Abstract
Current therapeutic approaches for osteoporosis predominantly involve antiresorptive agents, but the emergence of bone anabolic therapy, such as romosozumab, presents a promising alternative. Romosozumab, a monoclonal antibody targeting sclerostin, exhibits both bone anabolic and antiresorptive effects, offering the potential to enhance bone mineral density and mitigate fracture risk. Evidence from several studies demonstrating the efficacy of romosozumab is now established in improving bone mineral density and reducing fracture rates in postmenopausal women and men. This review critically evaluates the role of romosozumab in osteoporosis management, emphasizing findings from real-world studies to facilitate its practical application in clinical settings. Adverse effects, comparative effectiveness with other osteoporotic agents, and challenges in sequential therapy are also discussed, providing insights for informed decision-making by physicians, particularly in the context of pre-treatment considerations. Additionally, the review examines global prescribing guidelines and highlights challenges associated with romosozumab utilization in special patient subgroups, aiming to optimize its clinical use.
Keywords: osteoporosis, romosozumab, bone mineral density, minimal trauma fracture
The increasing prevalence of osteoporosis poses a significant burden among the aging population. The incidence of osteoporotic fractures is significant, with 50% of women and 22% of men older than age 50 years experiencing an osteoporotic fracture in their lifetime [1, 2]. Apart from associated morbidity and mortality, there are also substantial financial burdens on both individuals and society [3]. Osteoporosis is often underdiagnosed and undertreated. Even on recognition, usually following a minimal trauma fracture, undertreatment remains widespread. Traditional first-line therapies include antiresorptive or RANKL inhibitors (denosumab) that reduce the risk of minimal trauma fractures. Bone anabolic agents are also available (eg, teriparatide, abaloparatide) but are used primarily in patients who have experienced treatment failure with antiresorptive agents. Large randomized controlled trials have shown the effectiveness of zolendronic acid, alendronate, and denosumab in reducing vertebral and nonvertebral fractures in addition to gains in bone mineral density (BMD) [4-6]. Counselling patients about osteoporosis being a lifelong disease requiring ongoing therapy is necessary. In the past decade, bone anabolic therapy has emerged as a strategy to increase BMD and decrease fracture risk but cost and accessibility have limited its role as a first-line therapy.
Multiple molecular pathways regulate bone formation and resorption. Bone remodelling is governed by the coupling of osteoblast and osteoclast activity. Factors such as menopause, alcohol use, and low caloric intake disrupt this balance. Signalling through Wnt pathways via beta-catenin is central to the differentiation and proliferation of osteoblasts [7]. Mature osteoblasts generate osteoid, which, when mineralized, are termed osteocytes and are the dominant source of sclerostin [8]. Sclerostin, an antagonist of the Wnt-β-catenin pathway, binds to the Wnt LRP 5/6 coreceptors, leading to phosphorylation and ultimately degradation of β-catenin, thus inhibiting bone formation [9]. Romosozumab is a monoclonal antibody that binds to sclerostin and exhibits both bone anabolic and antiresorptive effects through these mechanisms [10]. The action of romosozumab-induced bone formation is dominant for the first few months, followed by sustained inhibition of bone resorption for the entire duration of the treatment [11].
Romosozumab is prescribed as 2 injections per month (105 mg each) for a total 12-month course. Following Food and Drug Administration approval in 2019, it has been approved for use in 37 countries including Australia, Japan, the United States, and within the United Kingdom. In Australia, it has been available for use for osteoporosis treatment since early 2020. Currently, the Australian Pharmaceutical Benefits Scheme only subsidizes romosozumab treatment for high-risk patients whose osteoporosis has been refractory to antiresorptive treatment demonstrated by a further fracture on treatment. However, evidence predominantly suggests superior BMD gains when romosozumab is given to treatment-naïve patients before antiresorptive drugs, which is incongruous with regulations for subsidization in Australia [12]. Here, if used as first-line treatment for high-risk patients, the outlay to patients without a subsidy is significantly more costly than antiresorptive therapies. In the United States, romosozumab is indicated in postmenopausal women with a history of osteoporotic fracture, multiple risk factors for fracture or have failed, or are intolerant to, other available osteoporosis therapy. In the United Kingdom, the NICE guidelines recommend romosozumab as a treatment option only for postmenopausal women who have experienced a major osteoporotic fracture within the past 24 months.
The role of romosozumab in the clinical management of osteoporosis is examined here with an emphasis on real-world outcomes, potential adverse effects, and the influence of other osteoporotic agents when used either before or after romosozumab treatment. Additionally, emerging evidence on the effectiveness of romosozumab in specific patient subgroups is investigated, including individuals with rheumatoid arthritis, nonweightbearing conditions, and end-stage kidney disease.
Summary of Current Literature
Clinical Trials Summary
Several studies have evaluated the efficacy of romosozumab in a clinical trial setting (Table 1, Fig. 1). First, The Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) was a randomized double-blinded trial that enrolled 7180 postmenopausal women with a T-score of −2.5 to −3.5 at the total hip or femoral neck [10]. Patients were required to be ambulatory, aged 55 to 90 years, and have a T-score of −2.5 to −3.5 SD to be eligible to participate. Exclusion criteria included previous hip fracture, severe or more than 2 moderate vertebral fractures, metabolic bone disease or conditions affecting bone metabolism, osteonecrosis of the jaw, 25-hydroxyvitamin D level <20 ng/mL, abnormal serum calcium, or recent use of drugs affecting bone metabolism. Patients either received romosozumab or placebo monthly for 12 months followed by open-label denosumab for a further 12 months. Of those enrolled, 6026 patients completed all 24 months of the trial.
Table 1.
Summary of clinical trials of romosozumab
| Trial | Population and outcome | Study design | Results | Adverse events |
|---|---|---|---|---|
| FRAME (2016) [10] | Fracture incidences among postmenopausal osteoporotic ♀in ROMO-to-DMAB vs placebo-to-DMAB groups. | Randomized double-blinded trial. Patients either received ROMO or placebo monthly for 12 months followed by open-label DMAB for a further 12 months. |
Vertebral fractures: 73% lower risk in ROMO group (0.5%) than placebo group (1.8%) (P < .001). Nonvertebral fractures: 1.6% in romosozumab group, 2.1% in placebo group (hazard ratio, 0.75; 95% CI, 0.53-1.05; P = .10). |
Overall adverse events and serious adverse events were balanced between groups. Two events of osteonecrosis of the jaw in ROMO group though one of these was in the context of a dental procedure and the other in the context of ill-fitting dentures. One event of atypical femoral fracture after first dose of ROMO. |
| ARCH (2017) [13] | Fracture incidences in postmenopausal osteoporotic ♀ in ROMO-to-ALN vs ALN-to-ALN groups. | Randomized double-blinded trial. Patients either received monthly ROMO or weekly oral ALN for 12 months followed by open-label ALN in both groups. |
Vertebral fractures: 48% lower risk in ROMO-to-ALN (6.2%) than ALN-to-ALN group (11.9%) (P < .001). Nonvertebral fractures: 19% lower risk in ROMO-to-ALN group (8.7%) vs ALN-to-ALN group (10.6%) (hazard ratio 0.81, P = .04). |
Overall adverse events and serious adverse events were balanced between groups. Events observed in ALN open-label period: 4 events of atypical femoral fracture in ALN-to-ALN group and 2 in ROMO-to-ALN group. Adjudicated serious cardiovascular adverse events were more frequent in ROMO group during the double-blind period. |
| STRUCTURE (2017) [14] | BMD outcomes in postmenopausal osteoporotic ♀ aged 55-90 years after 1 year of ROMO vs TPTD. | Randomized open-label active-controlled study. ♀ who had taken oral bisphosphonate for at least 3 years before screening and ALN the year before were assigned to receive either ROMO monthly or TPTD daily for 12 months. |
Over the 12 months, mean percentage change from baseline in hip BMD was 2.6% in the ROMO group and −0.6% in the TPTD group (P < .0001). | Overall balanced between treatment groups. Most frequently reported adverse events were nasopharyngitis (13% of ROMO group vs 10% of TPTD group) and hypercalcemia (<1% of the ROMO group vs 10% of the TPTD group). No serious adverse events were concluded to be treatment-related. |
| BRIDGE (2018) [15] | Safety and efficacy of ROMO in osteoporotic ♂. | Randomized placebo-controlled double-blind study. ♂ received either ROMO or placebo monthly for 12 months with a further 3 month follow-up to evaluate development of antibodies against ROMO. |
BMD: greater mean increase in ROMO group vs placebo group in lumbar spine (12.1% vs 1.2%, P < .001), total hip (2.5% vs −0.5%; P < .001) and femoral neck (2.2% vs −0.2%; P < .001) at 12 months. | Overall incidence rate of treatment-emergent adverse events was 75.5% in the ROMO group and 80.2% in the placebo group. Incident fractures: 1.8% in ROMO group, 2.5% in placebo group. Positively adjudicated cardiovascular serious events (ROMO vs placebo): overall (4.9% vs 2.5%), ischemic events [1.8% vs 0%], heart failure events [1.8% vs 1.2%], cardiovascular death in each group [0.6% vs 1.2%]. |
| VICTOR (2022) [16] | BMD outcomes in postmenopausal osteoporotic ♀ with a severe risk of fracture in ROMO-to-IBN vs ROMO-to-DMAB groups. | Prospective randomized controlled trial. ♀ received ROMO for 12 months and were then randomly assigned to receive 12 months further of either IBN or DMAB. |
The percentage (mean ± SE) BMD change from baseline at 18 and 24 months during sequential therapy were +12.8 ± 1.1% and +14.6 ± 1.3% in the IBN group and +16.6 ± 1.6% and +18.0 ± 1.3% in the DMAB group (all P < .001). | All events were generally minor with none resulting in treatment discontinuation. A total of 10 (16.1%) events occurred in the IBN group and 5 (8.1%) in the DMAB group. There was 1 (1.6%) new vertebral fracture in the DMAB group and no new fractures in the IBN group. |
| Inose et al Real-World Multicentre Retrospective Study (2022) [17] | Real-world effect of romosozumab on BMD increase and identifying factors that impact this in patients with high risk of fractures who completed 12 months of treatment | Multicenter descriptive retrospective study of 123 patients who had completed 12 months of romosozumab treatment | BMD: 14.6% increase at lumbar spine, 5.1% increase at femoral neck, 3.1% increase at total femur | N/A |
| Kobayakawa et al Real-World Prospective Cohort Study (2021) [18] | Real-world BMD outcomes after 12 months of treatment and the effect of prior osteoporosis treatment on this, changes in bone turnover markers, adverse events | Prospective multicenter cohort study of 230 patients who received romosozumab, 204 of which had completed 12 months of treatment | BMD: at 6 and 12 months, an increase from baseline of 7.4% and 12.2% for the lumbar spine, 1.8% and 5.8% for the total hip, and 2.9% and 6.0% for the femoral neck. Patients who switched from another osteoporosis drug had lower lumbar spine BMD gains. | 64 adverse events with most being temporary, mild, and not requiring drug discontinuation. 10 cases requiring discontinuation including 1 of osteonecrosis of the jaw in a patient who had received antiresorptive treatment for 6 months before romosozumab prescription and 1 case of cerebral infarction. |
Abbreviations: ALN, alendronate; DMAB, denosumab; IBN, ibandronate; ROMO, romosozumab; TPTD, teriparatide.
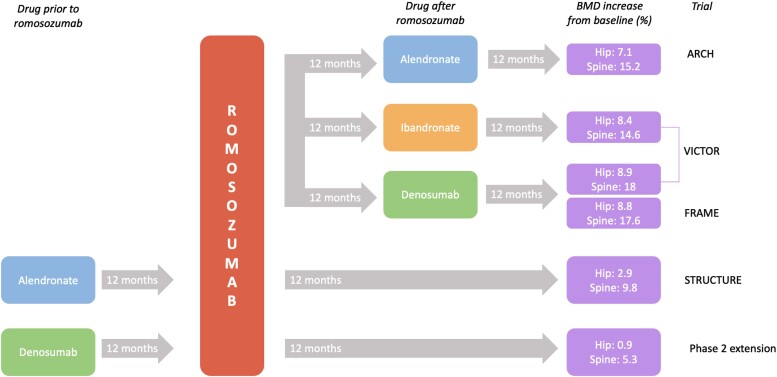
Figure 1.
Figure 1 incorporates a few studies examining BMD changes observed with romosozumab when used before/after other osteoporosis agents. Given differing study designs, the figure is included as a visual representation of different treatment sequences explored in the studies rather than as a direct comparison of outcomes.
Also examining postmenopausal women, the ARCH study was a blinded phase III trial enrolling 4093 postmenopausal osteoporotic women with a fragility fracture, assigning them to either receive romosozumab or oral alendronate for 12 months followed by open-label alendronate in both groups [13]. Patients were required to be ambulatory, aged 55 to 90 years, and have one of the following: BMD T-score −2.5 or less at total hip or femoral neck, either 1 or more moderate or severe vertebral fractures or 2 or more mild vertebral fractures; or BMD T-score −2.0 or less at the total hip or femoral neck and either 2 or more moderate or severe vertebral fractures or a fracture of the proximal femur sustained 3 to 24 months before randomization. Exclusion criteria were the same as that of the FRAME trial with the additional exclusion criteria of contraindications to alendronate including estimated glomerular filtration ratio <35 mL/min/1.73 m2.
Further analysis of this cohort was provided by the STRUCTURE trial, an open-label, active-controlled study that randomly assigned 436 women with postmenopausal osteoporosis who had taken an oral bisphosphonate for at least 3 years before screening and alendronate the year before screening to receive either romosozumab or teriparatide for 12 months [14]. Patients were ambulatory and aged 55 to 90 years with a BMD T-score of −2.5 or lower at the total hip, femoral neck, or lumbar spine. Those who had recently used other agents affecting bone metabolism, had low serum 25-hydroxyvitamin D concentration, or a history of other conditions affecting bone mass were excluded.
Despite men having lower rates of fractures compared with women, their outcomes postfracture are worse with higher mortality [19]. Analyzing romosozumab efficacy in a male cohort was performed by the BRIDGE study. Here, a phase III placebo-controlled double-blind study evaluated the safety and efficacy of romosozumab [15]. A total of 245 male subjects with osteoporosis received either romosozumab or placebo monthly for 12 months. Men were aged 44 to 90 years with BMD −2.5 or less, or −1.5 or less with a history of fragility fracture, and were required to have at least 1 hip evaluable for BMD measurement. Patients with T-scores of −3.5 or less at the total hip or femoral neck, history of hip fracture, metabolic or bone diseases, substantial laboratory abnormalities, or current use of medications affecting bone metabolism were excluded.
BMD Outcomes
The FRAME trial demonstrated a greater increase in BMD at both 6 and 12 months in comparison to placebo by 13.3 percentage points at the lumbar spine, 6.9 percentage points at the total hip. and 5.9 percentage points at the femoral neck (P < .001 for all comparisons) with ongoing BMD increases in the romosozumab group after transitioning to denosumab [10]. Similarly, the ARCH trial demonstrated romosozumab to rapidly increase BMD with greater gains in patients who had received romosozumab before alendronate in comparison to those who received alendronate alone. Additionally, the gains in the romosozumab-to-alendronate group at month 12 were maintained at month 36 after transition to alendronate. In the STRUCTURE trial, results showed that romosozumab resulted in BMD gains at the hip not observed in the teriparatide group (2.6% increase from baseline BMD in the romosozumab group and −0.6% in the teriparatide group) [14]. However, it is important to note that BMD results were measured at months 6 and 12. This is relevant because BMD gains in patients on teriparatide are typically seen at 18 to 24 months, later than this study's endpoint. Although data are more limited in men with osteoporosis, among the men in the BRIDGE trial, at 12 months it was found that the subjects in the romosozumab cohort had greater BMD gains at the lumbar spine, femoral neck, and total hip compared to the placebo group with these differences observed as early as month 6 [15]. Analysis was also undertaken accounting for subgroups of men. This accounted for differences in baseline testosterone level (<250 ng/dL, ≥250 ng/dL), minimum baseline BMD T-scores (≤−2.5, >−2.5), age groups (<70, ≥ 70 years), and baseline 10-year osteoporotic fracture risk (<median, ≥median). This concluded that romosozumab was effective for the treatment of osteoporosis in men, including those with hypogonadism.
Transition of romosozumab into clinical practice has yielded real-world studies, which are essential to determine a more representative clinical outcome outside the environment of a meticulous clinical trial. Inose et al completed a multicenter retrospective study on real-world patients receiving romosozumab for the treatment of osteoporosis aiming to investigate 12-month BMD changes and identify predictive factors [17]. A total of 106 patients with a high fracture risk who had completed 12 months of romosozumab treatment were evaluated from 4 hospitals. The study revealed that the treatment led to increases BMD at both the lumbar spine and the total femur. However, the changes at the total femur were not significant enough to be confidently attributed to romosozumab treatment. Additionally, administration of osteoporosis medications before romosozumab had a blunting effect on BMD gains with romosozumab, consistent with previous studies [20]. The bone turnover marker TRACP-5b value before romosozumab treatment was determined to be an independent predictor of lumbar spine BMD increase after 12 months of treatment, in which higher values before administration was associated with greater BMD gains after adjustment for age and sex.
Similarly, outcomes of romosozumab in real-world clinical practice were investigated by Kobayakawa et al through a prospective multicenter observational cohort study in Japanese osteoporotic patients [18]. Of the 230 patients prescribed romosozumab during the study period, 204 completed 12 months of treatment. BMD changes were observed to be higher in the romosozumab group compared to the denosumab group, with 5.3% greater increases at the lumbar spine, 2.4% greater increases at the total hip, and 2.9% greater increases at the femoral neck. Significant changes in bone turnover markers were also observed. Notably, patients switching from bisphosphonates and denosumab experienced significantly lower BMD increases than those in the treatment-naïve group.
Fracture Risk
The FRAME trial found that romosozumab was associated with a significant reduction in new vertebral fractures compared to placebo at 12 months as well as after sequential treatment with denosumab at 24 months [10]. The FRAME Extension Study followed 5743 of the originally enrolled women to 36 months, extending treatment with denosumab for an additional 12 months, concluding that fracture risk was significantly reduced and BMD gains were maintained in patients who received romosozumab rather than placebo in the first 12 months of the trial [21]. Romosozumab followed by denosumab was therefore established to be an effective regimen for the treatment of osteoporosis although further research comparing differences between alternate sequential therapy after romosozumab is needed. Similarly, the ARCH study found that patients in the romosozumab-to-alendronate group had a 48% lower risk of new vertebral fractures over the 24 months in comparison to the alendronate-to-alendronate group [13].
Adverse Events
Overall adverse events and serious adverse events in the FRAME trial, including hyperostosis, cardiovascular events, osteoarthritis, and cancer, appeared balanced between the romosozumab and placebo groups [10]. Serious adverse events possibly consistent with hypersensitivity in the romosozumab group occurred in 7 patients in the first year. Additionally, 2 events consistent with osteonecrosis of the jaw occurred in the romosozumab group; however, in both cases, there were confounding factors potentially contributing to the event raising questions regarding causality. As with the FRAME trial, overall adverse events and serious adverse events in the ARCH study were balanced between the groups, although unlike the FRAME trial, during the first 12 months, positively adjudicated serious cardiovascular events were more prevalent among patients receiving romosozumab than those receiving alendronate [13]. No adjudicated events of osteonecrosis of the jaw or atypical femoral fracture were observed in the 12-month double-blind period. During the open-label alendronate period, there was 1 event of osteonecrosis of the jaw in each treatment group and 6 total events of atypical femoral fracture (2 [<0.1%] in the romosozumab-to-alendronate group and 4 [0.2%] in the alendronate-to-alendronate group). In the BRIDGE study, romosozumab was also found to be relatively well-tolerated in men with adverse events and serious adverse events comparable between the 2 groups [15]. However, more subjects with positively adjudicated cardiovascular serious adverse events were seen in the romosozumab group, although more patients in the romosozumab group had positive findings in their cardiovascular disease history and less on cardioprotective medications at baseline compared to the placebo group.
As observed in clinical trials, romosozumab was found to generally be well-tolerated by the population in Kobayakawa et al's real-world study [18]. Sixty-four adverse events were observed, with most being temporary, mild, and not requiring drug discontinuation. There were 10 cases requiring discontinuation, of note 1 case of osteonecrosis of the jaw in a patient who had received antiresorptive treatment for 6 months before romosozumab prescription and 1 case of cerebral infarction.
Future Directions for Real-world Data
Taken together, real-world studies reflect significant BMD improvements but lack data on fracture outcomes, quality of life measures, and the potential risk of vascular events. The significant imbalance of vascular events identified in ARCH warrants in-depth investigation in future research. This study was the first time ischemic events were noted to be higher in the romosozumab cohort (cardiac ischemia: odds ratio, 2.65; 95% CI, 1.03-6.77) [13]. Since then, a large genome wide association study combined with mendelian randomization investigated association between sclerostin levels and cardiovascular risk and provided some data to show that lower levels of sclerostin may increase vascular risk and coronary artery calcification [22], although some methodological concerns of this study have been raised [23]. Others have shown that sclerostin may have a cardioprotective role (inhibition of calcified plaques), which may be reduced with romosozumab use [24]. In the United States, romosozumab has a boxed warning that advises that it may increase the risk of myocardial infarction (heart attack), stroke, and cardiovascular death, specifying romosozumab should not be initiated in patients who have had a heart attack or stroke within the preceding year. In Australia, the Therapeutic Goods Administration updated the Product Information and Consumer Medicine Information in December 2023, stating that romosozumab is now contraindicated in patients with previous myocardial infarction or stroke, with no qualification on timing. Although further research into this association and causation is needed, it is essential to exercise caution and closely monitor cardiovascular health in patients receiving romosozumab, especially in those with preexisting cardiovascular risk factors [25]. The authors’ practice is to avoid romosozumab in patients who are intermediate or high risk for vascular events without appropriate mitigation.
Sequence of Therapies
Ongoing pharmacological therapy for osteoporosis is often required because of the inability of osteoporosis drugs to fully restore bone health in many patients. Sequential therapies are therefore used, with the order of therapies influencing outcomes. Prescribing recommendations and subsidy criteria have limited the use of romosozumab to individuals with severe osteoporosis, leading to a predominantly pretreated patient population. The efficacy of the drug in pretreated bone presents challenges, particularly because of the potential lingering effects of bisphosphonates, which are known to suppress bone formation even after the initiation of romosozumab [26]. Notably, the STRUCTURE trial investigated outcomes when romosozumab was administered following antiresorptive treatment, eliminating the off-treatment requirement [14]. This approach resulted in only modest gains in hip BMD. Cosman et al explored the importance of treatment sequence in osteoporosis by reviewing the aforementioned clinical trials, concluding that larger BMD increases were observed in patients receiving romosozumab before, as opposed to after, an antiresorptive agent [12]. Updated guidelines have recommended romosozumab as first-line treatment in patients with multiple vertebral fractures or hip fracture and BMD in the osteoporotic range [27].
A retrospective study examining changes in bone turnover markers and BMD compared drug-naïve cases with cases where romosozumab was used after an antiresorptive agent [20]. In the drug-naïve group, the average change of bone markers reflected a high turnover of bone metabolism, which was lower in the dual therapy group. Additionally, in those who were treatment-naïve, statistically significant improvements in BMD were observed at the lumbar spine. In contrast, the group of patients switching to romosozumab from a previous antiresorptive agent did not undergo statistically significant BMD gains.
In larger randomized controlled trials (ARCH, FRAME) with greater fracture reductions, patients were excluded if they did not meet off-treatment requirements (eg, no oral bisphosphonate cumulative use >3 years, no dose received in 3 months before randomization) but these patients were not truly treatment-naïve and as such need to be interpreted accordingly [28]. However, this may reflect a more real-world experience with heterogenous treatment histories.
Treatment using denosumab followed by romosozumab is the authors’ most common experience regarding choice of sequential therapy, likely because of the prevalence of denosumab use. An extension of a romosozumab phase 2 dose-finding study included a cohort of patients who received denosumab for 12 months followed by romosozumab for 12 months [29]. These patients had BMD gains of 0.9% at the hip and 5.3% at the lumbar spine. Cessation of denosumab and the immediate loss of inhibition of bone resorption may yield a rebound effect that impacts the actions of romosozumab, possibly accounting for a blunted response in BMD in those following this sequential therapy compared to naïve bone [18]. Addressing the possible reduction in BMD gains resulting from previous denosumab treatment and minimizing the risk of increased bone turnover after discontinuing denosumab before transitioning to romosozumab is crucial. There is a need for innovative and effective strategies to optimize this specific therapeutic sequence.
Because of the restricted 12-month dosing period of romosozumab and concern about potential reduction in BMD after cessation, a continuous treatment strategy is necessary for managing ongoing osteoporosis. The VICTOR study, a prospective randomized controlled trial, aimed to assess the comparative efficacy of ibandronate and denosumab as sequential therapeutic options in patients with severe postmenopausal osteoporosis following romosozumab treatment [16]. Patients were randomly assigned to received either ibandronate or denosumab for an additional 12 months after romosozumab treatment. This concluded that BMD changes at the lumbar spine from 12 to 24 months were 2.5% in the ibandronate group and 5.4% in the denosumab group with comparably favorable trends of denosumab at the total hip and femoral neck. Several minor adverse events were reported in both groups, none of which resulted in drug discontinuation. This study supports denosumab as an option for sequential therapy, although the optimal timing for denosumab initiation following romosozumab has yet to be determined.
At present, romosozumab is not generally used beyond a single course with current prescribing guidelines reflective of this. The efficacy and safety of a second romosozumab course is reported in a phase 2 dose-finding study of postmenopausal women [30]. Participants received romosozumab or placebo for 24 months followed by placebo or denosumab for 12 months. All patients then received romosozumab for 12 months. Participants who received a second course of romosozumab following placebo who experienced BMD loss on placebo, experienced gains at the lumbar spine, total hip, and femoral neck comparative to gains seen in the group’s first romosozumab treatment course. This suggests resetting of skeletal responsiveness to romosozumab after 1 year off therapy. Participants who received a second course of romosozumab following denosumab had a small increase in BMD at the lumbar spine but no improvement in BMD at the total hip. This is in contrast to BMD loss after sequential therapy with denosumab followed by teriparatide [31]. In the small group of participants, no new safety findings were reported in the second course of romosozumab with the adverse event profile comparable to that of a single course of treatment. Although further research with larger sample sizes is needed to better understand the efficacy and safety of subsequent romosozumab treatment courses, current evidence suggests romosozumab may have potential in osteoporosis management with treatment-cycling [32].
Special Populations
Other subgroups in which there is significant potential for the dual action of romosozumab to improve outcomes include groups with complex causes of low bone density. Limited literature exists for the role of romosozumab in patients with glucocorticoid-induced osteoporosis, patients after spinal injury, lactation-induced osteoporosis, and patients with renal bone disease (chronic kidney disease [CKD]-mineral and bone disorder).
In a group of patients with rheumatoid arthritis receiving glucocorticoids, romosozumab was shown to have comparable effects to denosumab on BMD [33]. The effect of romosozumab was more modest than expected possibly because of the impact of glucocorticoids directly inhibiting the proliferation of osteoblasts. Treatment with romosozumab in the rare condition of pregnancy and lactation-induced osteoporosis has been reported in 1 case study, in which its use after 4 months of teriparatide was effective in increasing BMD without subsequent fracture [34].
Nonweightbearing Patients
An often understudied cohort are the nonweightbearing patients who have persistent refractory osteoporosis and high fracture rates [35]. Sclerostin release from osteocytes is inhibited with weightbearing resulting in the net effect of bone formation. Hence, when a patient is unable to bear weight, there is an elevated production of sclerostin, leading to diminished bone formation. The impact of romosozumab in this specific patient group remains understudied in humans but clinically it could be speculated that nonweightbearing regions with higher levels of sclerostin could benefit from romosozumab. Clinical trials in the spinal cord injury population examining this are ongoing in both subacute (NCT05101018) and chronic settings (NCT05180032).
Chronic Renal Disease
Bone complications are notorious in patients with chronic renal disease, in which disturbances in mineral metabolism, secondary hyperparathyroidism, and CKD-mineral and bone disorder often lead to osteoporosis from impaired bone mineralization and increased fracture risk. The safety and efficacy of romosozumab in postmenopausal osteoporosis patients with mild-to-moderate CKD was explored in a post hoc analysis of the FRAME and ARCH trials [36]. This demonstrated that in stages 1 to 3 of CKD, romosozumab resulted in greater BMD increases in comparison to alendronate and placebo with a similar safety profile between different levels of renal function. The pharmacokinetics and pharmacodynamics in stage 4 renal impairment and end-stage renal disease requiring hemodialysis were investigated with a single dose of romosozumab 210 mg [37]. Serum romosozumab levels were higher in these patients and, in addition, the dose was well-tolerated and results supported its use without dose adjustment in these patients. Data are limited on the outcomes in these patients though has been evaluated in small case studies. Mukaddam et al describes off-label use in a 37 -year-old African American male with severe osteoporosis on hemodialysis after multiple rib fractures and T8 compression fracture. BMD was improved at all sites: by 47% at the lumbar spine, 41% at the femoral neck, and 28% at the total hip [38]. The treatment was tolerated well with no adverse events observed.
Rheumatoid arthritis (RA) is an autoimmune disease associated with increased risk for osteoporotic fracture [39]. The effects of romosozumab on BMD in patients with RA and severe osteoporosis were compared to that of denosumab at 3 and 6 months in an open-label randomized study of 50 patients [40]. Romosozumab resulted in significantly greater increases in BMD than denosumab but BMD gains were lower in the RA population possibly because of steroid use in this group. This hypothesis is supported by observed decreases in sclerostin levels in patients with early RA after treatment with methylprednisolone [41].
Conclusions
Romosozumab is an effective first-line or subsequent treatment for osteoporotic men and postmenopausal osteoporotic women. It offers a unique approach to osteoporosis management, with its dual action as a bone anabolic and antiresorptive agent. Clinical trials and real-world studies have provided insights into the practical implications of romosozumab therapy, emphasizing the challenge of pretreatment and its impact on BMD gains. Future studies on its potential use in specific patient populations and its effects on fracture outcomes is warranted, as is further clarification on its role in vascular events. Ongoing real-world data are fundamental for determining the patient groups that will benefit most from this treatment and guiding prescribing guidelines that we have incorporated here through comprehensive review of the current literature. As we continue to gather data and refine our knowledge, we can better harness the potential of romosozumab to reduce the burden of osteoporosis, especially in high-risk populations.
Disclosures
There are no conflicts of interest to disclose. The authors did not receive any external funding for this manuscript.
Abbreviations
- BMD
bone mineral density
- CKD
chronic kidney disease
- FRAME
Fracture Study in Postmenopausal Women with Osteoporosis
- RA
rheumatoid arthritis
Contributor Information
Livia Liu, Department of Diabetes and Endocrinology, Royal North Shore Hospital, Sydney 2065, Australia.
Roderick J Clifton-Bligh, Department of Diabetes and Endocrinology, Royal North Shore Hospital, Sydney 2065, Australia; Faculty of Medicine and Health, University of Sydney, Sydney 2006, Australia.
Christian M Girgis, Faculty of Medicine and Health, University of Sydney, Sydney 2006, Australia; Department of Diabetes and Endocrinology, Westmead Hospital, Sydney 2145, Australia.
Matti L Gild, Email: matti.gild@sydney.edu.au, Department of Diabetes and Endocrinology, Royal North Shore Hospital, Sydney 2065, Australia; Faculty of Medicine and Health, University of Sydney, Sydney 2006, Australia.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Adachi JD, Ioannidis G, Pickard L, et al. The association between osteoporotic fractures and health-related quality of life as measured by the health utilities index in the Canadian multicentre osteoporosis study (CaMos). Osteoporos Int. 2003;14(11):895‐904. [DOI] [PubMed] [Google Scholar]
- 2. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(Suppl 2):S3‐S7. [DOI] [PubMed] [Google Scholar]
- 3. Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367(9527):2010‐2018. [DOI] [PubMed] [Google Scholar]
- 4. He B, Zhao JQ, Zhang MZ, Quan ZX. Zoledronic acid and fracture risk: a meta-analysis of 12 randomized controlled trials. Eur Rev Med Pharmacol Sci. 2021;25(3):1564‐1573. [DOI] [PubMed] [Google Scholar]
- 5. Serrano AJ, Begoña L, Anitua E, Cobos R, Orive G. Systematic review and meta-analysis of the efficacy and safety of alendronate and zoledronate for the treatment of postmenopausal osteoporosis. Gynecol Endocrinol. 2013;29(12):1005‐1014. [DOI] [PubMed] [Google Scholar]
- 6. Silva-Fernández L, Rosario MP, Martínez-López JA, Carmona L, Loza E. Denosumab for the treatment of osteoporosis: a systematic literature review. Reumatol Clin. 2013;9(1):42‐52. [DOI] [PubMed] [Google Scholar]
- 7. Karner CM, Long F. Wnt signaling and cellular metabolism in osteoblasts. Cell Mol Life Sci. 2017;74(9):1649‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li S-S, He S-H, Xie P-Y, et al. Recent progresses in the treatment of osteoporosis. Front Pharmacol. 2021;12:717065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vasiliadis ES, Evangelopoulos DS, Kaspiris A, Benetos IS, Vlachos C, Pneumaticos SG. The role of sclerostin in bone diseases. J Clin Med. 2022;11(3):806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532‐1543. [DOI] [PubMed] [Google Scholar]
- 11. Chavassieux P, Chapurlat R, Portero-Muzy N, et al. Bone-forming and antiresorptive effects of romosozumab in postmenopausal women with osteoporosis: bone histomorphometry and microcomputed tomography analysis after 2 and 12 months of treatment. J Bone Miner Res. 2019;34(9):1597‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cosman F, Kendler DL, Langdahl BL, et al. Romosozumab and antiresorptive treatment: the importance of treatment sequence. Osteoporos Int. 2022;33(6):1243‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417‐1427. [DOI] [PubMed] [Google Scholar]
- 14. Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390(10102):1585‐1594. [DOI] [PubMed] [Google Scholar]
- 15. Lewiecki EM, Blicharski T, Goemaere S, et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab. 2018;103(9):3183‐3193. [DOI] [PubMed] [Google Scholar]
- 16. Kobayakawa T, Miyazaki A, Takahashi J, Nakamura Y. Verification of efficacy and safety of ibandronate or denosumab for postmenopausal osteoporosis after 12-month treatment with romosozumab as sequential therapy: the prospective VICTOR study. Bone. 2022;162:116480. [DOI] [PubMed] [Google Scholar]
- 17. Inose H, Ariga A, Motoyoshi T, et al. The real-world effect of 12 months of romosozumab treatment on patients with osteoporosis with a high risk of fracture and factors predicting the rate of bone mass increase: a multicenter retrospective study. JBMR Plus. 2022;6(7):e10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobayakawa T, Suzuki T, Nakano M, et al. Real-world effects and adverse events of romosozumab in Japanese osteoporotic patients: a prospective cohort study. Bone Rep. 2021;14:101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bliuc D, Nguyen ND, Nguyen TV, Eisman JA, Center JR. Compound risk of high mortality following osteoporotic fracture and refracture in elderly women and men. J Bone Miner Res. 2013;28(11):2317‐2324. [DOI] [PubMed] [Google Scholar]
- 20. Inage K, Orita S, Eguchi Y, et al. Time-course changes in bone metabolism markers and density in patients with osteoporosis treated with romosozumab: a multicenter retrospective study. Yonsei Med J. 2021;62(9):829‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewiecki EM, Dinavahi RV, Lazaretti-Castro M, et al. One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: results of the FRAME extension study. J Bone Miner Res. 2019;34(3):419‐428. [DOI] [PubMed] [Google Scholar]
- 22. Zheng J, Wheeler E, Pietzner M, et al. Lowering of circulating sclerostin may increase risk of atherosclerosis and its risk factors: evidence from a genome-wide association meta-analysis followed by Mendelian randomization. Arthritis Rheumatol. 2023;75(10):1781‐1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Staley JR, Giannakopoulou O, Holdsworth G, Armstrong M. Genetic data do not provide evidence that lower sclerostin is associated with increased risk of atherosclerosis: comment on the article by Zheng et al. Arthritis Rheumatol. 2023. Doi: 10.1002/art.42751 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24. González-Salvatierra S, García-Fontana C, Lacal J, et al. Cardioprotective function of sclerostin by reducing calcium deposition, proliferation, and apoptosis in human vascular smooth muscle cells. Cardiovasc Diabetol. 2023;22(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vestergaard Kvist A, Faruque J, Vallejo-Yagüe E, Weiler S, Winter EM, Burden AM. Cardiovascular safety profile of romosozumab: a pharmacovigilance analysis of the US food and drug administration adverse event reporting system (FAERS). J Clin Med. 2021;10(8):1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gasser JA, Kneissel M, Thomsen JS, Mosekilde L. PTH and interactions with bisphosphonates. J Musculoskelet Neuronal Interact. 2000;1(1):53‐56. [PubMed] [Google Scholar]
- 27. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society guideline update. J Clin Endocrinol Metab. 2020;105(3):587‐594. [DOI] [PubMed] [Google Scholar]
- 28. Cosman F, CD, Adachi JD, et al. Protocol for: Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 2016;375(16):1532‐1543. [DOI] [PubMed] [Google Scholar]
- 29. McClung MR, Bolognese MA, Brown JP, et al. Skeletal responses to romosozumab after 12 months of denosumab. JBMR Plus. 2021;5(7):e10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kendler DL, Bone HG, Massari F, et al. Bone mineral density gains with a second 12-month course of romosozumab therapy following placebo or denosumab. Osteoporos Int. 2019;30(12):2437‐2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-switch study): extension of a randomised controlled trial. Lancet. 2015;386(9999):1147‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kumar S, Gild ML, McDonald MM, Kim AS, Clifton-Bligh RJ, Girgis CM. A novel sequential treatment approach between denosumab and romosozumab in patients with severe osteoporosis. Osteoporos Int. 2024;35(9):1669‐1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kobayakawa T, Miyazaki A, Kanayama Y, et al. Comparable efficacy of denosumab and romosozumab in patients with rheumatoid arthritis receiving glucocorticoid administration. Mod Rheumatol. 2023;33(1):96‐103. [DOI] [PubMed] [Google Scholar]
- 34. Kaneuchi Y, Iwabuchi M, Hakozaki M, Yamada H, Konno SI. Pregnancy and lactation-associated osteoporosis successfully treated with romosozumab: a case report. Medicina (Kaunas). 2022;59(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39(4):208‐214. [DOI] [PubMed] [Google Scholar]
- 36. Miller PD, Adachi JD, Albergaria BH, et al. Efficacy and safety of romosozumab among postmenopausal women with osteoporosis and mild-to-moderate chronic kidney disease. J Bone Miner Res. 2022;37(8):1437‐1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hsu CP, Maddox J, Block G, Bartley Y, Yu Z. Influence of renal function on pharmacokinetics, pharmacodynamics, and safety of a single dose of romosozumab. J Clin Pharmacol. 2022;62(9):1132‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mukaddam MA, Hvisdas C, Sulaj A, Patel K, Tran R. Experience with anti-sclerostin antibody for osteoporosis patient with end-stage renal disease on hemodialysis. J Endocr Soc. 2021;5(Supplement_1):A192‐A192. [Google Scholar]
- 39. Pietschmann P, Butylina M, Kerschan-Schindl K, Sipos W. Mechanisms of systemic osteoporosis in rheumatoid arthritis. Int J Mol Sci. 2022;23(15):8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mochizuki T, Yano K, Ikari K, Okazaki K. Effects of romosozumab or denosumab treatment on the bone mineral density and disease activity for 6 months in patients with rheumatoid arthritis with severe osteoporosis: an open-label, randomized, pilot study. Osteoporos Sarcopenia. 2021;7(3):110‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fassio A, Adami G, Giollo A, et al. Acute effects of glucocorticoid treatment, TNFα or IL-6R blockade on bone turnover markers and wnt inhibitors in early rheumatoid arthritis: a pilot study. Calcif Tissue Int. 2020;106(4):371‐377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.