico (IRCCS), which is part of an ongoing project about the feasibility of HRV as an autonomic metric when managing cardiovascular prevention in outpatients. We analyzed data from 133 subjects who attended our clinic to start a modification program aimed at improving their lifestyles and reducing their cardiometabolic risk. All subjects underwent the following assessments.
Clinical assessment
History, standard medical examination, anthropometric data (weight, height, waist circumference [WC], and BMI), hemodynamic data, and blood test data (fasting blood glucose, total cholesterol, HDL-C, low-density lipoprotein cholesterol, and triglycerides) were collected.
Cardiac autonomic regulation
In brief,20,22,23 electrocardiograms (ECGs), non-invasive (Finometer; Finapres Medical Systems) arterial pressure, and respiratory activity (piezoelectric belt; Marazza) were acquired on a computer. Beat-by-beat data series obtained during 5 minutes of rest and then 5 minutes of standing were analyzed offline with dedicated software (HeartScope) that automatically provided a series of indices describing HRV in the time domain: RR interval (in msec) and RR interval variability (assessed as total power, i.e., variance, in msec2), taken as simple classifiers typical of vagal control, and in the frequency domain: autoregressive spectral components both in the low frequency (LF; center frequency approximately 0.1 Hz) and in the high frequency (HF; centered with respiration, approximately 0.25 Hz), assessed in msec2 as well as in normalized units (nu).
To include an approximate evaluation of the effects of sympathetic activation produced by active standing, the stand–rest difference (Δ) in LFnu was also computed. Arterial pressure was assessed using an electronic sphygmomanometer. We used the ANSI21 as a proxy for CAR. Briefly,21-23 the index was derived through a combination of factor analysis results and a clinically optimized radar plot. By applying factor analysis to a multitude of HRV indices, RR (RR mean), RR interval variance (RR TP), and ΔRR LFnu were found highly representative of cardiac autonomic information (considering amplitude and oscillatory code modalities). Accordingly, we constructed the ANSI using the following procedure. First, the percentile rank (PR) transformations of RR mean, RR TP, and ΔRR LFnu were computed within each sex-by-age class while considering a benchmark population.21 In that way, new variables adjusted for age and sex effects could be obtained for a broader target population. Second, a radar plot was built for each subject using the values of these three PR-transformed variables, and the area of the triangle obtained was computed. Third, the PR transformation was applied to the triangle areas obtained for all subjects to create the ANSI as a composite normalized indicator in the 0 to 100 range, with higher values indicating better autonomic control. The ANSI is thus, by design, free of age and sex bias, and because the PR transformation is based on the ranking of subject values, the ANSI is robust to the possible presence of outlying subjects (i.e., subjects with variable values far distant from the central core of the data). The ANSI also correlates with cardiac baroreflex sensitivity, a powerful cardiac prognostic predictor.
Lifestyle assessment
An ad hoc questionnaire was employed to quantify lifestyle:7,25
(1) Information about the quality of nutrition was collected using the American Heart Association Healthy Diet Score (AHA diet score),26 which considers fruits/vegetables, fish, sweetened beverages, whole grains, and sodium consumption (adapted to Italian eating habits).
(2) Physical activity (total activity volume) was assessed with a modified version of the commonly used short version of the International Physical Activity Questionnaire,27 which focuses on the intensity (nominally estimated in metabolic equivalents according to the type of activity) and duration (in minutes) of physical activity.
(3) Stress, fatigue, and somatic symptom perceptions (subjective stress-related somatic symptom questionnaire [4SQ]) were collected using a self‐administered questionnaire7,25 on which participants provided ordinal scores from 0 (‘no feeling’) to 10 (‘strong feeling’). The 4SQ questionnaire considered 18 somatic symptoms, for a total score ranging from 0 to 180.
The population of our study was subdivided into two groups: those who met the MetS criteria24 (MetS subjects, n=58), and those who did not (no MetS subjects, n=75).
Statistical analysis
For statistical analyses, we used a non-parametric approach to avoid restrictive a priori conjectures about the data distribution.28 We applied alternative statistical testing procedures to obtain stronger empirical evidence from the comparisons between the no MetS and MetS groups performed for each variable.
Preliminarily, the hypotheses of equal distributions of specific (categorical) characteristics of interest (sex, smoking habits, and use of pharmacological treatments) across the no MetS and MetS groups were tested using the chi-square test and Fisher exact significance test (FT).28 Descriptive statistics for quantitative information (anthropometric, metabolic, hemodynamic, and lifestyle variables) and the cardiac autonomic indices were computed as the median±median absolute deviation both within the no MetS and MetS groups and for the subjects overall. For each quantitative variable, we then tested the null hypothesis of no difference between the groups using two-tailed exact Mann-Whitney (MW) and Kolmogorov-Smirnov (KS) tests.28
After that, to meet our primary objective of comparing CAR in patients with and without MetS, regardless of sex and age, we focused on potentially significant relationships between the ANSI and MetS. We first hypothesized that the MetS subjects would tend to have lower ANSI scores than the no MetS subjects. We verified that conjecture using one-tailed MW and KS tests plus the permutation Jonckheere-Terpstra (JT) test for ordered alternatives.28,29 We next hypothesized that ANSI scores would decrease as the number of MetS components increased. For that analysis, in addition to applying the JT test, we computed the Spearman’s rank correlation coefficient and tested the hypothesis of a non-negative correlation between ANSI and the number of MetS components against a negative correlation.
Finally, we depicted the distribution of ANSI scores within the two groups and the MetS component subgroups using violin plots.30 We also built the line plot of the median ANSI scores against the number of MetS components to better highlight the observed trend in ANSI as the number of MetS components increased.
We performed all the statistical analyses using R software version 4.3.1 (R Foundation for Statistical Computing),31 together with the R contributed packages “DescTools,” for the permutation JT test,29 and “ggplot2,” for the plots in Fig. 1.30 The nominal significance level was set at 0.05 for all the statistical tests.
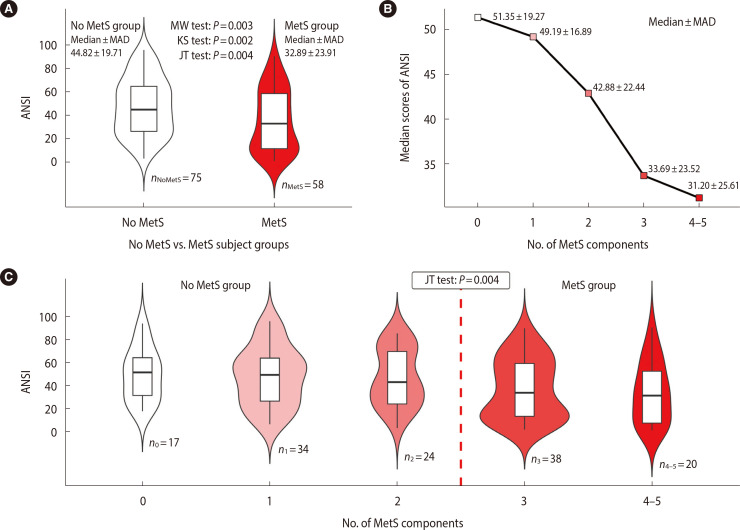
Figure 1.
Violin and line plots graphically showing the relationship between autonomic nervous system index (ANSI) and metabolic syndrome. (A) Violin plots (with the box plots inside) of the ANSI distribution in the no metabolic syndrome (MetS) and MetS groups. The displayed numbers are the group counts (lower part), median±median absolute deviation (MAD) of ANSI computed within the groups (top left and top right part), and P-values of the Mann-Whitney (MW), Kolmogorov-Smirnov (KS), and permutation Jonckheere-Terpstra (JT) tests (top central part). The MW and KS tests have the meaning described below Table 1 but were here applied as one-tailed tests. For the MW test, the null hypothesis H0:θ≥0 was tested against the alternative: H1:θ<0. For the KS test, the hypothesis H0:FnoMetS(x)≥FMetS(x) for every x was tested against H1:FnoMetS(x)<FMetS(x) for at least one x (i.e., roughly speaking, the alternative hypothesis means that the MetS population would have low ANSI scores with a higher probability than the no MetS population). For the permutation JT test, the null hypothesis H0:τnoMetS=τMetS was tested against the ordered decreasing alternative H1:τnoMetS>τMetS, where τnoMetS and τMetS are the effects of the no MetS and MetS populations, respectively, on ANSI. (B) Line plot of the median ANSI scores plotted against the number of MetS components. The displayed numbers are the median±MAD of the ANSI computed at every number of MetS components. (C) Violin plots (with the box plots inside) of the ANSI distribution within the five MetS component subgroups. The displayed numbers are the subgroup counts nc and the P-value of the permutation JT test, by which the null hypothesis H0:τ0=τ1=τ2=τ3=τ4–5 was tested against the ordered decreasing alternative H1:τ0≥τ1≥τ2≥τ3≥τ4–5 with at least a strict inequality, where τc is the effect on the ANSI of a number equal to c of the MetS components (for c=0, 1, 2, 3, and 4–5). The areas of the violins are proportional to the count of their corresponding group or subgroup.
RESULTS
Table 1 reports the anthropometric, metabolic, hemodynamic, medication, and lifestyle data collected for the 133 subjects in this study. Some 58 subjects met the criteria for MetS, and 75 did not. The no MetS subjects were characterized by a significant prevalence of females (n=73) compared with the MetS subjects (n=34) (chi-square test and FT, P<0.001), and reduced age (MW and KS, P<0.05). No significant differences were observed regarding smoking habits. The no MetS subjects were characterized by higher (chi-square test and FT, P<0.001) usage of adjuvant therapy for breast cancer (overall, 72 subjects were breast cancer survivors free of disease who attended our clinic to improve their lifestyle), and the MetS subjects were characterized by higher usage of statins (chi-square test and FT, P<0.001) or glucose-lowering drugs (chi-square test and FT, P<0.05). No significant differences were noted in antihypertensive treatments, absence of treatments, or use of other drugs or therapies.
Table 1.
Anthropometric, metabolic, hemodynamic, medication, and lifestyle data: summary statistics and 2-tailed non-parametric significance tests
| Variable | No MetS (n = 75) | MetS (n = 58) | All (n = 133) | P | P |
|---|---|---|---|---|---|
| Categorical variable | * | † | |||
| Female sex | 73 (97.33) | 34 (58.62) | 107 (80.45) | < 0.001∥ | < 0.001∥ |
| Smoking | 10 (13.33) | 7 (12.07) | 17 (12.78) | 1.000 | 1.000 |
| No drugs or therapies | 13 (17.33) | 13 (22.41) | 26 (19.55) | 0.609 | 0.513 |
| Breast cancer patients | 52 (69.33) | 20 (34.48) | 72 (54.14) | < 0.001∥ | < 0.001∥ |
| Breast cancer adjuvant therapies | 51 (68.00) | 17 (29.31) | 68 (51.13) | < 0.001∥ | < 0.001∥ |
| Antihypertensive drugs | 11 (14.67) | 12 (20.69) | 23 (17.29) | 0.497 | 0.368 |
| Statins | 2 (2.67) | 13 (22.41) | 15 (11.28) | < 0.001∥ | < 0.001∥ |
| Glucose-lowering drugs | 0 | 6 (10.34) | 6 (4.51) | 0.015∥ | 0.006∥ |
| Other drugs or therapies | 37 (49.33) | 36 (62.07) | 73 (54.89) | 0.198 | 0.163 |
| Quantitative variable | ‡ | § | |||
| Age (yr) | 49.00 ± 7.00 | 53.00 ± 9.00 | 51.00 ± 8.00 | 0.021∥ | 0.043∥ |
| Weight (kg) | 69.00 ± 10.00 | 87.25 ± 9.50 | 79.00 ± 13.00 | < 0.001∥ | < 0.001∥ |
| Height (cm) | 161.00 ± 4.00 | 164.50 ± 5.50 | 162.00 ± 6.00 | 0.018∥ | 0.026∥ |
| WC (cm) | 91.00 ± 10.00 | 106.50 ± 7.50 | 101.00 ± 10.00 | < 0.001∥ | < 0.001∥ |
| BMI (kg/m2) | 26.95 ± 3.42 | 32.84 ± 3.40 | 30.12 ± 4.17 | < 0.001∥ | < 0.001∥ |
| TC (mg/dL) | 210.00 ± 31.00 | 192.00 ± 28.50 | 198.00 ± 30.00 | 0.154 | 0.388 |
| HDL-C (mg/dL) | 61.00 ± 7.00 | 41.50 ± 5.00 | 54.00 ± 11.00 | < 0.001∥ | < 0.001∥ |
| LDL-C (mg/dL) | 128.00 ± 31.00 | 114.00 ± 19.00 | 124.00 ± 25.00 | 0.046∥ | 0.089 |
| TG (mg/dL) | 88.00 ± 24.00 | 148.00 ± 53.00 | 108.00 ± 32.00 | < 0.001∥ | < 0.001∥ |
| FBG (mg/dL) | 88.50 ± 5.50 | 105.00 ± 12.00 | 93.00 ± 8.00 | < 0.001∥ | < 0.001∥ |
| SAP (mmHg) | 114.00 ± 7.00 | 130.50 ± 12.00 | 120.00 ± 10.00 | < 0.001∥ | < 0.001∥ |
| DAP (mmHg) | 79.00 ± 6.00 | 83.50 ± 7.50 | 80.00 ± 7.00 | 0.001∥ | 0.009∥ |
| 4SQ score (a.u.) | 45.00 ± 23.00 | 32.50 ± 16.50 | 42.00 ± 22.00 | 0.210 | 0.186 |
| Stress perception score (a.u.) | 7.00 ± 2.00 | 5.00 ± 3.00 | 6.00 ± 3.00 | 0.161 | 0.256 |
| Fatigue perception score (a.u.) | 7.00 ± 2.00 | 7.00 ± 3.00 | 7.00 ± 3.00 | 0.668 | 0.759 |
| AHA diet score (a.u.) | 2.00 ± 1.00 | 2.00 ± 1.00 | 2.00 ± 1.00 | 0.796 | 0.256 |
| METs (MET/min/wk) | 537.00 ± 453.00 | 396.00 ± 396.00 | 513.00 ± 477.00 | 0.551 | 0.758 |
Values are presented as number (%) (categorical variables) or median±median absolute deviation (quantitative variables). The chi-square test and Fisher exact test were used to verify the distributional independence of each considered categorical variable from the subject subdivision into no MetS and MetS populations. For quantitative variables, the 2-tailed MW exact test was used to evaluate the null hypothesis H0:θ=0 against the alternative H1:θ≠0, where θ is the population MetS effect, i.e., the parameter representing the median difference XMetS−XnoMetS between the no MetS and MetS population distributions of variable X. The 2-tailed KS exact test was used to evaluate the null hypothesis of no difference against the presence of any difference between the no MetS and MetS cumulative distribution functions of variable X, i.e., H0:FnoMetS(x)=FMetS(x) for every x against H1:FnoMetS(x)≠ FMetS(x) for at least one x.
*Chi-square test; †Fisher test; ‡Mann-Whitney test; §Kolmogorov-Smirnov test; ∥Significant P-value.
MetS, metabolic syndrome; WC, waist circumference; BMI, body mass index; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; FBG, fasting blood glucose; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; 4SQ, subjective stress-related somatic symptom questionnaire; a.u., arbitrary unit; AHA, American Heart Association; MET, metabolic equivalent.
As expected, compared with the no MetS group, the MetS subjects had significantly different values in the MetS parameters: higher median values of SAP and diastolic arterial pressure, triglycerides, fasting blood glucose, and WC and smaller median value of HDL-C. No significant differences were detected in the perception of stress, fatigue, somatic symptoms, nutrition quality, or physical activity volume.
Table 2 reports the cardiac autonomic indices. The median values of RR TP (taken as a simple classifier typical of vagal control) and the alpha index (an overall marker of baroreflex control) were lower in MetS subjects. In contrast, the median values of SAP (recorded continuously during the period considered for the spectral analysis) and SAP LFa (LF component of SAP variability, considered as a marker of sympathetic activity to vasculature) were higher in the MetS group than in the no MetS group. All those differences were supported by significant MW and KS test results. On the other hand, no significant differences were noted in the RR LFnu and RR HFnu values, which are considered markers of prevalent sympathetic and vagal control of the sinoatrial node, respectively.
Table 2.
Cardiac autonomic indices: summary statistics and non-parametric 2-tailed Mann-Whitney and Kolmogorov-Smirnov exact significance tests
| Variable | No MetS | MetS | All | MW test P | KS test P |
|---|---|---|---|---|---|
| HR (bpm) | 68.12 ± 6.67 | 70.13 ± 8.28 | 68.67 ± 7.10 | 0.183 | 0.166 |
| RR mean (ms) | 880.80 ± 78.53 | 855.50 ± 109.94 | 873.70 ± 82.62 | 0.182 | 0.167 |
| RR TP (ms2) | 1,071.53 ± 553.56 | 603.78 ± 289.06 | 751.76 ± 410.29 | < 0.001* | < 0.001* |
| RR LFa (ms2) | 222.22 ± 142.35 | 148.58 ± 106.00 | 163.09 ± 114.79 | 0.182 | 0.358 |
| RR HFa (ms2) | 163.39 ± 135.66 | 108.66 ± 71.94 | 142.22 ± 108.19 | 0.016* | 0.028* |
| RR LFnu (nu) | 43.73 ± 15.24 | 50.30 ± 14.90 | 49.10 ± 15.57 | 0.310 | 0.211 |
| RR HFnu (nu) | 45.11 ± 14.26 | 38.72 ± 14.96 | 41.59 ± 15.28 | 0.193 | 0.396 |
| RR LF/HF | 0.95 ± 0.59 | 1.30 ± 0.72 | 1.14 ± 0.66 | 0.217 | 0.357 |
| ΔRR LFnu (nu) | 23.70 ± 19.08 | 17.21 ± 15.73 | 21.22 ± 16.21 | 0.128 | 0.263 |
| Alpha index (ms/mmHg) | 13.85 ± 4.71 | 6.92 ± 2.88 | 10.39 ± 4.65 | < 0.001* | < 0.001* |
| SAP mean (mmHg) | 117.61 ± 7.49 | 130.14 ± 13.46 | 122.10 ± 10.96 | < 0.001* | < 0.001* |
| SAP TP (mmHg2) | 13.27 ± 6.40 | 16.44 ± 9.54 | 13.34 ± 7.09 | 0.093 | 0.228 |
| SAP LFa (mmHg2) | 1.94 ± 0.89 | 3.30 ± 2.56 | 2.50 ± 1.47 | 0.032* | 0.011* |
Values are presented as median±median absolute deviation. The MW and KS tests were applied as described below Table 1.
*Significant P-value.
MetS, metabolic syndrome; MW, Mann-Whitney; KS, Kolmogorov-Smirnov; HR, heart rate; RR, RR interval; TP, total power; LF, low frequency; a, absolute value; HF, high frequency; nu, normalized unit; ΔRR LFnu, change induced by standing up on the LF component of RR variability expressed in nu; SAP, systolic arterial pressure.
As already noted, age and sex distributions differed significantly between the groups, rendering the observed differences difficult to interpret or misleading because age and sex have confounding effects. We relied, therefore, on the ANSI to investigate and interpret the differences between the no MetS and MetS subjects free from any age and sex bias. Fig. 1 illustrates the relationship between the ANSI and MetS.
Fig. 1A displays the comparison of ANSI between the MetS and no MetS groups. As expected, the median ANSI score was lower in the MetS group (32.89±23.91) than in the no MetS group (44.82±19.71). Moreover, the two violin plots show that the ANSI tendentially decreased in the MetS group, as evidenced by the significant results of all three one-tailed significance tests reported therein. In the red violin plot (MetS group), the widening density curve in the lower part of the violin denotes a higher concentration of MetS subjects than no MetS subjects at lower ANSI scores. All that suggests that CAR impairment is associated with MetS.
The line plot in Fig. 1B clearly shows a decreasing trend in the median ANSI scores as the number of MetS components increases, and that finding was confirmed by the significantly negative Spearman’s rank correlation coefficient between ANSI and the number of MetS components (r=−0.224, P=0.005).
The latter finding is shown more clearly in Fig. 1C, where the violin plots depict the entire distribution of ANSI scores within every MetS component subgroup. Interestingly, the pink violin plot corresponding to one MetS component has a widening density curve at medium-high ANSI scores, which is consistent with our hypothesis that no MetS subjects would tend to have higher ANSI scores than MetS subjects. However, from two MetS components onward, the highest concentrations of subjects are observed at progressively lower ANSI scores in a decreasing trend supported by the significant JT test result (P=0.004).
DISCUSSION
In this work, we found that ANSI, a unitary autonomic index free of age and sex bias used as a proxy for CAR, was significantly reduced in subjects with MetS. The CAR impairment was progressively more apparent as the number of MetS components increased, which can be taken as an approximate evaluation of clinical involvement. Furthermore, this simple index, which overcomes some drawbacks of HRV interpretation, successfully unveiled progressive impairment of autonomic control that was already present before the manifestation of major cardiometabolic disease.
A functional alteration in autonomic or vascular equilibrium has been considered an important element in the clinical evolution from risk to disease, and it is possible to reverse it with lifestyle modifications. More specifically, CAR impairment might play an important role in MetS development and its associated risks of cardiometabolic and oncologic diseases.32,33 Whether CAR impairment is a pathogenetic mechanism or a consequence is not fully clear,34 and the relationship might be bidirectional.10,11 Nevertheless, simplified clinical narratives attract clinical interest because of the complexity of CAR, which is based on many positive and negative feedback reflexes affected by vascular and metabolic alterations. In line with those considerations, we evaluated the feasibility of various practice methods for assessing CAR impairment. Previously, we observed a reduction in the overall gain of baroreflex sensitivity (alpha index) in both adults14 and children15 with high-normal (prehypertension) levels of arterial pressure, suggesting an impairment of ANS control before a clear diagnosis of hypertension and explaining (albeit in part) the increased cardiovascular risk independent of other cardiometabolic risk factors.16 In summary, our data provide evidence supporting the occurrence of multifaceted CAR alterations before the development of clinical hypertension. In this study, we report decreased ANSI in MetS subjects, suggesting that CAR impairments precede major cardiometabolic disease.
Here, CAR impairment was progressively more apparent as the number of MetS components increased, corroborating the idea that the presence of more numerous risk factors increases the cardiometabolic risk. Notably, CAR was impaired even in those presenting only one or two components and not diagnosed with MetS. This finding might reflect a continuum of cardiometabolic risk that can be missed when using conventional MetS definitions. Our results indicate that MetS has fundamental clinical importance—especially in terms of prevention—that is often not recognized by patients. Patients usually take a proactive role toward lifestyle modification, with consequent risk reduction, only after they are diagnosed with diseases such as diabetes, arterial hypertension, or cancer, which reduces the preventive value of improved lifestyles. The possibility of showing a clear alteration in the mechanisms involved in the pathogenesis of those chronic pathological conditions might have motivational value for improving exercise and nutrition habits.
Crucially, CAR alteration can be managed by behavioral interventions, such as regular aerobic exercise, reduction of fat mass, and stress management.7,19,35 Those interventions could, in turn, improve other alterations likely to be present in subjects with an elevated risk, such as optimizing glucose and lipid levels and improving immunological and endocrine control. Strong evidence supports CAR improvement following behavioral interventions. The variable derived from an HRV analysis that is most often considered when monitoring ANS improvement after aerobic training or other behavioral interventions is the total variance of RR interval variability (RR TP) (or other time domain indices, such as Standard deviation of the average NN intervals [SDANN] or Root Mean Square of the Successive Differences [RMSSD]),36 which is viewed as a marker of vagal control. That index, however, can be a suboptimal indicator, particularly in athletes or trained subjects33 or when the intervention can also affect other aspects of ANS control, such as a physiological increase in sympathetic drive with an orthostatic stimulus.
On the contrary, the unitary index ANSI (that is built considering the highly representative components of the cardiac autonomic information as indicated by factor analysis, considering a multitude of HRV indices) may carry more hidden information, rendering evident through coherent integration even small changes in multiple variables.21 Furthermore, the ANSI integrates autonomic information related to both rest and the dynamic response to sympathetic excitatory stimuli, such as standing up. Accordingly, we observed that MetS subjects were characterized by a slightly (but not significantly) higher RR LFnu value and a slightly (but not significantly) lower RR HFnu value (markers of prevalent sympathetic and vagal control to the sinoatrial node, respectively) and a reduced increase in sympathetic drive with an orthostatic stimulus (ΔRR LFnu) than no MetS subjects. On the other hand, the ANSI can unveil differences in CAR that depend on the subject group, and it depicted a significant decreasing trend in the median scores as the number of the MetS components increased.
In this study, we also observed a reduction of the alpha index, suggesting an impairment of the overall gain of baroreflex sensitivity, considered a powerful cardiac prognostic predictor. Furthermore, the combination of reduced cardiac baroreflex and impaired HRV determines a potent prognostic indicator for sudden death. However, although the calculation of the alpha index is non-invasive, it requires simultaneous recording of continuous non-invasive ECG and SAP signals and an ad hoc analysis. Those requirements assume the availability of economic and technical resources that might not be available everywhere. Indeed, ANSI requires only the HRV analysis starting from simple ECG recording, with consequent saving of money, time, and technical resources, rendering the study of the autonomic nervous system a more realistic tool in clinical practice. Notably, the ANSI presents a clear correlation with the alpha index, as observed in previous studies.22
The simultaneous analysis of arterial pressure variability, showing an increase in SAP LFa, suggests initial involvement of the arterial system with an attendant increase in risk.5
We did not observe any significant differences between the groups in the volume of physical activity, nutrition quality (as assessed by AHA diet score), or stress perception, suggesting that the observed autonomic impairment was not linked to those important parameters that can themselves affect autonomic control.22
We noticed that pharmacological treatment with statins or glucose-lowering drugs was more frequent in MetS subjects, as expected. At the same time, adjuvant therapy for breast cancer was higher in no MetS subjects (many cancer survivors free of metastasis attend our clinic to improve their lifestyle). Those differences might be a possible issue in interpreting our data. Nevertheless, statins and glucose-lowering drugs (more frequent in subjects characterized by worse CAR) can improve or not affect CAR control.37,38 In contrast, adjuvant therapy might impair CAR, particularly endocrine therapy, and it was more frequent in the group characterized by better CAR.39
This study has some limitations. First, we did not have information about other important control mechanisms, such as endocrine and immunological values, to verify a possible progressive impairment with the increasing number of MetS components. Second, the number of subjects was small, particularly the number of subjects with all five MetS components (so we combined them with those with four MetS components in the subgroup analysis). We used non-parametric statistical methods to help manage that issue. Third, an autoregressive spectral analysis of HRV is an indirect measure of ANS control. However, it is a non-invasive technique currently considered to be the de facto methodology for studying cardiac autonomic control. Moreover, the no MetS subjects were characterized by a significant prevalence of females (n=73) compared with the MetS subjects (n=34) and younger subjects; nonetheless, ANSI is, by design, free of age and sex bias, which makes this issue manageable.
In conclusion, we observed a progressive impairment of autonomic control before the manifestation of major cardiometabolic diseases, corroborating the importance of cardiovascular prevention strategies as soon as possible in subjects presenting even a few cardiometabolic risk factors. The possibility of studying ANS with a methodology convenient from both economical and organizational perspectives may be clinically advantageous, offering a new tool in the management of cardiometabolic risk in a clinical setting, particularly for MetS subjects who rarely realize the importance of behavioral strategies in improving autonomic balance and reducing cardiometabolic risk.
ACKNOWLEDGMENTS
This research was partially funded by the Italian Ministry of Health and a PON PhD scholarship.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: DL and NS; acquisition of data: DL, LG, MM, GB, AA, and WG; analysis and interpretation of data: LG, MM, GB, AA, and NS; drafting of the manuscript: DL, LG, and NS; critical revision of the manuscript: DL, LG, MM, GB, AA, WG, and NS; statistical analysis: NS; obtained funding: DL; administrative, technical, or material support: DL, MM, GB, and WG; and study supervision: DL.
REFERENCES
- 1.Haverinen E, Paalanen L, Palmieri L, Padron-Monedero A, Noguer-Zambrano I, Sarmiento Suárez R, et al. Comparison of metabolic syndrome prevalence using four different definitions: a population-based study in Finland. Arch Public Health. 2021;79:231. doi: 10.1186/s13690-021-00749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vishram JK, Borglykke A, Andreasen AH, Jeppesen J, Ibsen H, Jørgensen T, et al. Impact of age and gender on the prevalence and prognostic importance of the metabolic syndrome and its components in Europeans. The MORGAM Prospective Cohort Project. PLoS One. 2014;9:e107294. doi: 10.1371/journal.pone.0107294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–32. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson PM, Tuomilehto J, Rydén L. The metabolic syndrome: what is it and how should it be managed? Eur J Prev Cardiol. 2019;26(2 suppl):33–46. doi: 10.1177/2047487319886404. [DOI] [PubMed] [Google Scholar]
- 5.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol. 2009;587(Pt 23):5551–8. doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert EA, Straznicky NE, Dixon JB, Lambert GW. Should the sympathetic nervous system be a target to improve cardiometabolic risk in obesity? Am J Physiol Heart Circ Physiol. 2015;309:H244–58. doi: 10.1152/ajpheart.00096.2015. [DOI] [PubMed] [Google Scholar]
- 7.Giovanelli L, Palombo C, Pina M, Facchetti S, Malacarne M, Pagani M, et al. Progressive additive benefits of prehabilitation and subsequent bariatric surgery on cardiac autonomic regulation as assessed by means of a simple unitary composite index: preliminary data from an observational study. J Pers Med. 2022;12:1317. doi: 10.3390/jpm12081317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, et al. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97:2037–42. doi: 10.1161/01.CIR.97.20.2037. [DOI] [PubMed] [Google Scholar]
- 9.Yadav RL, Yadav PK, Yadav LK, Agrawal K, Sah SK, Islam MN. Association between obesity and heart rate variability indices: an intuition toward cardiac autonomic alteration: a risk of CVD. Diabetes Metab Syndr Obes. 2017;10:57–64. doi: 10.2147/DMSO.S123935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–52. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–90. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, et al. Overweight and sympathetic overactivity in Black Americans. Hypertension. 2001;38:379–83. doi: 10.1161/01.HYP.38.3.379. [DOI] [PubMed] [Google Scholar]
- 13.Smith MM, Minson CT. Obesity and adipokines: effects on sympathetic overactivity. J Physiol. 2012;590:1787–801. doi: 10.1113/jphysiol.2011.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucini D, Mela GS, Malliani A, Pagani M. Impairment in cardiac autonomic regulation preceding arterial hypertension in humans: insights from spectral analysis of beat-by-beat cardiovascular variability. Circulation. 2002;106:2673–9. doi: 10.1161/01.CIR.0000039106.89299.AB. [DOI] [PubMed] [Google Scholar]
- 15.Genovesi S, Pieruzzi F, Giussani M, Tono V, Stella A, Porta A, et al. Analysis of heart period and arterial pressure variability in childhood hypertension: key role of baroreflex impairment. Hypertension. 2008;51:1289–94. doi: 10.1161/HYPERTENSIONAHA.107.109389. [DOI] [PubMed] [Google Scholar]
- 16.Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–7. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Zhou L, Jiang H, Yu L. Editorial: autonomic nervous system and cardiovascular diseases: from brain to heart. Front Physiol. 2022;13:884832. doi: 10.3389/fphys.2022.884832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ondicova K, Mravec B. Role of nervous system in cancer aetiopathogenesis. Lancet Oncol. 2010;11:596–601. doi: 10.1016/S1470-2045(09)70337-7. [DOI] [PubMed] [Google Scholar]
- 19.Lucini D, Zuccotti GV, Scaramuzza A, Malacarne M, Gervasi F, Pagani M. Exercise might improve cardiovascular autonomic regulation in adolescents with type 1 diabetes. Acta Diabetol. 2013;50:341–9. doi: 10.1007/s00592-012-0416-z. [DOI] [PubMed] [Google Scholar]
- 20.Lucini D, Marchetti I, Spataro A, Malacarne M, Benzi M, Tamorri S, et al. Heart rate variability to monitor performance in elite athletes: criticalities and avoidable pitfalls. Int J Cardiol. 2017;240:307–12. doi: 10.1016/j.ijcard.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Sala R, Malacarne M, Solaro N, Pagani M, Lucini D. A composite autonomic index as unitary metric for heart rate variability: a proof of concept. Eur J Clin Invest. 2017;47:241–9. doi: 10.1111/eci.12730. [DOI] [PubMed] [Google Scholar]
- 22.Solaro N, Malacarne M, Pagani M, Lucini D. Cardiac baroreflex, HRV, and statistics: an interdisciplinary approach in hypertension. Front Physiol. 2019;10:478. doi: 10.3389/fphys.2019.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solaro N, Pagani M, Lucini D. Altered cardiac autonomic regulation in overweight and obese subjects: the role of age-and-gender-adjusted statistical indicators of heart rate variability and cardiac baroreflex. Front Physiol. 2021;11:567312. doi: 10.3389/fphys.2020.567312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA, et al. American Heart Association, author. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004;109:551–6. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 25.Lucini D, Solaro N, Lesma A, Gillet VB, Pagani M. Health promotion in the workplace: assessing stress and lifestyle with an intranet tool. J Med Internet Res. 2011;13:e88. doi: 10.2196/jmir.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 27.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 28.Hollander M, Wolfe DA, Chicken E. Nonparametric statistical methods. 3rd ed. John Wiley & Sons; 2014. [DOI] [Google Scholar]
- 29.Signorell A, Aho K, Alfons A, Anderegg N, Aragon T, Arachchige C, et al. DescTools: tools for descriptive statistics (R package DescTools version 0.99.48) [Internet] R Foundation for Statistical Computing; 2023. [cited 2024 Aug 31]. Available from: https://cran.r-project.org/package=DescTools . [Google Scholar]
- 30.Wickham H. ggplot2: elegant graphics for data analysis. 2nd ed. Springer-Verlag; 2016. [DOI] [Google Scholar]
- 31.R Core Team, author. R: a language and environment for statistical computing [Internet] R Foundation for Statistical Computing; 2024. [cited 2024 Aug 31]. Available from: https://www.R-project.org . [Google Scholar]
- 32.Licht CM, de Geus EJ, Penninx BW. Dysregulation of the autonomic nervous system predicts the development of the metabolic syndrome. J Clin Endocrinol Metab. 2013;98:2484–93. doi: 10.1210/jc.2012-3104. [DOI] [PubMed] [Google Scholar]
- 33.Lucini D, Cusumano G, Bellia A, Kozakova M, Difede G, Lauro R, et al. Is reduced baroreflex gain a component of the metabolic syndrome? Insights from the LINOSA study. J Hypertens. 2006;24:361–70. doi: 10.1097/01.hjh.0000202817.02836.9c. [DOI] [PubMed] [Google Scholar]
- 34.Tentolouris N, Argyrakopoulou G, Katsilambros N. Perturbed autonomic nervous system function in metabolic syndrome. Neuromolecular Med. 2008;10:169–78. doi: 10.1007/s12017-008-8022-5. [DOI] [PubMed] [Google Scholar]
- 35.Lucini D, Milani RV, Costantino G, Lavie CJ, Porta A, Pagani M. Effects of cardiac rehabilitation and exercise training on autonomic regulation in patients with coronary artery disease. Am Heart J. 2002;143:977–83. doi: 10.1067/mhj.2002.123117. [DOI] [PubMed] [Google Scholar]
- 36.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franco CC, Previate C, Trombini AB, Miranda RA, Barella LF, Saavedra LP, et al. Metformin improves autonomic nervous system imbalance and metabolic dysfunction in monosodium L-glutamate-treated rats. Front Endocrinol (Lausanne) 2021;12:660793. doi: 10.3389/fendo.2021.660793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millar PJ, Floras JS. Statins and the autonomic nervous system. Clin Sci (Lond) 2014;126:401–15. doi: 10.1042/CS20130332. [DOI] [PubMed] [Google Scholar]
- 39.Lucini D, Malacarne M, Oggionni G, Gatzmeier W, Santoro A, Pagani M. Endocrine adjuvant therapy might impair cardiac autonomic regulation in breast cancer survivors. Cardiol Cardiovasc Med. 2019;3:34–49. doi: 10.26502/fccm.92920052. [DOI] [Google Scholar]