In March 2022, a 46-year-old male patient (#1), was diagnosed with acute myeloid leukemia (AML) at our Outpatient Clinic in Rome. He was the last of four siblings. Cytogenetic analysis was normal (46, XY; 25 metaphases) as well as molecular studies (Table 1A), hence he was stratified in the intermediate-risk category according to the European Leukemia Net (ELN) 2017 classification.1 In April 2022, the patient received induction chemotherapy with the “7+3” scheme (cytarabine and daunorubicin) plus gemtuzumab ozogamicin as part of a phase III prospective clinical trial (GIMEMA AML1819 - clinicaltrials gov. Identifier: NCT04168502). Unfortunately, the bone marrow (BM) aspirate performed at day 30 after chemotherapy showed the persistence of blasts, hence he was withdrawn from the protocol. In May 2022, the patient underwent second-line chemotherapy with the FLA-IDA regimen (fludarabine, idarubicin and high-dose cytarabine), but re-evaluation of the BM aspirate at day 30 revealed again refractory disease. A BM biopsy confirmed the presence of blasts with focal aspects of BM fibrosis and dysplasia. Retesting for FLT3-internal tandem duplication (ITD) or -tyrosine kinase domain (TKD) mutations in the BM yielded negative results. As salvage therapy, the patient received one cycle of the demethylating agent decitabine plus the BCL2-inhibitor venetoclax in July 2022, achieving a confirmed complete remission (CR) at day 36 according to ELN criteria.1 Then, in September 2022, he was hospitalized to receive an allogeneic hematopoietic stem cell transplant (HSCT) from a matched unrelated donor (MUD) (10/10 HLA loci compatible). The BM aspirate at day 20 after transplant showed a CR with trilinear recovery and a 100% donor chimerism by capillary electrophoresis analysis of polymorphic short tandem repeats (STR). Unfortunately, in the months after the HSCT, he developed acute intestinal graft-versus-host disease (GVHD) and an erosive pansinusitis in March 2023. The BM aspirate performed at that time (day +180) showed 4% blasts by cytomorphology and an initial chimerism loss (65% donor and 35% host), which was compatible with an early relapse (6 months after the HSCT). Shortly after being hospitalized for a COVID-19 infection the patient died from neurologic and pulmonary complications in May 2023.
One of the patient’s brothers, patient #2, was 50 years old when, in March 2010, he was diagnosed with NPM1+ AML at the Hematology Center in Reggio Calabria. He was treated with “7+3” chemotherapy to which he was refractory, hence he received the FLA-IDA regimen as second-line treatment, which led to a CR. In September 2010, patient #2 underwent an allogeneic HSCT from his HLA-matched family donor, patient #1, who at the time was healthy. Post-transplant evaluation on a BM aspirate showed a CR with trilinear recovery. He remained in CR over the years until 2017 when, 7 years after the HSCT, due to progressive thrombocytopenia a BM aspirate was performed and minimal residual disease (MRD) analysis by leukemia-associated immunophenotyping (LAIP) showed 2% blasts, despite a 100% donor chimerism by capillary electrophoresis. Hence, it was decided to start treatment with azacytidine that led to MRD negativity, lasting for 3 more years. In September 2020 the BM aspirate showed 26% blasts, indicative of a clinical relapse. Chimerism analysis was repeated and showed a 100% donor origin. The patient received salvage therapy with decitabine plus venetoclax and obtained a CR. The response was maintained until January 2022 when a second clinical relapse occurred. At that point, his clinical conditions were very poor (repeated pulmonary infections and GVHD), hence he received palliative azacytidine - venetoclax until progression. In July 2023, he died from pulmonary complications.
Figure 1A briefly summarizes the clinical history of the two siblings. When patient #1 was admitted to our clinic in 2022 and was diagnosed with AML (see above), his brother (patient #2) had had a long clinical history of AML since 2010 (at that time he was in second relapse). Hence, suspecting a familial AML case, we sequenced the two patients and their two healthy siblings by next-generation sequencing (NGS) using a panel of genes linked to familial myeloid neoplasms (Table 1B),2 but found no predisposing aberrations.
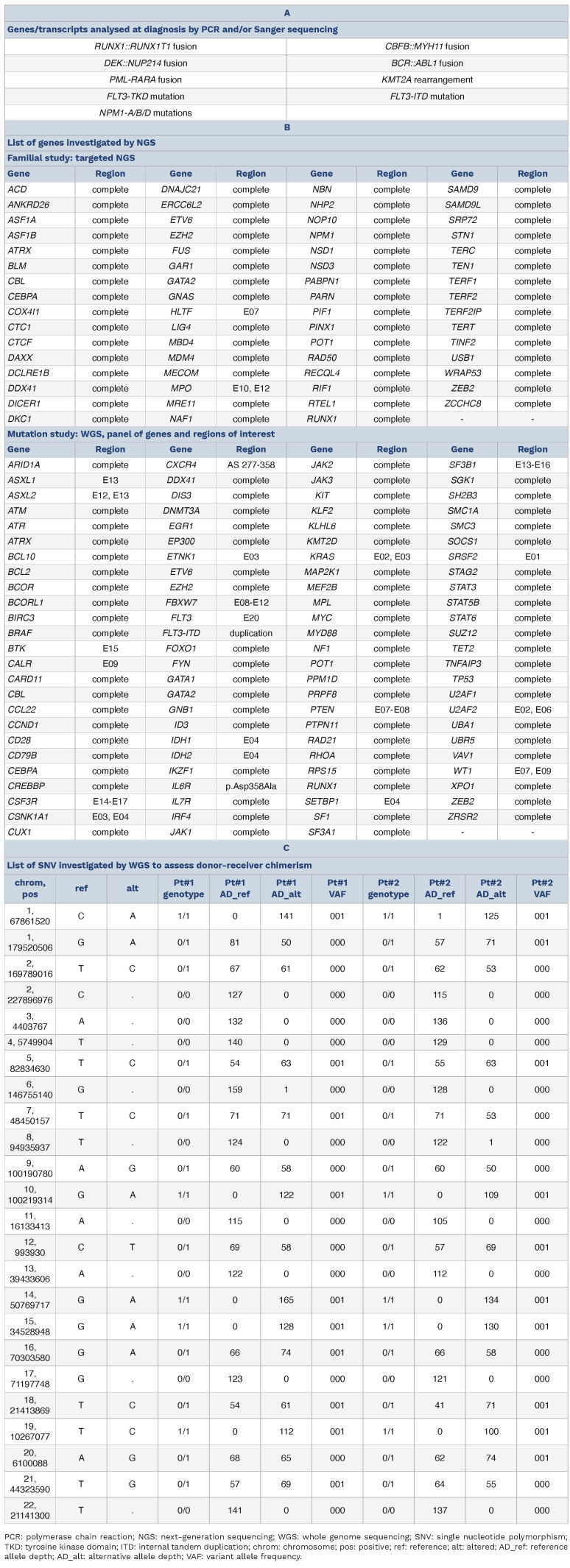
Table 1.
Molecular studies performed on the two leukemias.
The fact that patient #2 experienced an AML MRD+ with 100% donor chimerism 7 years after an allogeneic HSCT from his brother led us to hypothesize a donor-derived leukemia. To prove this, we sequenced a BM sample from patient #2 after his second relapse (November 2022) and a BM sample from patient #1 at the time of the diagnosis (March 2022) by whole genome sequencing (WGS). We then applied a bioinformatic pipeline focusing on a panel of recurrently mutated myeloid genes (Table 1). In the relapse sample of patient #2, we identified a pathogenic mutation in DNMT3A (p.Arg635Trp, variant allele frequency [VAF] 16%) (Figure 1B). The same was found in the diagnostic sample of patient #1 (VAF 42%), along with another DNMT3A mutation (p.Ser708Asn, VAF 41%), a BCOR mutation (p.Arg1514*, VAF 85%), an IDH2 mutation (p.Arg172Lys, VAF 42%) and a KIT mutation (p.Asp816Val, VAF 31%) (Figure 1). WGS data were also used to identify copy number variations (CNV) >1 Mb and structural variations (SV) and yielded negative results in both samples. The chimerism analysis performed by comparing specific single-nucleotide polymorphisms (SNP) by WGS (Table 1C) showed a 100% donor chimerism, which was further confirmed by capillary electrophoresis.
Donor-cell derived myeloid neoplasms (DDMN) are a rare complication of allogeneic HSCT. As of 2023, a total of 82 DDMN (56 AML, 23 myelodysplastic syndromes (MDS) and three myeloproliferative neoplasms [MPN]) have been reported.3 Several of these cases occurred as a result of the infusion of undetected donor leukemic cells into the recipient4,5 o r, occasionally, following solid organ transplantation.6 ,7 Above all, only three cases demonstrated the transfer of a pre-existing (pre)leukemic clone from the donor to the recipient: in the first two cases the donors’ stem cells carried an inv(3)(q21q26) and a t(1;5), respectively, resulting in the development of an AML both in the donor and in the recipient;8 in the third case a trisomy 11 in the donor’s stem cells at the time of donation gave rise to a donor-derived (DD)-AML in the recipient 14 years after the HSCT.9
In this report, we present a DD-AML case from a sibling donor and provide full molecular insight for both donor and recipient leukemias. The fact that the hematopoietic stem cell donor was familial and that both siblings developed an AML posed our case in the grey zone of a familial and/or donor-derived neoplasia. Increasing evidence is mounting on the role of predisposing germline variants in myeloid disorders; genetic testing is becoming paramount particularly in allogeneic HSCT donors with suspected hereditary predisposition.10 On this line, we performed a NGS study on the whole family and ruled out the presence of known germline variants associated with familial leukemia.
From a genetic standpoint, the two patient’s AML showed distinct somatic aberrations, with patient (#1) presenting mutations in DNMT3A (N=2), BCOR, KIT and IDH2, while his brother (#2) showed one mutation in DNMT3A which was co-shared between the two patients. DNMT3A mutations occur in ~25% of AML and have been linked to clonal hematopoiesis of indeterminate potential (CHIP),11 predisposing to the acquisition of further hits with an unfavorable effect on prognosis.12 Pathogenic mutations in BCOR occur in ~2% of all AML cases and are associated with MDS-related changes, bear an unfavorable prognosis and are classified as high-risk according to the ELN 2022 classification.12 Of note, we had originally classified patient #1 as intermediate risk since at that time our molecular biology workup was based on ELN 2017 and did not include this gene (Table 1). IDH2 mutations are found in ~10% of AML patients and lead to accumulation of the 2KG oncometabolite causing toxicity and epigenetic alterations.13 Finally, KIT mutations are rare in AML14 and are often described in systemic mastocytosis.
Figure 1.
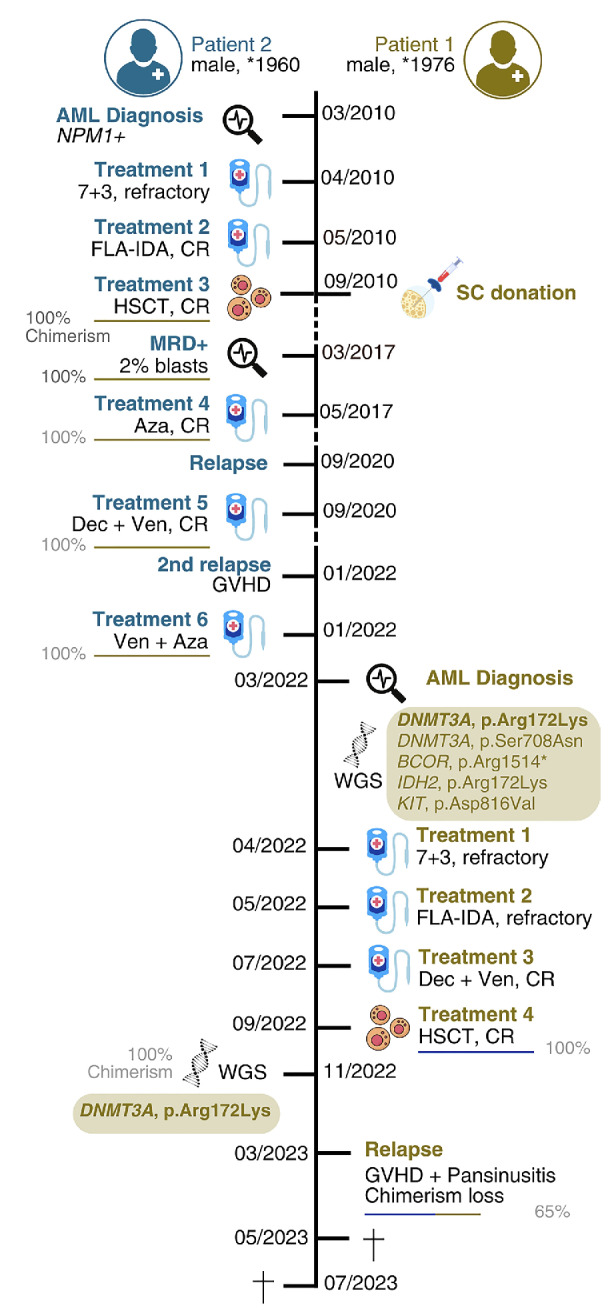
Overview of the clinical history of the two patients. The first patient (Pt #1) had a diagnosis of acute myeloid leukemia (AML) in 2022 which was highly refractory and relapsed early after allogeneic hematopoietic stem cell transplant (HSCT) (6 months) leading to death (overall survival [OS] 1.2 years). The second patient (Pt #2) had a diagnosis of AML in 2010, underwent an allogeneic HSCT from his brother (Pt #1) and remained in remission for 7 years. He eventually relapsed with a 100% donor chimerism, but benefitted from treatment with hypomethylating agents and venetoclax, leading to a longer OS of 12.8 years. CR: complete remission; Aza: azacytidine; Dec: decitabine; Ven: venetoclax; GO: gemtuzumab ozogamycin; GVHD: graft-versus-host disease; FLA-IDA: fludarabine, idarubicin and high-dose cytarabine.
Figure 2.
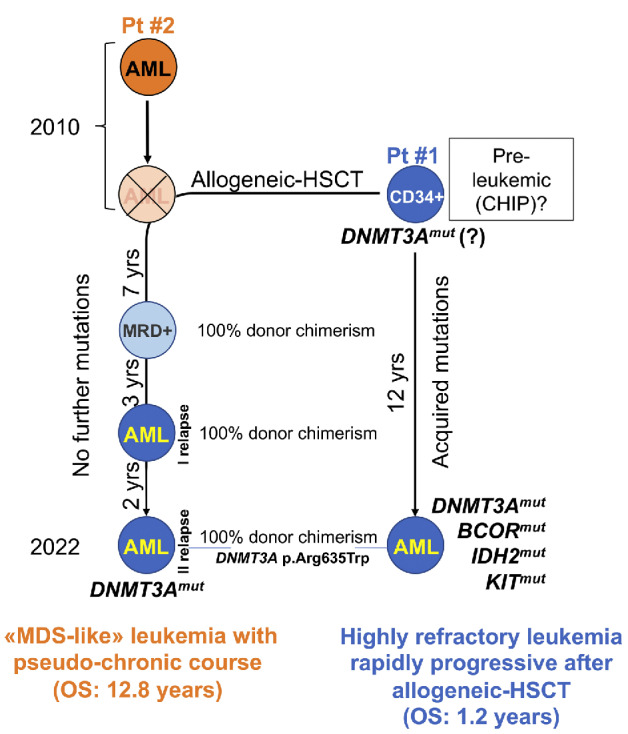
History of the two siblings acute myeloid leukemia. Based on the data provided by next-generation sequencing (NGS) studies on the 2 patients, the leading hypothesis is that patient’s (Pt) #1 CD34+ stem cells harbored a DNMT3A mutation predisposing to genomic instability at the time of donation to his brother, Pt #2. Years later, these DNMT3Amut CD34+ cells gave rise to a donor-derived acute myeloid leukemia (AML) in Pt #2, without acquiring further hits and hence with a myelodysplastic syndromes (MDS)-like pseudo-chronic clinical course (overall survival [OS] of 12.8 years); on the contrary, they acquired additional genomic aberrations in Pt #1, hence causing a rapidly progressing AML with a very short OS (1.2 years). MRD: minimal residual disease; HSCT: hematopoietic stem cell transplant; yrs: years; CHIP: clonal hematopoiesis of indeterminate potential.
Based on all of the above, a relapse 10 years after an allogeneic HSCT with a 100% donor chimerism and the co-sharing of the same DNMT3A variant (p.Arg635Trp) leads us to hypothesize that the donor’s hematopoietic stem cells might have carried this preleukemic hit at the time of the donation.
These progenitors might have evolved differently: in the recipient, they have not acquired further mutations and have given rise to a MDS-like AML with a pseudo-chronic clinical course and an OS of 13 years; in the donor, they have acquired further hits ultimately evolving into aggressive AML, with a very unfavorable OS of 1 year (Figure 2). It has been reported that DNMT3A mutations in HSC donor have been linked to GVHD after allogeneic HSCT,15 however in this case we did not observe this phenomenon in patient #2. Finally, based on VAF estimation we were unable to identify a distinct clonal hierarchy. This, together with the lack of patient’s #1 genomic material at time of diagnosis, hinders a verification of this hypothesis.
This case offers a precious possibility to interrogate on the cell-of-origin and the impact of preleukemic mutations in the development of AML. It also poses ethical questions in relation to the physician’s responsibility to inform donors and recipients when DDMN occur. Furthermore, this report further underlines the role of NGS as an essential tool to not only assess AML features refining patients’ risk stratification, but also to carry out familial studies and identify hereditary predispositions to myeloid neoplasms.
Funding Statement
Funding: The molecular studies as carried out in Munich Leukemia Laboratory (MLL) were part of the study: Solving Riddles Through Sequencing (SIRIUS) (clinicaltrials gov. Identifier: NCT05046444) and were sponsored in part by MLL and Illumina. This work was supported in part by the Italian Association for Cancer Research (AIRC, Milan, Italy), Metastases 5×1000 Special Program, and grant 21198 (to RF).
Data-sharing statement
NGS data of the two patients is available upon request.
References
- 1.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubota Y, Zawit M, Durrani J, et al. Significance of hereditary gene alterations for the pathogenesis of adult bone marrow failure versus myeloid neoplasia. Leukemia. 2022;36(12):2827-2834. [DOI] [PubMed] [Google Scholar]
- 3.Deshmukh KG, Kelemen K. Lessons learned from donor cell-derived myeloid neoplasms: report of three cases and review of the literature. Life (Basel). 2022;12(4):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niederwieser DW, Appelbaum FR, Gastl G, et al. Inadvertent transmission of a donor’s acute myeloid leukemia in bone marrow transplantation for chronic myelocytic leukemia. N Engl J Med. 1990;322(25):1794-1796. [DOI] [PubMed] [Google Scholar]
- 5.Sala-Torra O, Hanna C, Loken MR, et al. Evidence of donor-derived hematologic malignancies after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(5):511-517. [DOI] [PubMed] [Google Scholar]
- 6.Marchionni L, Lobo FP, Kostadinov R, et al. Donor-derived acute myeloid leukemia in solid organ transplantation. Am J Transplant. 2022;22(12):3111-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodó I, Peters M, Radich JP, et al. Donor-derived acute promyelocytic leukemia in a liver-transplant recipient. N Engl J Med. 1999;341(11):807-813. [DOI] [PubMed] [Google Scholar]
- 8.Glasser L, Meloni-Ehrig A, Greaves W, Demel KC, Butera J. Synchronous development of acute myeloid leukemia in recipient and donor after allogeneic bone marrow transplantation: report of a case with comments on donor evaluation. Transfusion. 2009;49(3):555-562. [DOI] [PubMed] [Google Scholar]
- 9.Dickson MA, Papadopoulos EB, Hedvat CV, Jhanwar SC, Brentjens RJ. Acute myeloid leukemia arising from a donor derived premalignant hematopoietic clone: a possible mechanism for the origin of leukemia in donor cells. Leuk Res Rep. 2014;3(2):38-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurnari C, Robin M, Godley LA, et al. Germline predisposition traits in allogeneic hematopoietic stem-cell transplantation for myelodysplastic syndromes: a survey-based study and position paper on behalf of the Chronic Malignancies Working Party of the EBMT. Lancet Haematol. 2023;10(12):e994-e1005. [DOI] [PubMed] [Google Scholar]
- 11.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377. [DOI] [PubMed] [Google Scholar]
- 13.Im AP, Sehgal AR, Carroll MP, et al. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid malignancies: associations with prognosis and potential treatment strategies. Leukemia. 2014;28(9):1774-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacher U, Haferlach T, Kern W, Haferlach C, Schnittger S. A comparative study of molecular mutations in 381 patients with myelodysplastic syndrome and in 4130 patients with acute myeloid leukemia. Haematologica. 2007;92(6):744-752. [DOI] [PubMed] [Google Scholar]
- 15.Gibson CJ, Kim HT, Zhao L, et al. Donor clonal hematopoiesis and recipient outcomes after transplantation. J Clin Oncol. 2022;40(2):189-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NGS data of the two patients is available upon request.