Abstract
Anticoagulants have long been fundamental in preventing and treating thromboembolic disorders, with a recent shift of focus towards direct oral anticoagulants, thanks to their ease of use, efficacy, and safety. Despite these advancements, bleeding complications remain a major concern with any anticoagulant, highlighting the need for safer drugs. Factor XI (FXI) inhibitors have emerged as promising agents in this regard, offering a novel approach by targeting upstream factors in the coagulation system. Phase II trials have shown encouraging outcomes, indicating a reduced bleeding risk compared to that associated with traditional anticoagulants, particularly in the context of cardiovascular disease management when combined with antiplatelet therapy. However, the variability in findings and limited efficacy data call for a cautious interpretation pending insights from phase III trials. These trials are essential for validating the potential of FXI inhibitors to balance bleeding risk reduction and maintain anticoagulant efficacy. This review explores the pharmacology, potential indications, clinical data, and future directions of FXI inhibitors, providing a perspective on their evolving role in anticoagulant therapy. It also provides a detailed analysis of data from published clinical trials on FXI inhibitors in various indications. Preliminary data from ongoing trials are also outlined. As the field moves forward, a cautiously optimistic outlook can be expected, focusing on comprehensive data from phase III trials to define the role of FXI inhibitors in various clinical scenarios.
Introduction
Anticoagulants are key in the prevention and treatment of arterial and venous thromboembolism (VTE). Historically, vitamin K antagonists such as warfarin were the mainstay of anticoagulant therapy, prized for their effectiveness in a broad range of thromboembolic conditions. The emergence of direct oral anticoagulants (DOAC) since 2010 has marked a significant shift in this landscape. DOAC have rapidly replaced vitamin K antagonists for various indications after demonstrating at least comparable efficacy in stroke prevention in atrial fibrillation (AF) patients1,2 and in the treatment of VTE, with enhanced safety.3,4 In this regard, a noteworthy advantage of DOAC is the reduction of the risk of serious bleeding, especially intracranial hemorrhage, compared to that associated with the use of vitamin K antagonists. Additionally, DOAC offer the convenience of fewer drug-drug and food-drug interactions, and fixed-dose administration without the need for routine monitoring of the anticoagulant effect.5
Despite the benefits of DOAC, thromboembolic diseases continue to pose substantial morbidity and mortality risks, and existing anticoagulants, by disrupting hemostasis, may result in bleeding complications that offset the benefits of antithrombotic treatment.6 This underscores the ongoing need for effective anticoagulants with lower bleeding risks. In response, research has shifted towards upstream factors in the coagulation system, focusing primarily on the contact pathway, where factor XI (FXI) has emerged as a promising target. These new anticoagulants aim to address unmet clinical needs, offering potentially safer alternatives with minimal bleeding risks.7,8 In the evolving landscape of anticoagulant therapy, patients requiring anticoagulation who are also at heightened risk of bleeding complications stand to gain considerably from the targeted action of FXI inhibitors. This review explores the pharmacological properties of these new oral and parenteral anticoagulants, analyzes clinical data on these agents, and provides a perspective on the opportunities and challenges faced by this emerging class of drugs.
Unmet clinical needs in antithrombotic treatment
The dilemma of bleeding risk and antithrombotic effect
The central challenge in anticoagulant therapy lies in achieving effective thrombosis prevention while minimizing the risk of bleeding. While DOAC have shown some improvement over vitamin K antagonists in this regard, they have not eliminated bleeding risks entirely.9
Anticoagulant treatment in patients with atrial fibrillation at high risk of bleeding
In a network meta-analysis of 23 randomized, controlled trials in AF patients, all DOAC significantly reduced the risk of intracranial hemorrhage compared to warfarin (odds ratio range, 0.31-0.46), with varying risks of major bleeding and gastrointestinal bleeding across the different DOAC.1 However, real-world data from European and Canadian studies involving 421,523 patients with non-valvular AF showed no clear superiority of DOAC over vitamin K antagonists with regard to major bleeding risks.10
The risk-benefit profile becomes even more precarious in specific groups of patients, such that concerns about bleeding risk lead to many AF patients either not receiving or being underdosed with DOAC.11,12
Atrial fibrillation patients with coronary stent implantation or acute ischemic stroke
About 10% of patients undergoing coronary stent implantation require anticoagulant treatment, predominantly due to coexisting AF. Combining antiplatelet therapy with anticoagulants further increases the bleeding risk associated with anticoagulant treatment, and major bleeding rates in these patients are 4% to 12% within the first year of triple therapy.13,14
This dilemma is further exacerbated in patients with AF and acute ischemic stroke, in whom the timing of initiation of anticoagulant treatment is critical to balance the risks of hemorrhagic transformation and recurrent ischemic stroke. The optimal window for initiating anticoagulant treatment for secondary stroke prevention is suggested to be 4 to 14 days after the stroke. Notably, large ischemic lesions, a high CHA2DS2-VASc score [CHADS, Congestive heart failure, Hypertension, Age 75 or more, Diabetes, Stroke; VASc, Vascular disease, Age 65-74, Sex category (female sex)], a high National Institutes of Health Stroke Scale score and the choice of antithrombotic treatment are all associated with a higher risk of both ischemic recurrence and bleeding.15,16
Elderly patients and patients with comorbidities requiring anticoagulant treatment
Elderly patients, often burdened with comorbidities and polypharmacy, are at increased risk of both bleeding and thrombosis, confounding the risk-benefit of anticoagulation.17,18 Patients with chronic conditions such as liver cirrhosis and
chronic kidney disease also present unique challenges. Cirrhotic patients appear to have a rebalanced hemostasis with hypercoagulable elements,19 and are at risk of thrombotic complications, particularly splanchnic vein thrombosis.20 Conversely, complications of portal hypertension, such as esophageal varices, increase the risk of bleeding.21 Chronic kidney disease carries a risk of both bleeding and thromboembolic events due to platelet dysfunction. The thromboembolic risk is markedly higher in patients with concurrent AF and chronic kidney disease than in those with either condition alone.22 Cancer patients, particularly those with metastatic disease or primary gastrointestinal and genitourinary malignancies, are at an increased risk of VTE recurrence as well as bleeding, making the management of anticoagulant therapy in these patients especially challenging.23-25 These complexities underline the need for safer anticoagulants, preferably with minimal renal clearance and enhanced efficacy, with FXI inhibitors emerging as potential solutions.
Catheter-related thrombosis
There is currently limited evidence guiding the optimal management of catheter-related thrombosis, often resulting in treatment challenges in clinical practice. Central venous catheters, widely used in cancer patients to deliver chemotherapy and to obtain blood samples, are associated with various complications, including catheter-related thrombosis. While symptomatic catheter-related thrombosis occurs in about 5% of cases, asymptomatic catheter-related thrombosis is more prevalent, with incidences ranging from 14-18%.26 In patients undergoing long-term total parenteral nutrition, the risk of catheter-related thrombosis is a major concern, posing challenges in the maintenance of venous access and successful total parenteral nutrition. Despite various efforts to mitigate these thrombotic complications, including the use of heparinized solutions and heparin-bonded catheters, the lack of high-quality clinical trials and consensus limits effective intervention.27 Thrombosis of hemodialysis catheters can lead to dysfunction and potentially render vessels unusable for future access, resulting in significant limb morbidity. The best preventative approach involves minimizing the use of central venous catheters and ensuring meticulous care and vigilant monitoring when it is indispensable, to reduce the risk of complications and preserve vascular access routes.28,29
Orthopedic surgery
In patients undergoing orthopedic surgery, especially total knee and hip arthroplasties, VTE prophylaxis is fundamental.30 Conventional anticoagulants, which target factor Xa or thrombin, are efficacious but associated with a marked risk of bleeding.31 This issue highlights the imperative for anticoagulation strategies that are both effective and safe. Against this backdrop, FXI inhibitors stand out as a promising avenue, potentially delivering effective thromboprophylaxis while minimizing bleeding risks, thus meeting the pressing demand for safer anticoagulants in patients undergoing arthroplasty procedures.
Patients with mechanical heart valves and external devices
Anticoagulation remains a pivotal yet challenging aspect in the management of valvular heart disease, particularly with mechanical heart valves. The standard treatment with warfarin has considerable limitations, prompting the search for alternatives. However, the use of DOAC in patients with mechanical heart valves has encountered setbacks, as evidenced by the failure of dabigatran and apixaban in major clinical trials, while rivaroxaban showed promise in smaller studies. Meanwhile, vitamin K antagonists continue to be the only approved oral anticoagulants for preventing valve thrombosis.32-34 The situation is further complicated in settings involving external surfaces such as left ventricular assist devices or extracorporeal membrane oxygenation, in which the safety profile and appropriate use of DOAC are yet to be clarified.
In patients with left ventricular assist devices, the challenge lies in balancing the risk of pump thrombosis against bleeding complications due to coagulopathies such as an acquired von Willebrand syndrome.35-37 Thus, there is a pressing need for randomized controlled trials to establish optimal antithrombotic regimens in these patients, particularly in the context of new valve designs and targeting different aspects of the coagulation cascade.
Factor XI as a therapeutic target in anticoagulation
The contact pathway of coagulation
The contact pathway of coagulation, comprising FXI, factor XII, prekallikrein, and high-molecular-weight kininogen, participates in procoagulant and proinflammatory responses, thereby connecting inflammation and coagulation processes. Available data imply that this pathway is more contributory to pathological thrombosis and inflammation than to physiological hemostasis.38-40 Contact activation occurs when negatively charged macromolecules, such as extracellular DNA, RNA from activated or dying cells, polyphosphates from activated platelets or microorganisms, or artificial surfaces, interact with plasma. This interaction leads to the downstream activation of both coagulation and inflammation. FXI functions as a serine protease responsible for thrombin generation through the activation of other coagulation factors. It acts as a bridge between tissue factor-mediated coagulation and the kallikrein-kinin system, primarily functioning in prolonged activation scenarios such as VTE. Notably, FXI is less crucial for initiating clot formation, but appears to play a significant role in clot stabilization and expansion.40
Elevated levels of factor XI
Elevated levels of FXI have been linked to an increased risk of cardiovascular events in patients with type 2 diabetes mellitus, suggesting that FXI plays a critical role in thrombosis in these patients.41 Similarly, circulating activated FXI has been identified as a predictor of arterial thromboembolic events in patients with stable coronary artery disease, further supporting the potential of FXI inhibitors as novel antithrombotic agents in these high-risk groups.42 Additionally, a recent case-control study found that elevated FXI levels, especially when persistently high, significantly increase the risk of VTE.43 This is further supported by a study that demonstrated the association of elevated FXI levels and activation with acute VTE and recurrent events,44 highlighting the value of inhibiting activated FXI (FXIa) in the setting of acute VTE. Although elevated FXI levels are associated with an increased risk of thrombotic events, the precise threshold beyond which FXI becomes a significant risk factor remains unclear.
Factor XI deficiency and its clinical implications
FXI deficiency is a rare disorder inherited as an autosomal recessive trait. Patients exhibit markedly prolonged activated partial thromboplastin times, yet even individuals with extremely low FXI levels may remain asymptomatic, indicating a complex relationship between FXI levels and clinical manifestations. Bleeding in FXI-deficient patients typically occurs following trauma or surgery, particularly in tissues with high fibrinolytic activity, such as the oral cavity, nasal passages, and the urinary tract.45,46 This pattern of bleeding underscores the role of FXI in clot stabilization rather than initiation, aligning with its involvement in the later stages of the coagulation cascade.47 Treatment options for FXI deficiency include antifibrinolytics, fresh-frozen plasma, plasma-derived FXI concentrates, and low-dose recombinant activated factor VII. However, despite cautious usage, especially in the perioperative period, FXI concentrates were associated with a risk of thrombotic complications, as evidenced by rare instances of transient ischemic attack and pulmonary embolism in treated patients.48
Low factor XI levels: available data
Evidence consistently points to a lower thrombotic risk among individuals with lower FXI levels. Patients with severe FXI deficiency had a significantly reduced incidence of deep vein thrombosis compared to the general population, indicating a potential protective effect.49 Similarly, a study suggested specific protection against ischemic stroke.50 Another cohort study further supported these findings, showing a decreased incidence of both VTE and cardiovascular events in individuals with FXI deficiency.51 A linear relationship between FXI activity levels and the risk of recurrent VTE has been observed, with patients having FXI activity below the 34th percentile experiencing significantly fewer recurrences,52 supporting the concept of FXI as a promising target for anticoagulation.
Differentiating thrombosis from hemostasis
The unique characteristics of FXI deficiency highlight the potential for targeting FXI in antithrombotic therapy, as patients often have extended clotting times without a severe bleeding tendency and show a reduced risk of certain thrombotic events.53
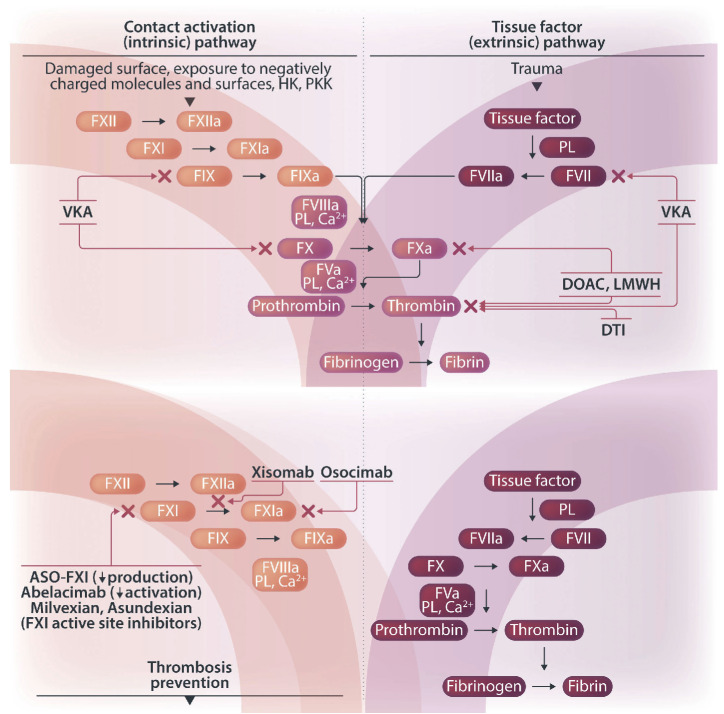
Thrombosis and hemostasis, while sharing enzymatic pathways, result in divergent physiological outcomes. Hemostasis, a natural response to vascular injury, results in the formation of a localized hemostatic plug to halt bleeding. Thrombosis, typically pathological, is characterized by the formation of intravascular thrombi that can interrupt blood flow and potentially lead to organ damage. Differentiating thrombosis from hemostasis is vital for the development of antithrombotic agents that prevent thrombosis without adversely affecting hemostasis, making the contact pathway a promising target for safer anticoagulants. Given its specific role in thrombosis without a significant impact on hemostasis, inhibition of upstream factors in this pathway can potentially decouple thrombosis from hemostasis, offering a promising strategy to reduce the risk of anticoagulant-related bleeding. This approach is particularly advantageous as it focuses on a single pathway rather than affecting multiple coagulation factors or engaging with the tissue factor/factor VII and common pathways (Figure 1). It potentially offers a safer alternative to currently available anticoagulants, which directly affect fibrin formation;53-55 however, while the involvement of the contact pathway in pathological thrombosis presents a compelling target for intervention, the redundancy and compensatory mechanisms within the coagulation system may pose challenges to the efficacy of targeting this pathway alone.
Preclinical studies
Preclinical studies in animal models have been instrumental in understanding the role of FXI in thrombosis. Notably, mice genetically modified to lack FXI showed protection against occlusive thrombosis in both venous and arterial systems, highlighting the role of FXI in thrombus formation.56,57 Additionally, targeting FXI with monoclonal antibodies, antisense oligonucleotides, and specific small molecules has been shown to effectively prevent thrombosis in various mammalian models.58-63 Pharmacological inhibition of FXI in low-density lipoprotein receptor-knockout mice reduced the extent of atherosclerotic lesions, suggesting that targeting FXI may even offer a safe and effective strategy to slow the progression of atherosclerosis.64
Figure 1.
Differentiating thrombosis from hemostasis for safer anticoagulation. Differentiating thrombosis from hemostasis is vital for safer anticoagulation. Hemostasis stops bleeding through a local plug, while thrombosis can obstruct blood flow, risking organ damage. The contact pathway, less involved in hemostasis but more in thrombosis, appears to offer a safer target for new anticoagulants. Inhibition of this pathway is less likely to cause bleeding than drugs affecting multiple coagulation factors or the common pathways. Targeted agents include antisense oligonucleotides, monoclonal antibodies and small molecules, focusing on factor XI to enhance safety profiles. HK: high molecular weight kininogen; PKK: prekallikrein; F: factor; VKA: vitamin K antagonists; PL: phospholipids; DOAC: direct oral anticoagulants; LMWH: low molecular weight heparins; DTI: direct thrombin inhibitors; ASO: antisense oligonucleotides.
The cumulative preclinical findings emphasize the efficacy and safety of FXI inhibition as a promising therapeutic approach to reduce thrombotic events while maintaining a favorable safety profile. Translating these findings into safe and effective clinical therapies requires careful navigation of the complex coagulation landscape in humans, in whom compensatory mechanisms and individual variability may influence therapeutic outcomes.
Available strategies to inhibit factor XI
Preclinical studies have set the foundation for the development of a range of anticoagulants targeting FXI, demonstrating their ability to prevent venous and arterial thrombosis in various animal models. Subsequent early phase human studies have confirmed the safety profile of these agents.65-72 A notable advantage of molecule-specific targeting is the substantial reduction in the risk of off-target adverse effects; however, establishing non-inferior efficacy in thrombosis prevention compared to standard anticoagulants, while offering a potentially lower risk of bleeding, is essential for the advancement of FXI-targeting strategies in clinical trials.8
Different strategies have emerged to inhibit FXI, including antisense oligonucleotides that reduce hepatic synthesis of FXI,59-62,64,65,73-76 monoclonal antibodies that inhibit FXI activation or its enzymatic activity,66-69,77-82 and small molecules designed to block the active site of FXI or induce its allosteric modulation.63,70-72,83-91 These strategies not only differ in their mechanism of action but also possess unique pharmacological properties that shape their potential clinical applications.
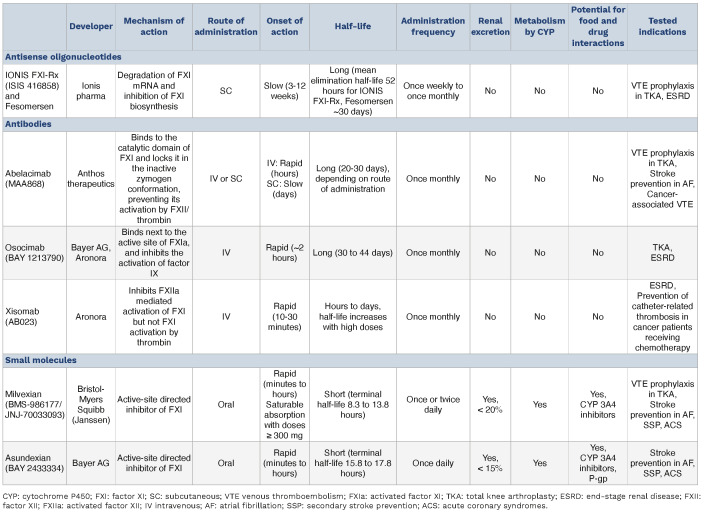
The pharmacological attributes of these agents, including their pharmacodynamics, pharmacokinetics, and possible drug interactions, are presented in Table 1.
Additional approaches, such as parenteral small molecules targeting the active site of FXI or exosites,92-95 DNA aptamers that selectively bind to and inhibit FXI96,97 and natural inhibitors of FXIa derived from snakes,98 vampire bats,99 and ticks,100 have been explored in vitro and in animal models. These agents have not yet progressed into clinical studies and therefore fall outside the scope of this review.
Antisense oligonucleotides
FXI, primarily synthesized in the liver, is an ideal target for antisense oligonucleotide therapy.101 Antisense oligonucleotides such as IONIS FXI-Rx and fesomersen (formerly known as IONIS-FXI-LRx) specifically inhibit the biosynthesis of FXI by binding to its mRNA, leading to its degradation, and reduce protein levels with a high degree of target selectivity.59 Antisense oligonucleotides have demonstrated effectiveness in various animal models59-62,64 and in human studies.65,73-76 They require parenteral administration by subcutaneous or intravenous injection, and have a slow onset of action (it typically takes 3 to 4 weeks to achieve therapeutic FXI levels), which limits their use in acute settings. Antisense oligonucleotides have been investigated in phase II clinical trials as VTE prophylaxis in patients undergoing total knee arthroplasty,73 and for the prevention of cardiovascular events in patients with end-stage renal disease.74 ,7 6
Monoclonal antibodies targeting factor XI
Monoclonal antibodies targeting FXI, such as abelacimab (MAA868), osocimab (BAY 1213790) and xisomab (AB023), have shown potential in selectively inhibiting FXI activation. Abelacimab acts by binding to the catalytic domain of FXI and locks it in an inactive conformation, thus preventing its activation by activated factor XII or thrombin.68 Osocimab binds adjacent to the active site of FXIa, preventing it from activating factor IX.66 In contrast, xisomab functions as a backdoor inhibitor of activated factor XII, reducing FXI activation by activated factor XII as well as reciprocal activation of factor XII by FXIa.79 Monoclonal antibodies require parenteral administration and are characterized by a rapid onset of FXI inhibition and relatively long half-lives, which enable their use in both acute and chronic settings and could allow for less frequent dosing. However, this extended activity also underscores the need for effective reversal strategies in the event of bleeding complications. Antibodies have also been studied in phase II clinical trials as VTE prophylaxis in patients undergoing total knee arthroplasty,7 7,7 8 as well as for the prevention of adverse cardiovascular outcomes in patients with end-stage renal disease,81 and are being investigated for stroke prevention in patients with AF.
Small molecules
Small molecule inhibitors of FXI, such as milvexian (BMS-986177/JNJ-70033093) and asundexian (BAY 2433334), directly inhibit FXI at its active site. They can be administered either orally or parenterally, and are designed for rapid FXI inhibition, making them suitable in both acute and chronic therapeutic settings, offering a more flexible and patient-friendly approach.70,71 Unlike antisense oligonucleotides and antibodies, these small molecules have shorter half-lives, requiring once or twice daily dosing, similar to DOAC. Parenterally administered small molecules, such as BMS-262084 and BMS-654457, have been primarily studied in animal models.93,94 Small molecule FXI inhibitors have been investigated for VTE prophylaxis in patients undergoing total knee arthroplasty,84 for secondary stroke prevention in patients with non-cardioembolic ischemic stroke,86,89 for stroke prevention in patients with AF,88 and as antithrombotic treatment following acute myocardial infarction.90
Table 1.
Characteristics, mechanism of action and pharmacological properties of factor XI and factor XII inhibitors.
Table 2.
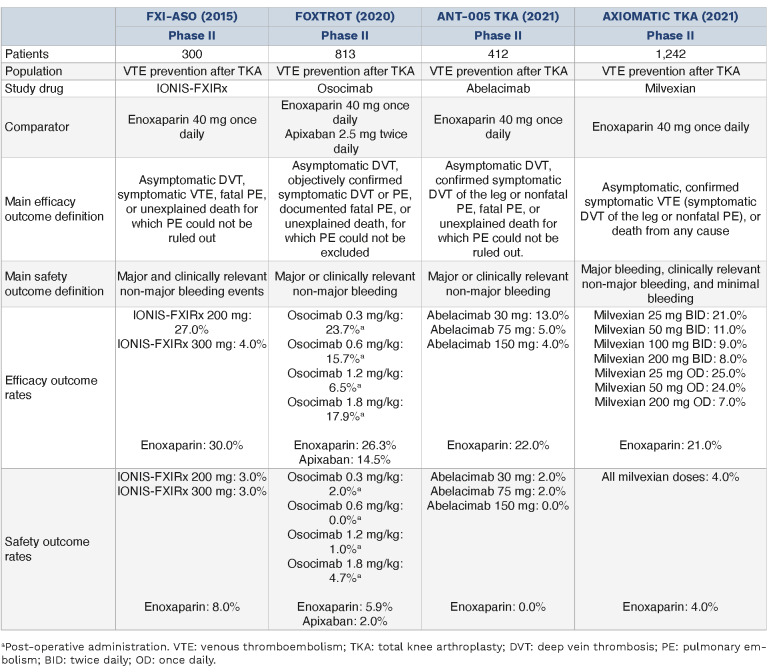
Overview of factor XI inhibitors in the prevention of venous thromboembolism after total knee arthroplasty.
Reversal strategies
The diversity of FXI inhibitor regimens, especially those of extended activity, underscores the need for a clear understanding of their implications for bleeding risk and, as with current anticoagulants, establishing reversal protocols is essential. In cases of bleeding or need for urgent surgery, evidence indicates that, similar to the management in FXI deficiency, antifibrinolytics and, in some cases, recombinant activated factor VII can effectively address bleeding without the need for FXI replacement. This approach is expected to be applicable for those undergoing therapy with FXI inhibitors.102 Currently, there is active evaluation of fully human antibody Fab fragments, known for their remarkably high affinity for FXIa inhibitors, focused on determining their ability to counteract the anticoagulant effects of the inhibitors.103 However, these strategies currently lack validation for reversal of FXI inhibitors and need to be further assessed in randomized controlled trials. Any development of specific antidotes should take into account the varied mechanisms of action and unique pharmacokinetic properties of FXI inhibitors, ensuring targeted and effective reversal strategies, thereby optimizing safety and therapeutic outcomes.
Factor XI inhibitors in completed and ongoing clinical trials
The following sections offer a comprehensive overview of the results already presented, along with insights into preliminary findings from ongoing phase III trials. Results of completed clinical trials are summarized in Tables 2-4. Ongoing clinical trials are outlined in Table 5.
Prevention of venous thromboembolism in patients undergoing total knee arthroplasty
In studies focused on the use of FXI inhibitors for VTE prophylaxis in patients undergoing total knee arthroplasty, significant findings demonstrate their potential efficacy and safety. In a randomized, open-label study, IONIS-FXRx at doses of 200 mg and 300 mg lowered FXI activity, with the 200 mg dose showing a 4% lower risk and the 300 mg dose a 26% lower risk of thrombotic events compared to enoxaparin. However, differences in major bleeding and clinically relevant non-major bleeding (CRNMB) were not statistically significant, with a risk difference of 6% for both doses.73
Table 3.
Overview of factor XI inhibitors in various cardiovascular indications (atrial fibrillation, acute myocardial infarction and stroke).
Table 4.
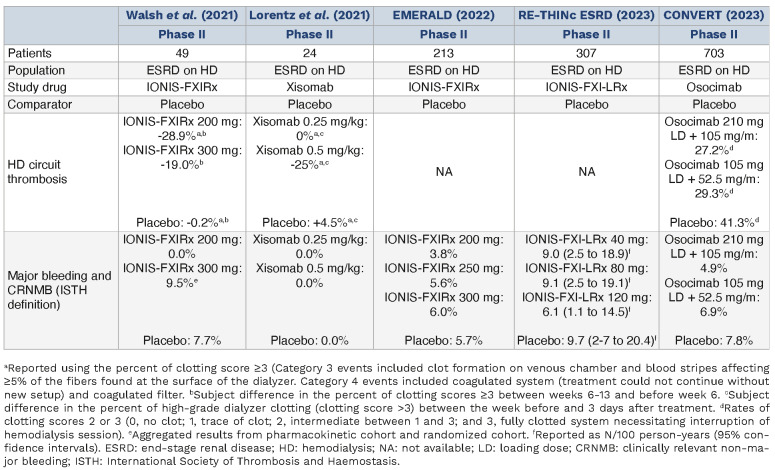
Factor XI inhibitors in end-stage renal disease patients undergoing hemodialysis.
The FOXTROT trial, comparing various osocimab doses (0.3 mg/kg to 1.8 mg/kg) with enoxaparin or apixaban, found that higher osocimab doses (0.6, 1.2, and 1.8 mg/kg) were non-inferior to enoxaparin, with the 1.8 mg/kg dose superior in reducing VTE events. Bleeding occurred in up to 4.7% of osocimab patients compared to 5.9% in enoxaparin-treated and 2% in apixaban-treated patients.78
In the ANT-005 TKA trial, abelacimab at 30 mg, 75 mg, and 150 mg was compared with enoxaparin. VTE occurred in 13%, 5%, and 4% of the abelacimab groups, respectively, against 22% in the enoxaparin group, showing higher doses of abelacimab were more effective. Bleeding rates were low and comparable across all groups.77
The AXIOMATIC-TKR trial found that higher doses of milvexian (50 mg, 100 mg, and 200 mg twice daily) significantly reduced VTE rates without major bleeding events, suggesting a favorable safety profile.84
A meta-analysis combining data from these studies reported significant reductions in VTE and major or CRNMB events with FXI inhibitors. The odds ratio for reducing VTE events was 0.50 (95% confidence interval [CI]: 0.36-0.69], while that for major or CRNMB events was 0.41 (95% CI: 0.22-0.75), indicating a substantial improvement over low molecular weight heparins.104 While these studies collectively suggest a promising role for FXI inhibitors in reducing VTE risk after total knee arthroplasty, variability in dosing regimens across trials underscores the need for standardized protocols and larger, multicenter phase III trials to confirm these findings and ensure generalizability. Further research, such as the ongoing REGN9933 study (NCT05618808), is expected to provide additional insights by August 2024; however, there are currently no phase III trials investigating FXI inhibitors in this setting.
Prevention of stroke and systemic embolism in patients with atrial fibrillation
In the realm of FXI inhibitors for stroke prevention in AF patients, key studies present varied results. The phase II PACIFIC-AF trial showed that asundexian potentially reduces bleeding events compared to apixaban, with incidence proportion ratios of 0.33 for pooled asundexian groups and 0.5 and 0.38 for the 20 mg and 50 mg doses, respectively.88 However, the full scope of the efficacy and safety of asundexian remains to be robustly analyzed.
The phase II AZALEA-TIMI 71 trial with abelacimab was stopped early due to significant reductions in major and CRNMB events, with hazard ratios (HR) of 0.33 and 0.23 for major and CRNMB, respectively; a 93% reduction in major gastrointestinal bleeding with the 150 mg dose was particularly noteworthy. There was, however, a slight, non-significant rise in the rates of ischemic stroke and systemic embolism with abelacimab.105
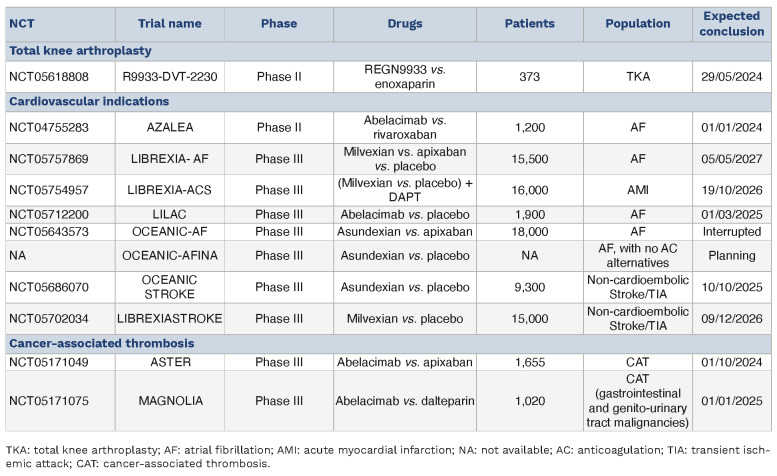
Table 5.
Ongoing clinical trials with factor XI inhibitors
In contrast, the phase III OCEANIC-AF trial, comparing asundexian with apixaban, was halted prematurely due to concerns of inferior efficacy with asundexian.106 The upcoming OCEANIC-AFINA trial will examine the efficacy of asundexian against placebo in older AF patients unsuitable for treatment with DOAC.107 The focus on older AF patients unsuitable for DOAC treatment underscores the potential of FXI inhibitors to safely address anticoagulation needs in populations in which traditional therapies pose significant risks. Additionally, the ongoing phase III trials LIBREXIA-AF (NCT05757869) and LILAC (NCT05712200) are assessing milvexian and abelacimab, respectively, for the prevention of ischemic stroke and systemic embolism in AF patients.
Antithrombotic treatment in acute ischemic stroke
In the evaluation of FXI inhibitors for the prevention of recurrent non-cardioembolic ischemic stroke, recent trials have yielded nuanced findings. The phase II PACIFIC-Stroke trial compared asundexian with placebo in conjunction with standard antiplatelet therapy, finding no significant differences in primary efficacy outcomes, including symptomatic and covert brain infarction. Asundexian 50 mg showed a non-significant reduction in ischemic stroke recurrence (HR=0.53, 90% CI: 0.24-1.17) and no significant increase in major or CRNMB events (HR=1.57, 90% CI: 0.91-2.71).89
Similarly, the AXIOMATIC-SSP trial assessed various doses of milvexian alongside dual antiplatelet therapy. Although fewer stroke recurrences were noted with most milvexian doses (except the 200 mg daily dose) compared to placebo at 90 days, no dose showed significant efficacy in reducing the composite outcome of symptomatic ischemic stroke or covert brain infarction. The risk of major bleeding also did not increase significantly across different doses.86
A meta-analysis of phase II trials found no significant reduction in the occurrence of ischemic stroke in patients treated with FXI inhibitors compared to controls (relative risk [RR]=0.89, 95% CI: 0.67-1.17) and a non-significant increase in major and CRNMB events (RR=1.19, 95% CI: 0.65-2.16), although with considerable statistical heterogeneity.108 Given the limited power of these phase II trials, ongoing phase III trials, OCEANIC-STROKE (NCT05686070) and LIBREXIA-STROKE (NCT05702034), are expected to provide more definitive conclusions on the efficacy and safety of FXI inhibitors in this context.
Antithrombotic treatment in acute myocardial infarction
In the context of FXI inhibition for treating patients with acute myocardial infarction the PACIFIC-AMI phase II trial stands out as a pivotal study. This trial investigated the combination of asundexian with dual antiplatelet therapy (aspirin and a P2Y12 inhibitor) in 1,601 patients with recent acute myocardial infarction. The findings showed no significant differences in Bleeding Academic Research Consortium (BARC) types 2, 3, or 5 bleeding between pooled recipients of asundexian doses and those receiving placebo (HR=0.98, 90% CI: 0.71-1.35). Additionally, the composite efficacy outcome of cardiovascular death, acute myocardial infarction, stroke, or stent thrombosis showed no notable difference when comparing pooled asundexian 20 and 50 mg doses with placebo (HR=1.05, 90% CI: 0.69-1.61).90 The lack of significant differences in efficacy outcomes raises questions about the benefit of adding a third drug to the treatment regimen, as it might not offer additional benefits over current antiplatelet and anticoagulant regimens for patients with acute myocardial infarction. The phase II LIBRXIA-ACS trial (NCT05754957) is ongoing, focusing on milvexian in a similar group of patients. However, due to the modest outcomes in current studies and the lack of phase III trial data, the definitive role of FXI inhibitors in the management of acute myocardial infarction remains uncertain.
Patients with end-stage renal disease requiring hemodialysis
In phase II trials investigating FXI inhibitors in patients with end-stage renal disease on hemodialysis, several studies have reported promising results. The IONIS-FXIRx study showed a reduction in FXI activity by as much as 70.7%, with significant decreases in hemodialysis circuit thrombosis—28.9% for the 200 mg dose and 19.0% for the 300 mg dose, versus a 1.6% reduction in the placebo group. Major bleeding events were notably rare.74 Similarly, in the EMERALD trial there were comparable rates of major and CRNMB events between patients receiving IONIS-FXIRx doses and placebo, ranging from 3.8% to 6.0% versus 5.7% in the placebo group.109 The RE-THINc ESRD trial reported similar rates of bleeding across recipients of all fesomersen doses and placebo.110 A study of xisomab in this setting highlighted its potential role in reducing dialyzer clotting and influencing biomarkers such as thrombin-antithrombin complexes and C-reactive protein levels.81 In the CONVERT trial, osocimab showed non-significant reductions in major and CRNMB rates and incidence of dialysis circuit thrombosis compared to placebo, with hazard ratios of 0.66 and 0.71 for high and low doses, respectively.111 The promising outcomes from these studies could herald a paradigm shift in the management of thrombotic complications for end-stage renal disease patients undergoing hemodialysis, potentially offering a safer anticoagulation strategy with FXI inhibitors that balances efficacy with a manageable bleeding risk profile. Nevertheless, phase III trials are needed for conclusive evidence.
Cancer-associated thrombosis
Cancer-associated thrombosis represents a significant challenge in oncology, necessitating a delicate balance between anticoagulant efficacy and safety.112 Two notable phase III trials, ASTER (NCT05171049) and MAGNOLIA (NCT05171075), are evaluating the effectiveness and safety of abelacimab in various cancer types, with ASTER focusing on a broad range of malignancies and MAGNOLIA on gastrointestinal malignancies, comparing abelacimab to dalteparin and apixaban, respectively. The role of abelacimab is supported by the observed reduction in gastrointestinal bleeding in the AZALEA-TIMI 71 trial.105 A phase II trial (NCT04465760) exploring xisomab in thrombosis prevention in cancer patients was terminated early due to manufacturing issues, with preliminary data from nine patients showing one episode of catheter-related thrombosis and no major or CRNMB events.113 Given the high prevalence of thrombotic complications in oncology, especially with central venous catheters used for chemotherapy and nutrition, the ability of FXI inhibitors to reduce thrombotic events while minimizing bleeding risks holds promise for enhancing patient care in various high-risk settings and could transform management strategies in oncology and related fields.
Conclusions and future directions
The diverse landscape of FXI inhibitors, from long-acting antisense oligonucleotides and monoclonal antibodies to the oral small molecule inhibitors, provides a range of dosing regimens and pharmaceutical properties that can cater to different clinical needs. Initially heralded as a promising solution to separate bleeding risk from anticoagulation efficacy in preclinical studies, FXI inhibitors have shown encouraging results in phase II trials. These trials collectively suggest a reduced bleeding risk compared to traditional anticoagulants, although results vary across different molecules and clinical scenarios. However, phase II trials are underpowered to provide clear evidence on efficacy, and confirmation from phase III trials is necessary. A number of meta-analyses have tried to provide some more robust results from currently available data, but the different dosages used in the dose-finding studies, as well as some heterogeneity in the definition of efficacy and bleeding outcome across studies, especially in cardiovascular settings, limit the validity of such analyses, suggesting the need for more standardized approaches. The unexpected efficacy concerns leading to the premature termination of the OCEANIC-AF trial led to greater caution and to some concerns about the ability of FXI inhibitors to reduce the risk of thrombosis in high-risk patients, despite the potential for bleeding risk reduction. It is important to note that other phase III trials, including OCEANIC-STROKE that is assessing the same drug, have not been stopped for similar reasons. Furthermore, uncertainties persist regarding reversal strategies for bleeding episodes and potential off-target adverse effects in patients receiving FXI inhibitors. Ongoing phase III trials are essential not only to validate the potential of FXI inhibitors initially observed in phase 2 studies, but also to explore these inhibitors’ full potential across various dosages and administration strategies. In addition to the critical outcomes of phase III trials, future research directions could include investigating combination therapy approaches that might synergize with FXI inhibitors for enhanced efficacy or safety profiles. Novel drug formulations aimed at improving delivery, reducing side effects, or tailoring dosing to patient-specific needs represent another promising area of investigation, potentially revolutionizing anticoagulant therapy. Upcoming results are expected to address the current unmet clinical needs and potentially open up new applications for FXI inhibitors in areas demanding high anticoagulant efficacy, such as patients with mechanical heart valves and external devices. While there are grounds for cautious optimism, the path forward calls for a nuanced understanding of each inhibitor’s unique attributes to fully integrate them into the anticoagulation therapy paradigm.
References
- 1.López-López JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359:j5058. Erratum in: BMJ. 2017;359: j5631,. Erratum in: BMJ. 2018;361: k2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnicelli AP, Hong H, Connolly SJ, et al. ; COMBINE AF. Direct oral anticoagulants versus warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation. 2022;145(4):242-255. Erratum in: Circulation. 2022;145(8): e640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Es N, Coppens M, Schulman S, Middeldorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975. [DOI] [PubMed] [Google Scholar]
- 4.Haas S, Farjat AE, Pieper K, et al. ; GARFIELD-VTE investigators. On-treatment comparative effectiveness of vitamin K antagonists and direct oral anticoagulants in GARFIELD-VTE, and focus on cancer and renal disease. TH Open. 2022;6(4):e354-e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen A, Stecker E, A Warden B. Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc. 2020;9(13):e017559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piran S, Schulman S. Treatment of bleeding complications in patients on anticoagulant therapy. Blood. 2019;133(5):425-435. [DOI] [PubMed] [Google Scholar]
- 7.Cohen O, Ageno W. Coming soon to a pharmacy near you? FXI and FXII inhibitors to prevent or treat thromboembolism. Hematology Am Soc Hematol Educ Program. 2022;2022(1):495-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredenburgh JC, Weitz JI. News at XI: moving beyond factor Xa inhibitors. J Thromb Haemost. 2023;21(7):1692-1702. [DOI] [PubMed] [Google Scholar]
- 9.Ballestri S, Romagnoli E, Arioli D, et al. Risk and management of bleeding complications with direct oral anticoagulants in patients with atrial fibrillation and venous thromboembolism: a narrative review. Adv Ther. 2023;40(1):41-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Ham HA, Souverein PC, Klungel OH, et al. Major bleeding in users of direct oral anticoagulants in atrial fibrillation: a pooled analysis of results from multiple population-based cohort studies. Pharmacoepidemiol Drug Saf. 2021;30(10):1339-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park S, Je NK. Underutilization of anticoagulants in patients with nonvalvular atrial fibrillation in the era of non-vitamin K antagonist oral anticoagulants. Int J Arrhythm. 2022;23:1. [Google Scholar]
- 12.Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123(7):638-645. [DOI] [PubMed] [Google Scholar]
- 13.Smits PC, Frigoli E, Tijssen J, et al. MASTER DAPT Investigators. abbreviated antiplatelet therapy in patients at high bleeding risk with or without oral anticoagulant therapy after coronary stenting: an open-label, randomized, controlled trial. Circulation. 2021;144(15):1196-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito Y, Kobayashi Y. Triple therapy: a review of antithrombotic treatment for patients with atrial fibrillation undergoing percutaneous coronary intervention. J Cardiol. 2019;73(1):1-6. [DOI] [PubMed] [Google Scholar]
- 15.Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF study. Stroke. 2015;46(8):2175-2182. [DOI] [PubMed] [Google Scholar]
- 16.Smythe MA, Parker D, Garwood CL, Cuker A, Messé SR. Timing of initiation of oral anticoagulation after acute ischemic stroke in patients with atrial fibrillation. Pharmacotherapy. 2020;40(1):55-71. [DOI] [PubMed] [Google Scholar]
- 17.Botto G, Ameri P, Cappellari M, et al. Unmet clinical needs in elderly patients receiving direct oral anticoagulants for stroke prevention in non-valvular atrial fibrillation. Adv Ther. 2021;38(6):2891-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah SJ, Singer DE, Fang MC, Reynolds K, Go AS, Eckman MH. Net clinical benefit of oral anticoagulation among older adults with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2019;12(11):e006212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Northup PG, Lisman T, Roberts LN. Treatment of bleeding in patients with liver disease. J Thromb Haemost. 2021;19(7):1644-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riva N, Ageno W. How to manage splanchnic vein thrombosis in patients with liver disease. Hematology Am Soc Hematol Educ Program. 2023;2023(1):281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauro E, Gadano A. What’s new in portal hypertension? Liver Int. 2020;40 Suppl 1:122-127. [DOI] [PubMed] [Google Scholar]
- 22.Ding WY, Gupta D, Wong CF, Lip GYH. Pathophysiology of atrial fibrillation and chronic kidney disease. Cardiovasc Res. 2021;117(4):1046-1059. [DOI] [PubMed] [Google Scholar]
- 23.Angelini DE, Radivoyevitch T, McCrae KR, Khorana AA. Bleeding incidence and risk factors among cancer patients treated with anticoagulation. Am J Hematol. 2019;94(7):780-785. [DOI] [PubMed] [Google Scholar]
- 24.Barca-Hernando M, Lopez-Ruz S, Marin-Romero S, et al. Risk of recurrent cancer-associated thrombosis after discontinuation of anticoagulant therapy. Res Pract Thromb Haemost. 2023;7(2):100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vedovati MC, Giustozzi M, Munoz A, et al. Risk factors for recurrence and major bleeding in patients with cancer-associated venous thromboembolism. Eur J Intern Med. 2023;112:29-36. [DOI] [PubMed] [Google Scholar]
- 26.Lee AY, Kamphuisen PW. Epidemiology and prevention of catheter-related thrombosis in patients with cancer. J Thromb Haemost. 2012;10(8):1491-1499. [DOI] [PubMed] [Google Scholar]
- 27.Zaccone V, Santoro L, Guerrieri E, et al. Prevention and treatment of catheter-related venous thrombosis in long-term parenteral nutrition: a SINuC position statement. Front Nutr. 2023;10:1106327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunawansa N, Sudusinghe DH, Wijayaratne DR. Hemodialysis catheter-related central venous thrombosis: clinical approach to evaluation and management. Ann Vasc Surg. 2018;51:298-305. [DOI] [PubMed] [Google Scholar]
- 29.Abrantes C, Soares E Sr, Valério P, Furtado T, Barreto C. Hemodialysis catheter-related thrombi: a challenging patient. Cureus. 2020;12(6):e8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson DR, Morgano GP, Bennett C, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019;3(23):3898-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gade IL, Kold S, Severinsen MT, et al. Venous thromboembolism after lower extremity orthopedic surgery: a population-based nationwide cohort study. Res Pract Thromb Haemost. 2020;5(1):148-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langenaeken T, Vanoppen A, Janssens F, et al. DOACs in the anticoagulation of mechanical valves: a systematic review and future perspectives. J Clin Med. 2023;12(15):4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang TY, Svensson LG, Wen J, et al. Apixaban or warfarin in patients with an On-X mechanical aortic valve. NEJM Evid. 2023;2(7):EVIDoa2300067. [DOI] [PubMed] [Google Scholar]
- 34.Ryu R, Tran R. DOACs in mechanical and bioprosthetic heart valves: a narrative review of emerging data and future directions. Clin Appl Thromb Hemost. 2022;28:10760296221103578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.den Exter PL, Beeres SLMA, Eikenboom J, Klok FA, Huisman MV. Anticoagulant treatment and bleeding complications in patients with left ventricular assist devices. Expert Rev Cardiovasc Ther. 2020;18(6):363-372. [DOI] [PubMed] [Google Scholar]
- 36.Morici N, Varrenti M, Brunelli D, et al. Antithrombotic therapy in ventricular assist device (VAD) management: from ancient beliefs to updated evidence. A narrative review. Int J Cardiol Heart Vasc. 2018;20:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sage W, Gottiparthy A, Lincoln P, Tsui SSL, Pettit SJ. Improving anticoagulation of patients with an implantable left ventricular assist device. BMJ Open Qual. 2018;7(4):e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Y. Contact pathway of coagulation and inflammation. Thromb J. 2015;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grover SP, Mackman N. Intrinsic pathway of coagulation and thrombosis. Arterioscler Thromb Vasc Biol. 2019;39(3):331-338. [DOI] [PubMed] [Google Scholar]
- 40.Raghunathan V, Zilberman-Rudenko J, Olson SR, Lupu F, McCarty OJT, Shatzel JJ. The contact pathway and sepsis. Res Pract Thromb Haemost. 2019;3(3):331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paszek E, Polak M, Bryk-Wiązania AH, Konieczyńska M, Undas A. Elevated plasma factor XI predicts cardiovascular events in patients with type 2 diabetes: a long-term observational study. Cardiovasc Diabetol. 2023;22(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paszek E, Pociask E, Ząbczyk M, et al. Active factor XI is associated with the risk of cardiovascular events in stable coronary artery disease patients. Atherosclerosis. 2022;346:124-132. [DOI] [PubMed] [Google Scholar]
- 43.Spiezia L, Forestan C, Campello E, Simion C, Simioni P. Persistently high levels of coagulation factor XI as a risk factor for venous thrombosis. J Clin Med. 2023;12(15):4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagy M, Robles AP, Visser M, et al. Predictive value for increased activated factor XI activity in acute venous thromboembolism. J Thromb Haemost. 2023;21(6):1610-1622. [DOI] [PubMed] [Google Scholar]
- 45.Barg AA, Livnat T, Kenet G. Factor XI deficiency: phenotypic age related considerations and clinical approach towards bleeding risk assessment. Blood. 2024:143(15):1455-1464. [DOI] [PubMed] [Google Scholar]
- 46.Mohammed BM, Matafonov A, Ivanov I, et al. An update on factor XI structure and function. Thromb Res. 2018;161:94-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewandowska MD, Connors JM. Factor XI deficiency. Hematol Oncol Clin North Am. 2021;35(6):1157-1169. [DOI] [PubMed] [Google Scholar]
- 48.Batty P, Honke A, Bowles L, et al. Ongoing risk of thrombosis with factor XI concentrate: 5 years experience in two centres. Haemophilia. 2015;21(4):490-495. [DOI] [PubMed] [Google Scholar]
- 49.Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost. 2011;105(2):269-273. [DOI] [PubMed] [Google Scholar]
- 50.Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111(8):4113-4117. [DOI] [PubMed] [Google Scholar]
- 51.Preis M, Hirsch J, Kotler A, et al. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood. 2017;129(9):1210-1215. [DOI] [PubMed] [Google Scholar]
- 52.Kyrle PA, Eischer L, Šinkovec H, Eichinger S. Factor XI and recurrent venous thrombosis: an observational cohort study. J Thromb Haemost. 2019;17(5):782-786. [DOI] [PubMed] [Google Scholar]
- 53.Gailani D, Bane CE, Gruber A. Factor XI and contact activation as targets for antithrombotic therapy. J Thromb Haemost. 2015;13(8):1383-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu C, Hutt E, Bloomfield DM, Gailani D, Weitz JI. Factor XI inhibition to uncouple thrombosis from hemostasis: JACC review topic of the week. J Am Coll Cardiol. 2021;78(6):625-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mackman N, Bergmeier W, Stouffer GA, Weitz JI. Therapeutic strategies for thrombosis: new targets and approaches. Nat Rev Drug Discov. 2020;19(5):333-352. [DOI] [PubMed] [Google Scholar]
- 56.Al-Horani RA, Afosah DK. Recent advances in the discovery and development of factor XI/XIa inhibitors. Med Res Rev. 2018;38(6):1974-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Smith PL, Hsu MY, et al. Effects of factor XI deficiency on ferric chloride-induced vena cava thrombosis in mice. J Thromb Haemost. 2006;4(9):1982-1988. [DOI] [PubMed] [Google Scholar]
- 58.Tucker EI, Marzec UM, White TC, et al. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113(4):936-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, Löwenberg EC, Crosby JR, et al. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116(22):4684-4692. [DOI] [PubMed] [Google Scholar]
- 60.Younis HS, Crosby J, Huh JI, et al. Antisense inhibition of coagulation factor XI prolongs APTT without increased bleeding risk in cynomolgus monkeys. Blood. 2012;119(10):2401-2408. [DOI] [PubMed] [Google Scholar]
- 61.Crosby JR, Marzec U, Revenko AS, et al. Antithrombotic effect of antisense factor XI oligonucleotide treatment in primates. Arterioscler Thromb Vasc Biol. 2013;33(7):1670-1678. Erratum in: Arterioscler Thromb Vasc Biol. 2013;33(8): e127,. Erratum in: Arterioscler Thromb Vasc Biol. 2013;33(10): e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prakash TP, Graham MJ, Yu J, et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42(13):8796-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong PC, Crain EJ, Bozarth JM, et al. Milvexian, an orally bioavailable, small-molecule, reversible, direct inhibitor of factor XIa: in vitro studies and in vivo evaluation in experimental thrombosis in rabbits. J Thromb Haemost. 2022;20(2):399-408. Erratum in: J Thromb Haemost. 2022;20(4): 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ngo ATP, Jordan KR, Mueller PA, et al. Pharmacological targeting of coagulation factor XI mitigates the development of experimental atherosclerosis in low-density lipoprotein receptor-deficient mice. J Thromb Haemost. 2021;19(4):1001-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crooke ST, Baker BF, Witztum JL, et al. The effects of 2’-O-methoxyethyl containing antisense oligonucleotides on platelets in human clinical trials. Nucleic Acid Ther. 2017;27(3):121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schaefer M, Buchmueller A, Dittmer F, Straßburger J, Wilmen A. Allosteric inhibition as a new mode of action for BAY 1213790, a neutralizing antibody targeting the activated form of coagulation factor XI. J Mol Biol. 2019;431(24):4817-4833. [DOI] [PubMed] [Google Scholar]
- 67.Thomas D, Thelen K, Kraff S, et al. BAY 1213790, a fully human IgG1 antibody targeting coagulation factor XIa: first evaluation of safety, pharmacodynamics, and pharmacokinetics. Res Pract Thromb Haemost. 2019;3(2):242-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koch AW, Schiering N, Melkko S, et al. MAA868, a novel FXI antibody with a unique binding mode, shows durable effects on markers of anticoagulation in humans. Blood. 2019;133(13):1507-1516. [DOI] [PubMed] [Google Scholar]
- 69.Yi BA, Freedholm D, Widener N, et al. Pharmacokinetics and pharmacodynamics of abelacimab (MAA868), a novel dual inhibitor of factor XI and factor XIa. J Thromb Haemost. 2022;20(2):307-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perera V, Wang Z, Luettgen J, et al. First-in-human study of milvexian, an oral, direct, small molecule factor XIa inhibitor. Clin Transl Sci. 2022;15(2):330-342. Erratum in: Clin Transl Sci. 2022;15(4): 1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas D, Kanefendt F, Schwers S, Unger S, Yassen A, Boxnick S. First evaluation of the safety, pharmacokinetics, and pharmacodynamics of BAY 2433334, a small molecule targeting coagulation factor XIa. J Thromb Haemost. 2021;19(10):2407-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kubitza D, Heckmann M, Distler J, Koechel A, Schwers S, Kanefendt F. Pharmacokinetics, pharmacodynamics and safety of BAY 2433334, a novel activated factor XI inhibitor, in healthy volunteers: a randomized phase 1 multiple-dose study. Br J Clin Pharmacol. 2022;88(7):3447-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Büller HR, Bethune C, Bhanot S, et al. FXI-ASO TKA Investigators. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372(3):232-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walsh M, Bethune C, Smyth A, et al. CS4 Investigators. Phase 2 study of the factor XI antisense inhibitor IONIS-FXIRx in patients with ESRD. Kidney Int Rep. 2021;7(2):200-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Yu RZ, Henry S, Geary RS. Pharmacokinetics and clinical pharmacology considerations of GalNAc3-conjugated antisense oligonucleotides. Expert Opin Drug Metab Toxicol. 2019;15(6):475-485. [DOI] [PubMed] [Google Scholar]
- 76.Willmann S, Marostica E, Snelder N, et al. PK/PD modeling of FXI antisense oligonucleotides to bridge the dose-FXI activity relation from healthy volunteers to end-stage renal disease patients. CPT Pharmacometrics Syst Pharmacol. 2021;10(8):890-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verhamme P, Yi BA, Segers A, et al. ANT-005 TKA Investigators. Abelacimab for prevention of venous thromboembolism. N Engl J Med. 2021;385(7):609-617. [DOI] [PubMed] [Google Scholar]
- 78.Weitz JI, Bauersachs R, Becker B, et al. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the FOXTROT randomized clinical trial. JAMA. 2020;323(2):130-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan NC, Weitz JI. AB023, a novel antibody that binds factor XI and blocks its activation by factor XIIa. Arterioscler Thromb Vasc Biol. 2019;39(4):533-535. [DOI] [PubMed] [Google Scholar]
- 80.Lorentz CU, Verbout NG, Wallisch M, et al. Contact activation inhibitor and factor XI antibody, AB023, produces safe, dosedependent anticoagulation in a phase 1 first-in-human trial. Arterioscler Thromb Vasc Biol. 2019;39(4):799-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lorentz CU, Tucker EI, Verbout NG, et al. The contact activation inhibitor AB023 in heparin-free hemodialysis: results of a randomized phase 2 clinical trial. Blood. 2021;138(22):2173-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nowotny B, Thomas D, Schwers S, et al. First randomized evaluation of safety, pharmacodynamics, and pharmacokinetics of BAY 1831865, an antibody targeting coagulation factor XI and factor XIa, in healthy men. J Thromb Haemost. 2022;20(7):1684-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dilger AK, Pabbisetty KB, Corte JR, et al. Discovery of milvexian, a high-affinity, orally bioavailable inhibitor of factor XIa in clinical studies for antithrombotic therapy. J Med Chem. 2022;65(3):1770-1785. [DOI] [PubMed] [Google Scholar]
- 84.Weitz JI, Strony J, Ageno W, et al. AXIOMATIC-TKR Investigators. Milvexian for the prevention of venous thromboembolism. N Engl J Med. 2021;385(23):2161-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perera V, Abelian G, Li D, et al. Single-dose pharmacokinetics of milvexian in participants with mild or moderate hepatic impairment compared with healthy participants. Clin Pharmacokinet. 2022;61(6):857-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharma M, Molina CA, Toyoda K, et al. Safety and efficacy of factor XIa inhibition with milvexian for secondary stroke prevention (AXIOMATIC-SSP): a phase 2, international, randomised, double-blind, placebo-controlled, dose-finding trial. Lancet Neurol. 2024;23(1):46-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heitmeier S, Visser M, Tersteegen A, et al. Pharmacological profile of asundexian, a novel, orally bioavailable inhibitor of factor XIa. J Thromb Haemost. 2022;20(6):1400-1411. Erratum in: J Thromb Haemost. 2022;20(10): 2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piccini JP, Caso V, Connolly SJ, et al. PACIFIC-AF Investigators. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): a multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet. 2022;399(10333):1383-1390. [DOI] [PubMed] [Google Scholar]
- 89.Shoamanesh A, Mundl H, Smith EE, et al. PACIFIC-Stroke Investigators. Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet. 2022;400(10357):997-1007. [DOI] [PubMed] [Google Scholar]
- 90.Rao SV, Kirsch B, Bhatt DL, et al. PACIFIC AMI Investigators. A multicenter, phase 2, randomized, placebo-controlled, double-blind, parallel-group, dose-finding trial of the oral factor XIa inhibitor asundexian to prevent adverse cardiovascular outcomes after acute myocardial infarction. Circulation. 2022;146(16):1196-1206. Erratum in: Circulation. 2022;146(23): e330. [DOI] [PubMed] [Google Scholar]
- 91.Beale D, Dennison J, Boyce M, et al. ONO-7684 a novel oral FXIa inhibitor: safety, tolerability, pharmacokinetics and pharmacodynamics in a first-in-human study. Br J Clin Pharmacol. 2021;87(8):3177-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gómez-Outes A, Suárez-Gea ML, Lecumberri R, Rocha E, Pozo-Hernández C, Vargas-Castrillón E. New parenteral anticoagulants in development. Ther Adv Cardiovasc Dis. 2011;5(1):33-59. [DOI] [PubMed] [Google Scholar]
- 93.Wong PC, Crain EJ, Watson CA, Schumacher WA. A small-molecule factor XIa inhibitor produces antithrombotic efficacy with minimal bleeding time prolongation in rabbits. J Thromb Thrombolysis. 2011;32(2):129-137. [DOI] [PubMed] [Google Scholar]
- 94.Wong PC, Quan ML, Watson CA, et al. In vitro, antithrombotic and bleeding time studies of BMS-654457, a small-molecule, reversible and direct inhibitor of factor XIa. J Thromb Thrombolysis. 2015;40(4):416-423. [DOI] [PubMed] [Google Scholar]
- 95.Pollack CV Jr, Kurz MA, Hayward NJ. EP-7041, a factor XIa inhibitor as a potential antithrombotic strategy in extracorporeal membrane oxygenation: a brief report. Crit Care Explor. 2020;2(9):e0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Donkor DA, Bhakta V, Eltringham-Smith LJ, Stafford AR, Weitz JI, Sheffield WP. Selection and characterization of a DNA aptamer inhibiting coagulation factor XIa. Sci Rep. 2017;7(1):2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Woodruff RS, Ivanov I, Verhamme IM, Sun MF, Gailani D, Sullenger BA. Generation and characterization of aptamers targeting factor XIa. Thromb Res. 2017;156:134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen W, Carvalho LP, Chan MY, Kini RM, Kang TS. Fasxiator, a novel factor XIa inhibitor from snake venom, and its site-specific mutagenesis to improve potency and selectivity. J Thromb Haemost. 2015;13(2):248-261. [DOI] [PubMed] [Google Scholar]
- 99.Ma D, Mizurini DM, Assumpção TC, et al. Desmolaris, a novel factor XIa anticoagulant from the salivary gland of the vampire bat (Desmodus rotundus) inhibits inflammation and thrombosis in vivo. Blood. 2013;122(25):4094-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Decrem Y, Rath G, Blasioli V, et al. Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J Exp Med. 2009;206(11):2381-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Greco A, Laudani C, Spagnolo M, et al. Pharmacology and clinical development of factor XI inhibitors. Circulation. 2023;147(11):897-913. [DOI] [PubMed] [Google Scholar]
- 102.Salomon O, Gailani D. A proposal for managing bleeding in patients on therapeutic factor XI(a) inhibitors. J Thromb Haemost. 2022;20(1):32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Akbulut AC, Arisz RA, Baaten CCFMJ, et al. Blood coagulation and beyond: Position Paper from the Fourth Maastricht Consensus Conference on Thrombosis. Thromb Haemost. 2023;123(8):808-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Presume J, Ferreira J, Ribeiras R, Mendes M. Achieving higher efficacy without compromising safety with factor XI inhibitors versus low molecular weight heparin for the prevention of venous thromboembolism in major orthopedic surgery - systematic review and meta-analysis. J Thromb Haemost. 2022;20(12):2930-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ruff C. AZALEA TIMI 71 A multicenter randomized active controlled study to evaluate the safety and tolerability of two blinded doses of abelacimab compared with open label rivaroxaban in patients with atrial fibrillation. https://timi.org/wp-content/uploads/2023/11/Christian-Ruff-AZALEA-TIMI-71-A-Multicenter-RandomiZed-Active-ControLled-Study-to-Evaluate-the-Safety-and-Tolerability-of-Two-Blinded-Doses-of-Abelacimab-Compared-with-Open-Labe.pdf. Accessed March 21, 2024. [Google Scholar]
- 106.Jorgal A. OCEANIC-AF study stopped early due to lack of efficacy. https://www.bayer.com/media/en-us/oceanic-af-study-stopped-early-due-to-lack-of-efficacy/. Accessed March 21, 2004. [Google Scholar]
- 107.Jorgal A. New asundexian phase III study to include patients with atrial fibrillation ineligible for oral anticoagulant treatment. https://www.bayer.com/media/en-us/new-asundexian-phase-iii-study-to-include-patients-with-atrial-fibrillation-ineligible-for-oral-anticoagulant-treatment/. Accessed March 21, 2024. [Google Scholar]
- 108.Palaiodimou L, Papagiannopoulou G, Katsanos AH, et al. Efficacy and safety of oral factor XIa inhibitors in stroke prevention: a systematic review and meta-analysis. J Clin Med. 2023;12(17):5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clinicaltrial.gov. Bethesda (MD): National Library of Medicine (US). A study of ISIS 416858 administered subcutaneously to participants with end-stage renal disease (ESRD) on hemodialysis (EMERALD). https://www.clinicaltrials.gov/study/NCT03358030?tab=results. Accessed March 21, 2024. [Google Scholar]
- 110.Clinicaltrial.gov. Bethesda (MD): National Library of Medicine (US). Factor XI LICA to reduce events such as heart attack and stroke in patients whose kidneys are no longer able to work as they should and require treatment to filter wastes from the blood: focus is on the safety of BAY2976217 and the way the body absorbs, distributes and removes the study drug (RE-THINc ESRD). https://clinicaltrials.gov/study/NCT04534114?tab=results. Accessed March 21, 2004. [Google Scholar]
- 111.Weitz JI, Tankó LB, Floege J, et al. CONVERT Investigators. Anticoagulation with osocimab in patients with kidney failure undergoing hemodialysis: a randomized phase 2 trial. Nat Med. 2024;30(2):435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clinicaltrial.gov. Bethesda (MD): National Library of Medicine (US). Xisomab 3G3 for the prevention of catheter-associated thrombosis in patients with cancer receiving chemotherapy. https://www.clinicaltrials.gov/study/NCT04465760. Accessed March 21, 2024. [Google Scholar]
- 113.Fujisaki T, Sueta D, Yamamoto E, et al. Comparing anticoagulation strategies for venous thromboembolism associated with active cancer: a systematic review and meta-analysis. J Am Coll Cardiol CardioOnc. 2024;6(1):99-113. [DOI] [PMC free article] [PubMed] [Google Scholar]